|
1
|
Barsouk A, Aluru JS, Rawla P, Saginala K
and Barsouk A: Epidemiology, risk factors, and prevention of head
and neck squamous cell carcinoma. Med Sci (Basel).
11(42)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marur S and Forastiere AA: Head and neck
squamous cell carcinoma: Update on epidemiology, diagnosis, and
treatment. Mayo Clin Proc. 91:386–396. 2016.PubMed/NCBI View Article : Google Scholar
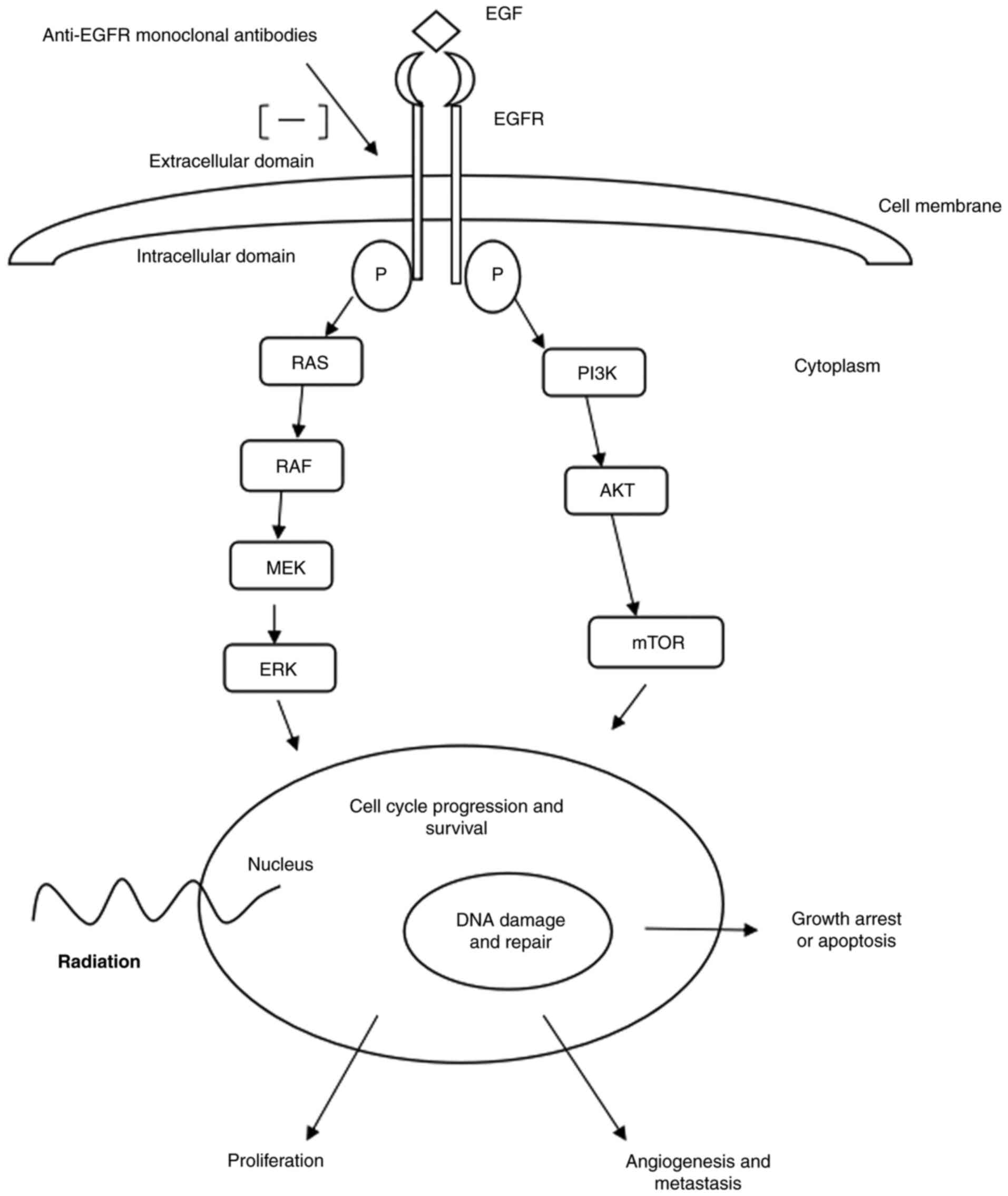
|
|
3
|
Adelstein DJ, Li Y, Adams GL, Wagner H,
Kish JA, Ensley JF, Schuller DE and Forastiere AA: An intergroup
phase III comparison of standard radiation therapy and two
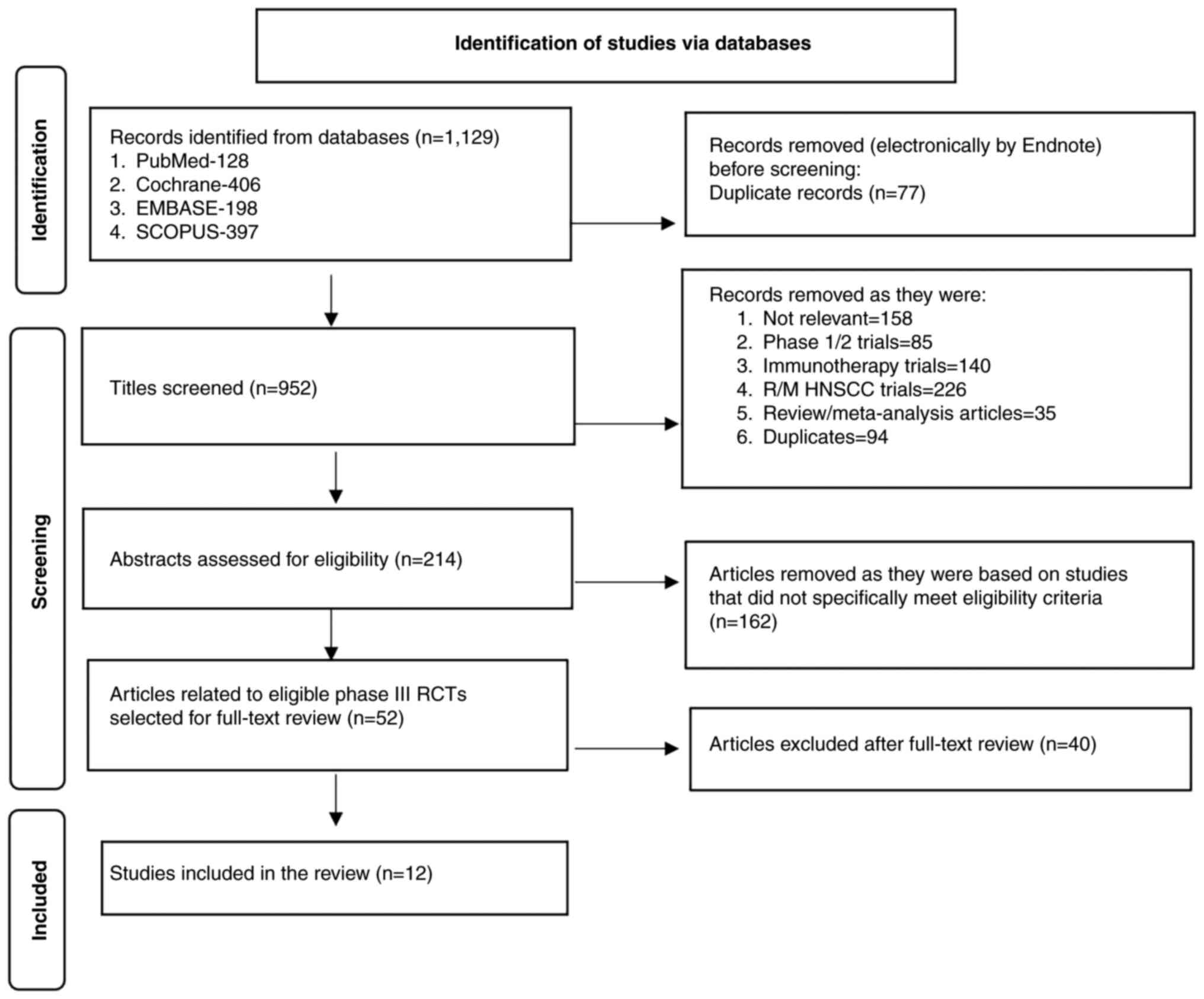
schedules of concurrent chemoradiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098.
2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pignon JP, Bourhis J, Domenge C and
Designé L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: Three meta-analyses of updated
individual data. MACH-NC collaborative group. meta-analysis of
chemotherapy on head and neck cancer. Lancet. 355:949–955.
2000.PubMed/NCBI
|
|
6
|
Pignon JP, le Maître A, Maillard E and
Bourhis J: MACH-NC Collaborative Group. Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lacas B, Carmel A, Landais C, Wong SJ,
Licitra L, Tobias JS, Burtness B, Ghi MG, Cohen EEW, Grau C, et al:
Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An
update on 107 randomized trials and 19,805 patients, on behalf of
MACH-NC group. Radiother Oncol. 156:281–293. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trotti A, Bellm LA, Epstein JB, Frame D,
Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L and Zilberberg MD:
Mucositis incidence, severity and associated outcomes in patients
with head and neck cancer receiving radiotherapy with or without
chemotherapy: A systematic literature review. Radiother Oncol.
66:253–262. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bossi P and Platini F: Radiotherapy plus
EGFR inhibitors: Synergistic modalities. Cancers Head Neck.
2(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Harari PM and Huang SM: Head and neck
cancer as a clinical model for molecular targeting of therapy:
Combining EGFR blockade with radiation. Int J Radiat Oncol Biol
Phys. 49:427–433. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Iberri DJ and Colevas AD: Balancing safety
and efficacy of epidermal growth factor receptor inhibitors in
patients with squamous cell carcinoma of the head and neck.
Oncologist. 21(391)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fasano M, Della Corte CM, Viscardi G, Di
Liello R, Paragliola F, Sparano F, Iacovino ML, Castrichino A,
Doria F, Sica A, et al: Head and neck cancer: The role of anti-EGFR
agents in the era of immunotherapy. Ther Adv Med Oncol.
13(1758835920949418)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kalyankrishna S and Grandis JR: Epidermal
growth factor receptor biology in head and neck cancer. J Clin
Oncol. 24:2666–2672. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang SM and Harari PM: Epidermal growth
factor receptor inhibition in cancer therapy: Biology, rationale
and preliminary clinical results. Invest New Drugs. 17:259–269.
1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bai J, Guo XG and Bai XP: Epidermal growth
factor receptor-related DNA repair and radiation-resistance
regulatory mechanisms: A mini-review. Asian Pac J Cancer Prev.
13:4879–4881. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dassonville O, Formento JL, Francoual M,
Ramaioli A, Santini J, Schneider M, Demard F and Milano G:
Expression of epidermal growth factor receptor and survival in
upper aerodigestive tract cancer. J Clin Oncol. 11:1873–1878.
1993.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ang KK, Andratschke NH and Milas L:
Epidermal growth factor receptor and response of head-and-neck
carcinoma to therapy. Int J Radiat Oncol Biol Phys. 58:959–965.
2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liang K, Ang KK, Milas L, Hunter N and Fan
Z: The epidermal growth factor receptor mediates radioresistance.
Int J Radiat Oncol Biol Phys. 57:246–254. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bonner JA, Maihle NJ, Folven BR,
Christianson TJ and Spain K: The interaction of epidermal growth
factor and radiation in human head and neck squamous cell carcinoma
cell lines with vastly different radiosensitivities. Int J Radiat
Oncol Biol Phys. 29:243–247. 1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Moher D, Shamseer L, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P and Stewart LA: PRISMA-P Group.
Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015 statement. Syst Rev. 4(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan
PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar
AK, et al: Randomized phase III trial of concurrent accelerated
radiation plus cisplatin with or without cetuximab for stage III to
IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 32:2940–2950.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Patil VM, Noronha V, Joshi A, Agarwal J,
Ghosh-Laskar S, Budrukkar A, Murthy V, Gupta T, Mahimkar M, Juvekar
S, et al: A randomized phase 3 trial comparing nimotuzumab plus
cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy
alone in locally advanced head and neck cancer. Cancer.
125:3184–3197. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eriksen JG, Maare C, Johansen J, Primdahl
H, Evensen JF, Kristensen CA, Andersen LJ and Overgaard J:
Evaluation of the EGFR-inhibitor zalutumumab given with primary
curative (CHEMO) radiation therapy to patients with squamous cell
carcinoma of the head and neck: Results of the DAHANCA 19
randomized phase 3 trial. Int J Radiat Oncol Biol Phys.
88(465)2014.
|
|
26
|
Gebre-Medhin M, Brun E, Engström P, Cange
HH, Hammarstedt-Nordenvall L, Reizenstein J, Nyman J, Abel E,
Friesland S, Sjödin H, et al: ARTSCAN III: A randomized phase III
study comparing chemoradiotherapy with cisplatin versus cetuximab
in patients with locoregionally advanced head and neck squamous
cell cancer. J Clin Oncol. 39:38–47. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Siu LL, Waldron JN, Chen BE, Winquist E,
Wright JR, Nabid A, Hay JH, Ringash J, Liu G, Johnson A, et al:
Effect of standard radiotherapy with cisplatin vs accelerated
radiotherapy with panitumumab in locoregionally advanced squamous
cell head and neck carcinoma: A randomized clinical trial. JAMA
Oncol. 3:220–226. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Geoffrois L, Martin L, De Raucourt D, Sun
XS, Tao Y, Maingon P, Buffet J, Pointreau Y, Sire C, Tuchais C, et
al: Induction chemotherapy followed by cetuximab radiotherapy is
not superior to concurrent chemoradiotherapy for head and neck
carcinomas: Results of the GORTEC 2007-02 phase III randomized
trial. J Clin Oncol. 36:3077–3083. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Merlano MC, Denaro N, Vecchio S, Licitra
L, Curcio P, Benasso M, Bagicalupo A, Numico G, Russi E, Corvo' R,
et al: Phase III randomized study of induction chemotherapy
followed by definitive radiotherapy + cetuximab versus
chemoradiotherapy in squamous cell carcinoma of head and neck: the
INTERCEPTOR-GONO study (NCT00999700). Oncology. 98:763–770.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hitt R, Mesía R, Lozano A, Iglesias
Docampo L, Grau JJ, Taberna M, Rubió-Casadevall J, Martínez-Trufero
J, Morillo EDB, García Girón C, et al: Randomized phase 3
noninferiority trial of radiotherapy and cisplatin vs radiotherapy
and cetuximab after docetaxel-cisplatin-fluorouracil induction
chemotherapy in patients with locally advanced unresectable head
and neck cancer. Oral Oncol. 134(106087)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mehanna H, Robinson M, Hartley A, Kong A,
Foran B, Fulton-Lieuw T, Dalby M, Mistry P, Sen M, O'Toole L, et
al: Radiotherapy plus cisplatin or cetuximab in low-risk human
papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An
open-label randomised controlled phase 3 trial. Lancet. 393:51–60.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gillison ML, Trotti AM, Harris J, Eisbruch
A, Harari PM, Adelstein DJ, Jordan RCK, Zhao W, Sturgis EM,
Burtness B, et al: Radiotherapy plus cetuximab or cisplatin in
human papillomavirus-positive oropharyngeal cancer (NRG Oncology
RTOG 1016): A randomised, multicentre, non-inferiority trial.
Lancet. 393:40–50. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rischin D, King M, Kenny L, Porceddu S,
Wratten C, Macann A, Jackson JE, Bressel M, Herschtal A, Fisher R,
et al: Randomized trial of radiation therapy with weekly cisplatin
or cetuximab in low-risk HPV-associated oropharyngeal cancer (TROG
12.01)-a trans-tasman radiation oncology group study. Int J Radiat
Oncol Biol Phys. 111:876–886. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sobin LH, Gospodarowicz MK and Christian
Wittekind C (eds): International Union Against cancer (UICC): TNM
classification of malignant tumours. 7th edition. Oxford,
Wiley-Blackwell, 2009.
|
|
35
|
Fleming ID, Cooper JS, Henson DE, Hutter
RVP, Kennedy BJ and Murphy GP (eds): American Joint Committee on
Cancer (AJCC). AJCC cancer staging manual. 5th edition.
Philadelphia: J. B. Lippincott, 1997.
|
|
36
|
Greene FL, Page DL, Fleming ID, Fritz A
and Balch CM: American Joint Committee on Cancer. AJCC cancer
staging manual. 6th edition. New York, NY: Springer-Verlag,
2002.
|
|
37
|
Rosenthal DI, Mendoza TR, Chambers MS,
Asper JA, Gning I, Kies MS, Weber RS, Lewin JS, Garden AS, Ang KK,
et al: Measuring head and neck cancer symptom burden: The
development and validation of the M. D. Anderson symptom inventory,
head and neck module. Head Neck. 29:923–931. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bonner JA, Harari PM, Giralt J, Cohen RB,
Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-Year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash and survival. Lancet
Oncol. 11:21–28. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Roman J, Dissaux G, Gouillou M, Gobel Y,
Potard G, Leclere JC, Conan-Charlet V, Gujral D, Abgral R, Guibourg
B, et al: Prolonged overall treatment time and lack of skin rash
negatively impact overall survival in locally advanced head and
neck cancer patients treated with radiotherapy and concomitant
cetuximab. Target Oncol. 12:505–512. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Caudell JJ, Torres-Saavedra P, Rosenthal
DI, Axelrod R, Nguyen-Tan PF, Sherman E, Weber RS, Galvin JM,
El-Naggar AK, Konski AA, et al: Long-term update of NRG/RTOG 0522:
A randomized phase 3 trial of concurrent radiation and cisplatin
with or without cetuximab in locoregionally advanced head and neck
cancer. Int J Radiat Oncol Biol Phys. 116:533–543. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Eriksen JG, Maare C, Johansen J, Primdahl
H, Evensen J, Kristensen CA, Andersen LJ and Overgaard J: 5-Y
update of the randomized phase III trial DAHANCA19: Primary (Chemo)
RT +/- zalutumumab in HNSCC. Radiother Oncol. 127:S137–S138.
2018.
|
|
42
|
Ringash J, Waldron JN, Siu LL, Martino R,
Winquist E, Wright JR, Nabid A, Hay JH, Hammond A, Sultanem K, et
al: Quality of life and swallowing with standard chemoradiotherapy
versus accelerated radiotherapy and panitumumab in locoregionally
advanced carcinoma of the head and neck: A phase III randomised
trial from the Canadian cancer trials group (HN.6). Eur J Cancer.
72:192–199. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cohen EE, Karrison TG, Kocherginsky M,
Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB,
Yunus F, et al: Phase III randomized trial of induction
chemotherapy in patients with N2 or N3 locally advanced head and
neck cancer. J Clin Oncol. 32:2735–2743. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tao Y, Geoffrois L, Martin L, De Raucourt
D, Miny J, Maingon P, Lafond C, Tuchais C, Sire C, Babin E, et al:
Impact of p16 expression on induction taxotere-cisplatin-5 FU (TPF)
followed by cetuximab-radiotherapy in N2b-N3 head and neck squamous
cell carcinoma (HNSCC): Results of GORTEC 2007-02 phase III
randomized trial. J Clin Oncol. 35 (15 Suppl)(S6070)2017.
|
|
45
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jones DA, Mistry P, Dalby M, Fulton-Lieuw
T, Kong AH, Dunn J, Mehanna HM and Gray AM: Concurrent cisplatin or
cetuximab with radiotherapy for HPV-positive oropharyngeal cancer:
Medical resource use, costs, and quality-adjusted survival from the
De-ESCALaTE HPV trial. Eur J Cancer. 124:178–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Garrido G, Tikhomirov IA, Rabasa A, Yang
E, Gracia E, Iznaga N, Fernández LE, Crombet T, Kerbel RS and Pérez
R: Bivalent binding by intermediate affinity of nimotuzumab: A
contribution to explain antibody clinical profile. Cancer Biol
Ther. 11:373–382. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Berger C, Krengel U, Stang E, Moreno E and
Madshus IH: Nimotuzumab and cetuximab block ligand-independent EGF
receptor signaling efficiently at different concentrations. J
Immunother. 34:550–555. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Noronha V, Joshi A, Patil VM, Agarwal J,
Ghosh-Laskar S, Budrukkar A, Murthy V, Gupta T, D'Cruz AK, Banavali
S, et al: Once-a-week versus once-every-3-weeks cisplatin
chemoradiation for locally advanced head and neck cancer: A phase
III randomized noninferiority trial. J Clin Oncol. 36:1064–1072.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mashhour K and Hashem W: Cisplatin weekly
versus every 3 weeks concurrently with radiotherapy in the
treatment of locally advanced head and neck squamous cell
carcinomas: What is the best dosing and schedule? Asian Pac J
Cancer Prev. 21:799–807. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Menon N, Patil V, Noronha V, Joshi A,
Bhattacharjee A, Satam BJ, Mathrudev V, Ghosh Laskar S and Prabhash
K: Quality of life in patients with locally advanced head and neck
cancer treated with concurrent chemoradiation with cisplatin and
nimotuzumab versus cisplatin alone-additional data from a phase 3
trial. Oral Oncol. 122(105517)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Radiation Therapy With Durvalumab or
Cetuximab in Treating Patients With Locoregionally Advanced Head
and Neck Cancer Who Cannot Take Cisplatin-Full Text View-ClinicalTrials.gov, 2022. Available from:
https://clinicaltrials.gov/ct2/show/NCT03258554.
|
|
53
|
Mell LK, Torres-Saavedra P, Wong S, Chang
S, Kish JA, Minn AJ III, Jordan R, Liu T, Truong MT, Winquist E, et
al: Radiotherapy with durvalumab vs cetuximab in patients with
locoregionally advanced head and neck cancer and a contraindication
to cisplatin: Phase II results of NRG-HN004. Int J Radiat Oncol
Biol Phys. 114(1058)2022.
|
|
54
|
Tao Y, Biau J, Sun X, Sire C, Martin L,
Alfonsi M, Prevost JB, Modesto A, Lafond C, Tourani JM, et al:
Pembrolizumab versus cetuximab concurrent with radiotherapy in
patients with locally advanced squamous cell carcinoma of head and
neck unfit for cisplatin (GORTEC 2015-01 PembroRad): A multicenter,
randomized, phase II trial. Ann Oncol. 34:101–110. 2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Randomized Trial of
Avelumab-cetuximab-radiotherapy Versus SOCs in LA SCCHN
(REACH)-Full Text View-ClinicalTrials.gov, 2022. Available from:
https://clinicaltrials.gov/ct2/show/NCT02999087.
|
|
56
|
Tao Y, Aupérin A, Sun X, Sire C, Martin L,
Coutte A, Lafond C, Miroir J, Liem X, Rolland F, et al:
Avelumab-cetuximab-radiotherapy versus standards of care in locally
advanced squamous-cell carcinoma of the head and neck: The safety
phase of a randomised phase III trial GORTEC 2017-01 (REACH). Eur J
Cancer. 141:21–29. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bourhis J, Tao Y, Sun X, Sire C, Martin L,
Liem X, Coutte A, Pointreau Y, Thariat J, Miroir J, et al: LBA35
Avelumab-cetuximab-radiotherapy versus standards of care in
patients with locally advanced squamous cell carcinoma of head and
neck (LA-SCCHN): Randomized phase III GORTEC-REACH trial. Ann
Oncol. 32 (Suppl 5)(S1310)2021.
|
|
58
|
Temam S, Kawaguchi H, El-Naggar AK,
Jelinek J, Tang H, Liu DD, Lang W, Issa JP, Lee JJ and Mao L:
Epidermal growth factor receptor copy number alterations correlate
with poor clinical outcome in patients with head and neck squamous
cancer. J Clin Oncol. 25:2164–2170. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Chung CH, Ely K, McGavran L,
Varella-Garcia M, Parker J, Parker N, Jarrett C, Carter J, Murphy
BA, Netterville J, et al: Increased epidermal growth factor
receptor gene copy number is associated with poor prognosis in head
and neck squamous cell carcinomas. J Clin Oncol. 24:4170–4176.
2006.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bossi P, Resteghini C, Paielli N, Licitra
L, Pilotti S and Perrone F: Prognostic and predictive value of EGFR
in head and neck squamous cell carcinoma. Oncotarget.
7:74362–74379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Posner MR and Wirth LJ: Cetuximab and
radiotherapy for head and neck cancer. N Engl J Med. 354:634–636.
2006.PubMed/NCBI View Article : Google Scholar
|