Introduction
Head and neck squamous cell carcinoma (HNSCC) is
reported as the seventh most common type of cancer worldwide
(1). In total, >50% of patients
with head and neck cancer present with loco-regionally advanced
disease at the time of diagnosis (2). The standard treatment for locally
advanced head and neck squamous cell carcinoma (LAHNSCC) is
concurrent chemoradiation or surgery followed by adjuvant treatment
(3,4). The MACH-NC meta-analyses had shown an
overall survival benefit with concurrent chemotherapy compared to
radiotherapy (RT) alone (5-7).
However, the combined modality treatment is associated with higher
rates of acute and late toxicities (8). Even following combined modality
treatment, ~50% of patients experience relapse (9). Therefore, to improve treatment efficacy
and prevent the incidence of adverse events, novel small molecules
designed to target various different signaling pathways are
increasingly being tested alongside RT or chemoradiation (9-13).
In total, 80-90% of HNSCC cases are reported to
exhibit upregulated expression levels of EGFRs. EGFR upregulation
in cancer cells is associated with local treatment failure,
resistance to radiation, increased incidence of distant metastases
and decreased survival. In cancer cells with upregulated EGFR
expression, radiation can activate the EGFR pathway, resulting in
re-proliferation of tumor cells and DNA repair, resulting in
treatment failure. Anti-EGFR monoclonal antibodies block the
activation of the EGFR pathway, resulting in inhibition of tumor
cell proliferation, tumor angiogenesis and the development of
metastasis. Anti-EGFR monoclonal antibodies also play a role in the
enhancement of the effect of radiation through suppression of DNA
repair during RT (10-20).
Thus, improved disease control is considered to be achieved through
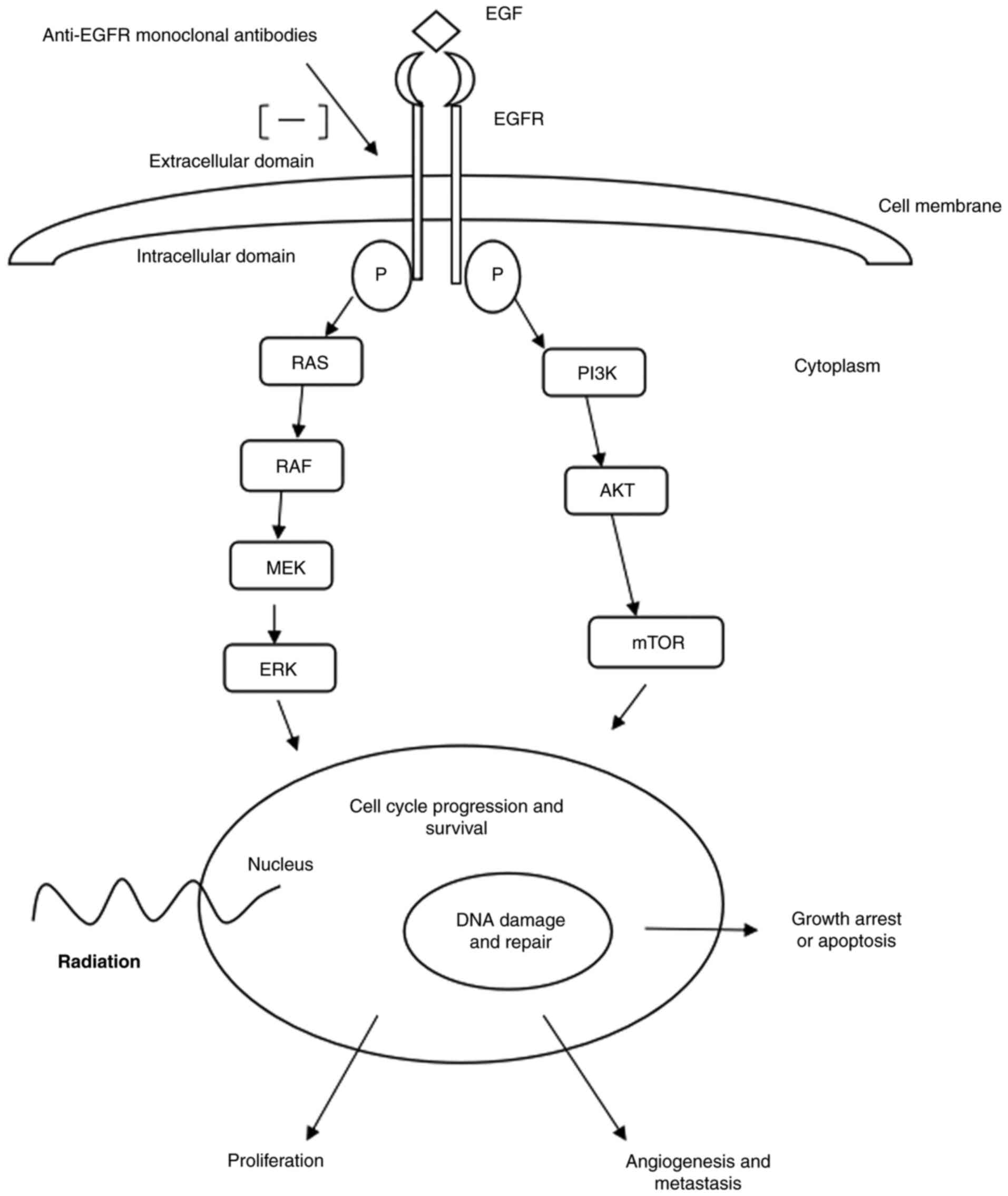
the synergistic action of radiation and EGFR blockade. Fig. 1 demonstrates the EGFR pathway by
which EGFR blockade is considered to work in the treatment of
LAHNSCC.
The objective of the present systematic review was
to gather evidence from a list of published phase III clinical
trials that investigated the efficacy of anti-EGFR monoclonal
antibody therapy for the definitive treatment of LAHNSCC, including
human papillomavirus (HPV)+-oropharyngeal carcinoma
(OPC). Ultimately, the present study aimed to compare the
effectiveness of anti-EGFR therapy in combination with RT or
chemoradiotherapy with that of the non-surgical standard treatment
method for the treatment of LAHNSCC.
Materials and methods
Inclusion/exclusion criteria
The inclusion criteria were as follows: i) Articles
published in peer-reviewed journals; ii) phase III randomized
clinical trials (RCTs); iii) patients with LAHNSCC and patients
with HPV+-OPC; iv) patients that received an anti-EGFR
agent in combination with RT or chemoradiotherapy; and v) patients
that received non-surgical standard treatment (RT or
chemoradiotherapy). The exclusion criteria were as follows: i)
Phase I or phase II clinical trials; ii) observational studies; and
iii) studies involving patients with nasopharyngeal carcinoma.
Data sources and literature
search
The present study was conducted based on the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
(PRISMA) guidelines (21). The
PubMed (www.ncbi.nlm.nih.gov/pubmed/), SCOPUS (https://www.scopus.com/), EMBASE (https://www.embase.com/) and COCHRANE (https://www.cochranelibrary.com/) databases were
searched without any language or date limits. Searches were
performed using the following key words: ‘head and neck squamous
cell carcinoma’, ‘head and neck cancer’, ‘locally advanced’,
‘anti-EGFR antibody’, ‘panitumumab’, ‘cetuximab’, ‘nimotuzumab’ and
‘zalutumumab’. A sensitive search filter was used to identify RCTs.
The original literature search was conducted in November 2022, and
this was later updated in January 2024.
Study selection
Titles from the search results were exported to
Endnote (https://www.myendnoteweb.com/EndNoteWeb.html) by one
author before any duplicates were removed. Titles pertaining to
phase I/II studies, immunotherapy trials or trials involving
patients with recurrent/metastatic HNSCC were all removed by the
same author. The remaining titles were then screened further for
relevance. Abstracts of potentially eligible studies were
independently analyzed by two authors and articles pertaining to
eligible studies were selected for full-text review. Disagreements
were resolved through discussion among the authors at each step.
Phase III RCTs that evaluated the role of an anti-EGFR agent in
combination with RT or chemoradiotherapy were included in the
present systematic review. Cetuximab, nimotuzumab, panitumumab and
zalutumumab were the four anti-EGFR agents studied. The following
data were extracted from each study: Patient population,
intervention, parameters being compared, outcomes and adverse
events. The search syntax is provided in Table SI, Table
SII, Table SIII and Table SIV.
Results
Description of included studies
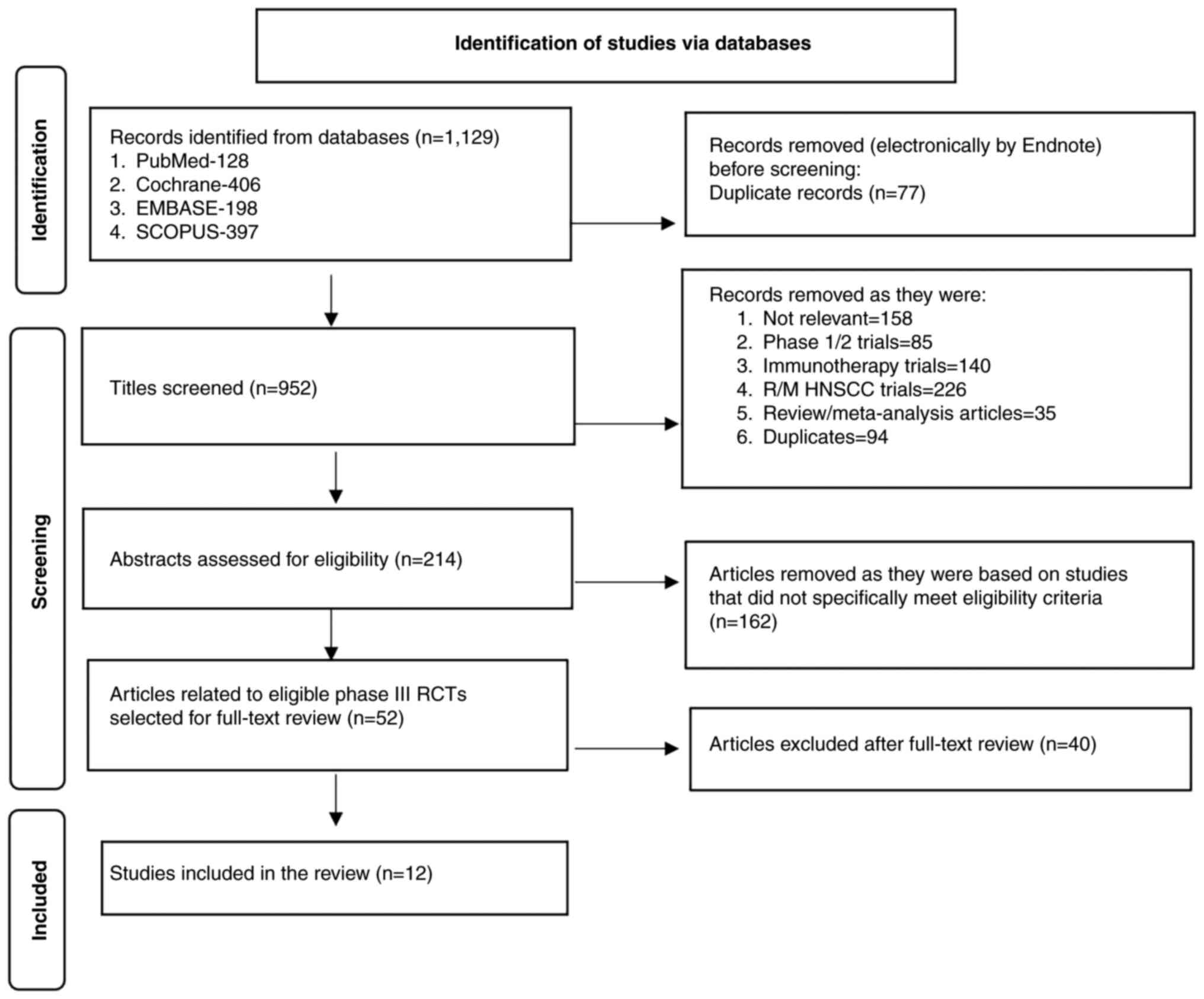
The updated literature search performed in January,
2024 identified 1,129 titles from the four databases. After
removing duplicates, 952 titles were screened, among which 214
titles were selected for abstract review to identify potentially
eligible studies. After abstract review, 162 records were excluded,
and the remaining 52 articles were selected for full-text review.
From these papers, 12 original articles on 12 unique phase III
clinical studies were selected for the present review. The PRISMA
flow chart of study selection for the present review is shown in
Fig. 2. A detailed summary of the 12
RCTs (22-33)
that tested the effectiveness of anti-EGFR monoclonal antibodies
for the treatment of LAHNSCC is provided in Table I.
 | Table IRCTs testing the effectiveness of
anti-EGFR monoclonal antibody in treating locally advanced head and
neck squamous cell carcinoma. |
Table I
RCTs testing the effectiveness of
anti-EGFR monoclonal antibody in treating locally advanced head and
neck squamous cell carcinoma.
| A, RT + concurrent
monoclonal anti-EGFR antibody vs. RT alone (1 RCT) |
|---|
| Author/s, year | Anti-EGFR
agent | Design | Population | Intervention | Control | Follow-up | Primary
endpoint | Secondary
endpoints | (Refs.) |
|---|
| Bonner et
al, 2006, 2010 | Cetuximab | RCT | Patients with
locoregionally advanced head and neck cancer of oropharynx,
hypopharynx, larynx (n=424) | High dose RT+
cetuximab High dose RT (Investigators were required to choose one
of the following three RT regimens: 1.70.0 Gy in 35 fractions once
daily, 2. 72.0-76.8 Gy in 60-64 fractions twice daily 3. 72.0 Gy in
42 fractions for concomitant boost schedule) + Weekly cetuximab
administered 1 week before RT at a dose of 400 mg/m2,
followed by 250 mg/m2 weekly for the duration of RT.
(n=211) | High dose RT alone
High dose RT (Investigators were required to choose one of the
following three RT regimens: 1.70.0 Gy in 35 fractions once daily
2.72.0-76.8 Gy in 60-64 fractions twice daily 3. 72.0 Gy in 42
fractions for concomitant boost schedule; (n=213) | 54 months | LRC locoregional
control of disease duration: Interventio- 24.4 months; control-
14.9 months; HR for locoregional progression/death, 0.68 (95% CI,
0.52-0.89); P=0.005 | OS: After a median
follow-up duration of 54 months, the median OS was 49 months in the
intervention arm compared with 29.3 months in the control arm; HR
fordeath, 0.74 (95% CI, 0.57-0.97); P=0.03. PFS: Intervention-17.1
months; control-12.4 months; HR for disease progression/death,
0.70;95% CI, 0.54-0.90; P=0.006 ORR: Intervention-74%; control-64%;
OR, 0.57 (95% CI, 0.36-0.90); P=0.02. Adverse effects (grade ≥3):
No difference in terms of adverse events between the intervention
and control arms except for acneiform rash and infusion reaction.
Cetuximab arm had higher rates of acneiform rash (P=<0.001) and
infusion reaction. (P=0.01) | (22,38) |
| B, Concurrent
chemoradiotherapy + anti-EGFR monoclonal antibody vs. concurrent
chemoradiotherapy alone (3 RCTs) |
| Author/s, year | Anti-EGFR
agent | Design | Population | Intervention | Control | Follow-up | Primary
endpoint | Secondary
endpoints | (Refs.) |
| Ang et al,
2014 (RTOG 0522) | Cetuximab | RCT | Patients with stage
III or IV non-metastatic squamous cell carcinoma of the oropharynx,
hypopharynx, or larynx Patients were stratified by tumour site
(larynx vs. other), Nodal stage (N0 vs. N1-N2b vs. N2c-N3), Zubrod
performance status (0 vs. 1), Use of IMRT (yes v no) and receipt of
pre-treatment fused PET scan or CT scan (yes vs. No) (n=891) | Chemo-RT +
cetuximab RT (72 Gy in 42 fractions over 6 weeks or 70 Gy in 35
fractions over 6 weeks using twice-a-day dosing once a week for 5
weeks) + cisplatin 100 mg/m2 D1 and D22 + Cetuximab (400
mg/m2 1 week before RT and at 250 mg/m2
weekly during RT; Used IMRT or three- dimensional conformal RT
(3DCRT) techniques for radiation planning and delivery (n=444) | Chemo-RT alone RT
(72 Gy in 42 fractions over 6 weeks or 70 Gy in 35 fractions over 6
weeks using twice-a-day dosing once a week for 5 weeks) + cisplatin
100 mg/m2 D1 and D22; Used IMRT or three-dimensional
conformal RT (3DCRT) techniques for radiation planning and delivery
(n=447) | 3.8 years | PFS The 3-year PFS
was 58.9% (95% CI, 54.2-63.6%) in the intervention arm (cetuximab +
cisplatin-RT) vs. 61.2% (95% CI, 56.7-65.8%) in the control arm
(cisplatin-RT; P=0.76). | The 3-year OS was
75.8% (95% CI, 71.7-79.9%) in the intervention arm vs. 72.9% (95%
CI, 68.7-77.1%) in the control arm (P=0.32). The 3-year LRF was
25.9% (95% CI, 21.7-30.1%) in the intervention arm compared with
19.9% (95% CI, 16.2-23.7%) in the control arm (P=0.97). The rates
of distant metastasis in the two arms were not significantly
different (13.0 vs. 9.7%; P=0.08). Adverse events: Patients who
received cetuximab + cisplatin experienced more treatment- related
grade 5 adverse events (10 vs. 3 events; P=0.05) compared with the
control patients. The rate of grade 3 to grade 4 radiation
mucositis was higher in the cetuximab arm compared with the control
arm (43.2% vs. 33.3%). Intervention patients experienced higher
rates of grade 3 to grade 4 skin reactions, fatigue, anorexia, and
hypokalaemia during the first 90 days of therapy compared with the
control patients. | (23,40)a |
| Patil et al,
2019 | Nimotuzumab | RCT | Non-metastatic,
stage III or IV SCC of oropharynx, larynx, hypopharynx, or oral
cavity (n=536) | Chemo-RT plus +
nimotuzumab High dose RT 5days/week, (gross tumour and lymph node
disease received 70 Gy at 2 Gy/fraction, uninvolved nodal regions
in the neck received 46-50 Gy. Other altered fractionation
schedules were allowed if the biological equivalent dose for tmour
control was similar to 70 Gy at 2 Gy/ fraction) 2D-RT, 3DCRT or
IMRT + Cisplatin 30 mg/m2 weekly + Nimotuzumab 200 mg iv
weekly (n=268) | Chemo-RT alone High
dose RT 5days/week, (gross tumour and lymph node disease received
70 Gy at 2 Gy/fraction, uninvolved nodal regions in the neck
received 46-50 Gy. Other altered fractionation schedules were
allowed if the biological equivalent dose for tmour control was
similar to 70 Gy at 2 Gy/fraction) 2D-RT, 3DCRT or IMRT + Cisplatin
30 mg/m2 weekly (n=268) | 39.13 months | PFS The 2-year PFS
were 61.8% (95% CI, 55.2-67.7) in the intervention arm and 50.1%
(95% CI, 43.7-56.2) in the control arm (HR, 0.69; 95% CI,
0.53-0.89, P=0.004) | OS: 2-year OS,
63.8% (95% CI, 57.3-69.6) for intervention vs. 57.7% for control
(95% CI, 50.9-63.6; P=0.163). LRC: 2-year LRC, 67.5% (95% CI,
60.9-73.3) for intervention vs. 57.6% (95% CI, 50.9-63.6) for
control; HR, 0.67 (95% CI, 0.50-0.89; P=0.006). DFS: HR, 0.71; 95%
CI, 0.55-0.92; P=0.008. Adverse events: Higher incidence of grade
3-5 mucositis in the nimotuzumab + cisplatin RT arm. The incidence
of other grade 3-5 adverse effects was similar in the two groups.
There was no difference the global health status QoL scores over
time (P=0.396) between the nimotuzumab +chemoRT arm and the control
arm (chemoRT) | (24,51)a |
| Eriksen et
al, 2014, 2018 (DAHANCA 19) | Zalutumumab | RCT | Biopsy-verified
HNSCC of the oral cavity, oropharynx, hypopharynx, and larynx
(n=619) | Chemo-RT +
zalutumumab Accelerated RT (66-68 Gy, 2 Gy/fraction, 6
fractions/week)+ nimorazole + weekly cisplatin 40 mg/m2
in stage III and IV + Zalutumumab 8 mg/kg weekly. Used IMRT
(n=310) | Chemo-RT alone
Accelerated RT (66-68 Gy,2Gy/ fraction,6 fractions/ week) +
nimorazole + weekly cisplatin 40 mg/m2 in stage III and
IV; Used IMRT (n=309) | 36 months | LRC: 3-year LRC,
78% (intervention) vs. 79% (control) HR, 0.8; 95% CI, 0.6-1.2;
5-year LRC, 70.0% (intervention) vs. 74% (control) HR, 1.10; 95%
CI, 0.81-1.50 | Disease-specific
survival (DSS; HR, 1.0; 95% CI, 0.7-1.7) and OS (HR, 0.9; 95% CI,
0.6-1.3) were similar in the intervention and control arms DSS:
3-year DSS; HR, 1; 95% CI, 0.7-1.7 5-year DSS; HR, 1.12; 95% CI,
0.79-1.60. OS: 3-year OS; HR, 0.9; 95% CI, 0.6-1.3 5-year OS; HR,
1.17; 95% CI, 0.89-1.52. Adverse events: Skin rash occurred in 94%
of patients in the zalutumumab arm, (29% experienced grade 3-4
rash) and 13% of patients stopped zalutumumab due to skin rash.
Patients in the zalutumumab arm experienced significant rates of
confluent mucositis (70% vs. 56%, P=0.001) and grade 3-4 in-field
skin reaction (27% vs. 4% P<0.0001) | (25,41) |
| C, RT + anti-EGFR
monoclonal antibody vs. concurrent chemoradiotherapy alone (2
RCTs) |
| Author/s, year | Anti-EGFR
agent | Design | Population | Intervention | Control | Follow-up | Primary
endpoint | Secondary
endpoints | (Refs.) |
| Gebre-Medhin et
al, 2021 (ARTSCAN III trial) | Cetuximab | RCT | Patients ≥18 years
with previously untreated squamous cell carcinoma of oropharynx,
hypopharynx or larynx, stage III-IV according to UICC TNM
classification, 7th edition, without distant metastasis. Aimed for
curative treatment with definitive RT (n=298) | RT +cetuximab RT
(68 Gy to the primary tumour and lymph node metastases, 54.4 Gy to
elective neck volumes given as daily fractions of 2.0 Gy and 1.6 Gy
respectively, 5 days per week for seven weeks + Cetuximab (400
mg/m2 as loading dose 1 week before start of RT,
followed by 250 mg/m2 per week for 7 weeks Patients with
T3-T4 tumour underwent another random assignment of 63 Gy or 73.1
Gy given to the primary tumour as daily fractions of 2.15 Gy. Used
IMRT, helical tomotherapy or volumetric arc therapy for radiation
planning. (n=149) | Chemo-RT RT (68 Gy
to the primary tumour and lymph node metastases, 54.4 Gy to
elective neck volumes given as daily fractions of 2.0 Gy and 1.6 Gy
respectively,5 days per week for seven weeks + Cisplatin 40
mg/m2 per week for seven weeks Patients with T3-T4
tumour underwent another random assignment of 63 Gy or 73.1 Gy
given to the primary tumour as daily fractions of 2.15 Gy Used
IMRT, helical tomotherapy or volumetric arc therapy for radiation
planning. (n=149) | 39 months | OS: 3-year OS, 78%
(95% CI, 71-85) vs. 88% (95% CI, 83-94); adjusted HR, 1.63; 95% CI,
0.93-2.86; P=0.086. Post hoc subgroup analyses: HR for OS, 5.70
(95% CI, 1.67-19.5) for patients with p16+-OPC.
Cetuximab + RT vs. cisplatin + RT (P=0.03) | LRC: Locoregional
failure at 3 years, 23% (95% CI, 16-31) vs. 9% (95% CI, 4-14).
Adjusted cause-specific HR, 2.49; 95% CI, 1.33-4.66; P=0.0045.
Distant failure: NS. Adverse events: Acute mucositis (P=0.035),
skin reactions (P=0.001) and acneiform rashes (P<0.001) were
more common in the cetuximab group compared with the cisplatin
group. Nausea (P=0.001), vomiting (P=0.015), acute kidney injury
(P<0.001), tinnitus (P=0.002), dysphagia (P=0.033) and
neutropenia (P<0.001) were significantly more common in the
cisplatin arm. Late toxicities such as taste alteration and hearing
impairment were more common in the cisplatin arm, while late pain
and mucosal toxicities were more common in the cetuximab arm.. | (26) |
| Siu et al,
2017 (HN.6 trial) | Panitumumab | RCT | Patients with
locoregionally advanced SCC of the oral cavity, oropharynx larynx
or hypopharynx (n=320) | Arm B
RT+panitumumab Accelerated RT (70 Gy in 35 fractions over 6 weeks)
+ concurrent panitumumab (9 mg/kg) every 3 weeks, starting 1 week
before RT (on days-7,15 and 36) Used IMRT or three-dimensional
conformal RT (3DCRT) techniques for radiation planning and delivery
(n=160) | Arm A Chemo-RT
Standard fractionation RT (70 Gy 35 fractions over 7 weeks) +
concurrent cisplatin 100 mg/m every 3 weeks (on days 1,22 and 43 of
RT Used IMRT or three-dimensional conformal RT (3DCRT) techniques
for radiation planning and delivery (n=160) | 46 months | PFS 2-year PFS, 76%
(95% CI, 68-82) vs. 73% (95% CI, 65-79); HR, 0.95; 95% CI,
0.60-1.50; stratified log-rank test, P=0.83P=0.83 | OS: NS (arm B vs.
arm A; HR, 0.89; 95% CI, 0.54-1.48; stratified log rank test
P=0.66). The 2-year OS was 85% (95% CI, 78-90) for arm A, and 88%
(95% CI, 82-92) for arm B. Recurrence: NS for 2-year cumulative
incidence of local recurrence, regional recurrence, or distant
recurrence. Adverse events: Ototoxic effects such as hearing loss
and tinnitus, GI symptoms such as nausea, vomiting and dehydration,
renal toxic effects, and weight loss were more common in the
cisplatin + standard RT arm. Skin toxicity and grade ≥3 mucositis
were more common in the accelerated RT + panitumumab group. The
incidence of non- hematologic adverse events of grade ≥3 was
similar in both arms (88% in arm A vs. 92% in arm B; P=0.25). There
was no significant difference in the quality of life (QoL)
parameters or swallowing outcomes between the two arms. At 1 year
post treatment no difference was found between the arms in terms of
FACT-H&N change from baseline: -1.70 (Control arm) and -4.81
(intervention), P=0.194 Swallowing related QOL (measured using
SWAL-QOL and MDADI) declined from baseline to every subsequent time
point. | (27,42)a |
| D, Three-drug
induction chemotherapy followed by anti-EGFR monoclonal antibody +
RT vs. chemoradiotherapy alone (2 RCTs) |
| Author/s, year | Anti-EGFR
agent | Design | Population | Intervention | Control | Follow-up | Primary
endpoint | Secondary
endpoints | (Refs.) |
| Geoffrois et
al, 2018 (GORTEC 2007-02) | Cetuximab | RCT | Stage III to IV
non-metastatic SCC of the oral cavity, oropharynx, hypopharynx, or
larynx, with heavy nodal disease (N2b, N2c or N3), which was prone
to distant metastasis (n=360) | TPF + cetuximab+ RT
3 cycles of TPF(docetaxel 75 mg/m2 on day 1+cisplatin 75 mg/m2 on
day 1 + FU 750 mg/m2 on days 1-5) followed by cetuximab at a
loading dose of 400 mg/m2 eight days before starting RT and a
weekly dose of 250 mg/m2 for 7 weeks + RT RT dose was 70
Gy, in 35 fractions, 2 Gy per day, 5 days per week for 7 weeks
delivered by 3-D conformal or IMRT technique. (n=181) | Chemo-RT 3 cycles
of concurrent chemotherapy (carboplatin 70 mg/m2/day on
days 1-4, and FU 600 mg/m2/day on days 1-4 continuous
infusion every 3 weeks, during weeks 1,4 and 7 of RT) + RT. The RT
dose was 70 Gy, in 35 fractions 2 Gy per day, 5 days per week for 7
weeks delivered by 3 D conformal or IMRT technique (n=179) | 2.8 years for
intervention arm, 2.6 years for the control arm | PFS: 2-year PFS,
0.36 (intervention) vs. 0.38 (control); HR, 0.93 (95% CI,
0.73-1.20; P=0.58) | OS: 2-year OS-NS;
(HR, 1.12, 95% CI, 0.86-1.46; P=0.39). LRC: 2-year LRC-NS; (HR,
0.98, 95% CI, 0.74-1.3;P=0.90). RDM: If first event, HR, 0.54 (95%
CI, 0.30-0.99; P=0.05) in favour of TPF + cetuximab-RT arm. If
first or later event, HR, 0.62 (95% CI, 0.40-0.95; P=0.03) in
favour of TPF + cetuximab- RT arm. Adverse events: The TPF +
cetuximab-RT arm experienced higher rates of grades 3 and 4 fever
(9% vs. 0.6%; P<0.001), grade 3 and 4 neutropenia (26% vs. 6%;
P<0.001) and febrile neutropenia (17% vs. 0%; P<0.001). Grade
3 and 4 skin reactions were also more common in the cetuximab-RT
arm (53% vs. 29%; P<0.001). Grade 3-4 mucositis was reported by
48% patients in the intervention arm and 50% patients in the
control arm (p=0.7) Grade 3-4 skin reactions: 11 and 0% (outside RT
fields) and 53 and 29% (inside RT fields) for the intervention and
control arm, respectively (P=0.001); Grade 3-4 hypersensitivity to
cetuximab occurred in 7% of patients (in the intervention arm);
treatment- related deaths, 6.6 and 0.6% in intervention and control
arm, respectively (P=0.0016). | (28,44)a |
| Merlano et
al, 2020 (The INTERCEPTOR- GONO Study) | Cetuximab | RCT | Previously
untreated stage III-IV, T1-4, N0-N3 histologically confirmed
LASCCHN of oral cavity, oropharynx, larynx, hypopharynx Performance
Status 0-1 (n=242) | Arm A(IBRT arm)
TPF+ cetuximab- (loading dose, 400 mg/m2; 1 week before
RT, then 250 mg/m2once a week for 7 weeks of RT +RT
(70Gy 2Gy/day 5days/week) Used IMRT or three-dimensional conformal
RT (3-DCRT) techniques for radiation planning and delivery
(n=121) | Arm B (CRT arm) RT
(70 Gy 2 Gy/day 5days/week; + concurrent cisplatin (100
mg/m2 every three weeks. Used IMRT or three-dimensional
conformal RT (3-DCRT) techniques for radiation planning and
delivery (n=121) | NA | OS: Median OS, 59
months in both arms; HR, 1.05 (95% CI, 0.71-1.54; P=0.8) | PFS: Median PFS,
31.6 (arm A) vs. 40.3 months (arm B); HR, 1.03 (95% CI, 0.72-1.48;
P=0.48). ORR: 79% (95% CI, 0.55-0.72) in arm A, vs. 76% (95% CI,
0.55-0.73) in arm B, P=0.47. Adverse events: Severe neutropenia and
skin toxicity were more common in the IBRT arm (P=0.04 and P=0.017,
respectively). Weight loss was more common in the chemo-RT arm
(P=0.017). | (29) |
| E, induction
chemotherapy followed by RT + anti-EGFR monoclonal antibody vs.
induction chemotherapy followed by chemoradiotherapy (1 RCT) |
| Author/s, year | Anti-EGFR
agent | Design | Population | Intervention | Control | Follow-up | Primary
endpoint | Secondary
endpoints | (Refs.) |
| Hitt et al,
2022 | Cetuximab | RCT | Patients with
unresectable locally advanced head and neck cancer received 3
cycles of TPF (docetaxel, cisplatin, and fluorouracil) (n=407) | Cetuximab (400
mg/m2 on day 1 followed by 250 mg/m2
cetuximab weekly during RT) + RT (conventionally fractionated form
at 2 Gy/fraction for 35 fractions at 1 fraction per day; total dose
was 70 Gy over 7 weeks. Used the 3-DCRT technique for all patients
(n=202) | Cisplatin (100
mg/m2 on days 1, 22 and 43 of RT) + RT (conventionally
fractionated form at 2 Gy/fraction for 35 fractions at 1 fraction
per day; total dose was 70 Gy over 7 weeks. Used the 3-DCRT
technique for all patients (n=205) | 41.1 months
(intervention) 43.9 months (control) | OS: Median OS, 63.6
(control) and 42.9 (intervention) months; HR, 1.106 (90% CI,
0.888-1.378; P=0.4492) | PFS: Median PFS,
39.9 (control) and 20.2 (intervention) months; HR, 1.190 (95% CI,
0.925-1.530; P=0.1759). ORR: Complete response or partial response,
76.1% (control) and 79.7% (intervention); P=0.3809. Adverse events:
Rates of AEs of special interest occurring in >10% of patients
in the control arm. were mucosal inflammation (74.2%), radiation
dermatitis (43.4%), dysphagia (28.3%), neutropenia (22.9%), anemia
(18.5%), vomiting (17.6%) and ototoxicity (10.7%) Rates of AEs of
special interest occurring in >10% of patients in the
intervention arm were: mucosal inflammation (79.7%), radiation
dermatitis (46.5%), dysphagia (26.7%) and skin toxicity due to
cetuximab (21.8%). Late AEs included neurotoxicity (11.2% cisplatin
+ RT arm vs. 4.0% in the cetuximab + RT arm; P=0.0058), xerostomia
(22.0% in the cisplatin + RT arm vs. 27.7% in the cetuximab + RT
arm; P=0.1777), and asthenia (5.9% in the cisplatin + RT arm vs.
5.9% in the cetuximab + RT arm; P=0.9703). Improvement of QoL
dimensions was demonstrated in the cetuximab + RT arm compared with
cisplatin + RT arm in terms of physical functioning (P=0.0287),
appetite loss (P=0.0248), and social contact (P=0.0153). | (30) |
| F, Anti-EGFR
therapy in HPV+-oropharyngeal cancer (3 RCTs) |
| Author/s, year | Anti-EGFR
agent | Design | Population | Intervention | Control | Follow-up | Primary
endpoint | Secondary
endpoints | (Refs.) |
| Mehanna et
al, 2019 (De-ESCALaTE trial) | Cetuximab | RCT | Patients with
HPV+ low-risk oropharyngeal cancer (non- smokers or
life-time smokers with a smoking history of <10 years;
n=334) | Cetuximab (400
mg/m² loading dose followed by weekly infusions of 250 mg/m² for
seven weeks during RT) + RT(IMRT, 70 Gy,35 fractions) (n=168) | Cisplatin (100
mg/m² on days 1, 22 and 43 of RT) + RT (IMRT,70 Gy,35 fractions )
(n=166) | 25.9 months | Overall severe
toxicities, and all grade toxicities: Overall severe (grades 3-5)
toxicity did not differ significantly between the groups. The mean
number of events per patient was 4.81 (95% CI, 4.23-5.40) for
cisplatin arm and 4.82 (95% CI, 4.22-5.43) for cetuximab arm
(P=0.98). The incidence of overall toxicity of all grades was also
similar (mean number of events per patient, 29.2, 95% CI, 27.3-31.0
in the cisplatin arm; and 30.1, 95% CI, 28.3-31.9 for the cetuximab
arm P=0.49) | OS: 2-year OS,
89.4% in the cetuximab group vs. 97.5% in the cisplatin group; HR,
5.0 (95% CI, 1.7-14.7); log-rank P=0.0012. Recurrence rate: 2-year
recurrence rate, 16.1% in the cetuximab group vs. 6% in the
cisplatin group; HR, 3.4 95% CI, 1.6-7.2; log-rank P=0.0007. QoL:
social functioning and swallowing: Ns for mean global QoL scores on
EORTC QLQ-C30; did not differ significantly between the groups at
any of the time points, mean difference at 24 months was 1.51
points (in favour of cisplatin; P=0.09976). Social functioning: At
the end of treatment, there was a significant difference in social
functioning in favour of cetuximab (mean difference, 8.67 points;
P=0.0374; disappeared 6 months later). At 12 and 24 months, there
was a significant difference in role functioning in favour of
cisplatin (difference in mean scores of 8.3 points; P=0.0173). None
of these differences reached the minimum for clinically meaningful
difference of 10 points; Swallowing, Ns; at 24 months, mean
difference, 6.90 points in favour of cisplatin; P=0.1279. Adverse
events: Acute period severe short-term toxicities did not differ
significantly; mean number of events/patient, 4.43 (95% CI,
3.84-4.97) for cisplatin arm and 4.35 (95% CI, 3.9-4.97; for
cetuximab arm; P=0.84) Acute period rates of all grade toxicities
were similar. Mean number of events/patient, 19.96 (95% CI,
18.8-21.1) in the cisplatin arm and 20.35 (95% CI, 19.18-21.52) in
the cetuximab arm and (P=0.64). Late toxicity: rates of
all-grade(P=0.49) late toxicities and severe (P=0.53) late
toxicities were similar, but types of toxicities differed between
the two groups. In the cetuximab group, the most common toxicity
was gastrointestinal, mean number of events per patient: 1.9
(acute) and 0.2 (late), and higher rates of skin toxicity and
infusion reactions. In the cisplatin group, the most common acute
toxicity was gastrointestinal, mean number of events per patient:
2.12. The most common late toxicities were gastrointestinal (mean
number of events/patient, 0.2), and labyrinthine (mean number of
events/patient, 0.1). Haematological, metabolic and renal late
toxicities were more in the cisplatin group than in the cetuximab
group. | (31,46)a |
| Gillison et
al, 2019 (NRG RTOG 1016), | Cetuximab | RCT | Patients with
HPV+-OPC (n=805) | RT (Accelerated
IMRT 70 Gy in 35 fractions over 6 weeks at 6 fractions/week) +
cetuximab (400 mg/m² 5-7 days before RT initiation, followed by
cetuximab 250 mg/m² weekly for 7 doses; n=399) | RT (Accelerated
IMRT 70 Gy in 35 fractions over 6 weeks at 6 fractions/week) +
cisplatin (100 mg/m² on days 1 and 22. (n=406) | 4.5 years | OS: RT + cetuximab
group did not reach the non-inferiority criteria for OS; HR, 1.45;
one-sided 95% upper CI, 1.94. For non- inferiority, P=0.5056 and
one-sided log- rank P=0.0163. Estimated OS at 5 years, 77.9% (95%
CI, 73.4-82.5) in the cetuximab group vs. 84.6% (95% CI, 80.6-88.6)
in the cisplatin group | PFS: PFS was
significantly lower in the cetuximab group compared with the
cisplatin group (HR, 1.72; 95% CI, 1.29-2.29; P=0.0002). 5-year
PFS, 67.3% (95% CI, 62.4-72.2) vs. 78.4% (95% CI, 73.8-83.0). LRF
and distant metastasis: The rate of LRF was significantly higher in
the cetuximab group (HR, 2.05; 95% CI, 1.35-3.10; P=0.0005); 5-year
proportions: 17.3% (95% CI, 13.7-21.4) vs. 9.9% (95% CI, 6.9-13.6).
Distant metastasis, ns. No significant difference in distant
metastasis between the two groups. Cetuximab vs. cisplatin HR, 1.49
(95% CI, 0.94-2.36; P=0.09). 5-year proportions: 11.7 vs. 8.6%.
QoL: EORTC QLQ-H&N35 completion patterns were similar between
the two groups. Patient-reported severity of swallowing issues was
increased in both groups at the end of treatment compared with
pre-treatment scores. However, between the groups, there was no
statistically significant difference in the change of scores from
baseline (mean, 47.4 vs. 48.0; P=0.86). At 1 year, the cetuximab
group exhibited a significant increase in symptoms from
pre-treatment levels compared with the cisplatin group although it
was not a clinically important difference (7.6 vs. 2.5; P=0.0382).
Adverse events: Proportions of acute moderate to severe toxicity:
77.4% (95% CI, 73.0-81.5) in the cetuximab group vs. 81.7% (95% CI,
77.5-85.3) in the cisplatin group; P=0.1586. Late moderate to
severe toxicity: 16.5% (95% CI, 12.9-20.7) in the cetuximab group
vs. 20.4% (95% CI, 16.4-24.8) in the cisplatin group; P=0.1904.
Feeding tube issues, at treatment completion: 57.3% (95% CI,
52.2-62.2) of patients in the cetuximab group and 61.5% (95% CI,
56.5-66.3) of patients in the cisplatin group had a feeding tube.
At 1 year after treatment, the proportions dropped to 8.4% (95% CI,
5.8-11.8) in the cetuximab group and 9.2% (95% CI, 6.5-12.7) in the
cisplatin group (P=0.79). | (32) |
| Rischin et
al, 2021 (TROG12.01 trial), | Cetuximab | RCT | Patients with low
risk p16+- OPC AJCC 7th edition stage III (excluding
T1-2N1) or stage IV (excluding T4 and/or N3 and/or N2b-c if smoking
history >10 years and/or distant metastases; n=182) | Intensity modulated
RT (70 Gy in 35 fractions over 7 weeks) with cetuximab with a
loading dose of 400 mg/m2 in the week before RT, then
250 mg/m2 weekly for 7 doses (n=90) | Intensity modulated
RT (70 Gy in 35 fractions over 7 weeks) weekly cisplatin (40
mg/m2 for 7 cycles; n=92) | 4.1 years | Symptom severity:
There was no difference in the MDASI-HN symptom severity AUC
between the arms; difference in AUC between cetuximab- cisplatin
was 0.05 (95% CI, -0.19-0.30; P=0.66) | FFS: The 3-year FFS
was superior in the cisplatin arm (93 vs. 80%; HR, 3.0; 95% CI,
1.2-7.7; P=0.015). OS: There was no significant difference in
3-year OS; 98 and 96% (HR, 2.3; 95% CI, 0.4-12.7; P=0.32) for the
cisplatin arm and the cetuximab arm, respectively. MDASI-HN scores:
There were no significant differences in other MDASI-HN scores,
including modified symptom severity (P=0.97), symptom interference
score (P=0.071), mucositis symptoms (P=0.91) or common symptoms
(P=0.92). FDG-PET complete response rates at 13 weeks post-RT: The
FDG-PET complete response rate at 20 weeks (13 weeks after
completion of RT) was 79% (95% CI, 69-87) in the cisplatin arm and
69% (95% CI, 58-78) in the cetuximab arm (P=0.16). Acute and late
toxicities and rates of enteral feeding: Radiation dermatitis and
acneiform rash were more common in the cetuximab arm, while febrile
neutropenia, emesis, dry mouth and fatigue were more common in the
cisplatin arm. There was no significant difference in late
toxicities. No patient was on enteral feeding at 12 months in
either of the two arms. Freedom from distant failure was better in
the cisplatin +RT arm compared to the cetuximab+RT arm (3-year
Freedom from Failure rates were 97% vs. 88%, HR, 4.1; 95% CI,
1.2-14.9 P=0.18) | (33) |
All trials included patients >18 years of age.
Whilst the majority of the trials included patients with
malignancies originating from the oropharynx, hypopharynx, larynx
and oral cavity, in two of the studies (22,23), the
oral cavity subsite was not included. In total, three studies
(31-33)
compared the efficacy of cetuximab + RT against cisplatin + RT for
HPV+-OPC. The majority of the studies used The American
Joint Committee on Cancer (AJCC)/Union for International Cancer
Control 7th edition staging system (34), except for the trial performed by
Bonner et al (22), which
used the AJCC 1998 system (35) and
the RTOG 0522 trial (23), which
used the AJCC 6th edition guidelines (36).
Only one study (22)
compared the effectiveness of an anti-EGFR agent with that of RT
alone, whilst three studies (23-25)
assessed the potential benefit of adding an anti-EGFR agent into
the chemoradiotherapy regimen compared with concurrent
chemoradiotherapy alone. In two of the included studies (26,27),
patients in the treatment group received an anti-EGFR agent + RT,
whilst patients in the control group received chemoradiotherapy
alone. In two other studies (28,29),
patients in the intervention group received induction chemotherapy
prior to treatment with an anti-EGFR agent + RT, whereas patients
in the control group received chemoradiotherapy alone. In one study
(30), patients who responded to
induction chemotherapy were randomized to evaluate the
effectiveness of the anti-EGFR agent + RT compared with that of
cisplatin + RT. In three studies (31-33),
the efficacy of cetuximab + RT was evaluated compared with that of
cisplatin + RT in HPV+-OPC.
Cetuximab was the anti-EGFR agent evaluated in nine
of the studies (22,23,26,28-33).
Nimotuzumab, panitumumab and zalutumumab were the anti-EGFR
monoclonal antibodies evaluated in three studies (24,25,27).
Docetaxel+ cisplatin+ fluorouracil (TPF) induction chemotherapy was
given to the intervention arm before cetuximab + RT in two studies
(28,29), whilst in one study (29), TPF induction therapy was given in
both the control and intervention arms. In one study accelerated RT
was used both in the intervention arm and the control arm (25), while in another study the
intervention arm received accelerated RT and control arm received
conventional RT (27). In all other
trials, conventional RT was used in both the control and
intervention arms. Chemoradiotherapy was the standard treatment
strategy used in the control arm in all but one study (22), in which RT alone was given as the
standard therapeutic method. Regarding concurrent chemotherapy,
cisplatin administered three times per week was used in six trials
(23,27,29-32),
whilst weekly cisplatin was used in four trials (24,25,26,33) and
one trial used carboplatin + 5-flurouracil for concurrent
chemotherapy (28). The radiation
dose was in the 60-70 Gy range in these studies. While the phase
III trial performed by Bonner et al (22) used two dimensional-RT, in total, four
trials used intensity-modulated RT (IMRT) or three-dimensional
conformal RT (3DCRT) techniques for radiation planning and delivery
(23,27-29).
By contrast, four trials used IMRT for radiation planning and
delivery for all patients. (25,31-33).
In the trial by Patil et al (24), 2D-RT, 3DCRT or IMRT was used. In
addition, one study (26) used IMRT,
helical tomotherapy or volumetric arc therapy for radiation
planning. The trial by Hitt et al (30) used the 3DCRT technique for all
patients.
Outcomes
Of the 12 trials included in the present review,
four studies (23,24,27,28)
assessed progression-free survival (PFS) as the primary endpoint,
whilst four studies (26,29,30,32)
assessed OS as the primary endpoint. Locoregional control (LRC) was
the primary outcome in two studies (22,25).
Overall severe toxicity was assessed as the primary outcome in the
De-ESCALaTE trial by Mehanna et al (31). Symptom severity as assessed using the
MD Anderson Symptom Inventory Head and Neck Symptom Severity Scale
(MDASI-HN) was the primary endpoint in the TROG 12.01 trial
(33,37).
Since the studies were heterogeneous in terms of
treatment combinations, for the purpose of cataloging the evidence,
the studies were categorized into six groups based on the nature of
the treatment combinations involved. The groups were as follows: i)
RT + concurrent anti-EGFR monoclonal antibody vs. RT alone; ii)
Concurrent chemoradiotherapy + anti-EGFR monoclonal antibody vs.
concurrent chemoradiotherapy alone; iii) RT + anti-EGFR monoclonal
antibody vs. concurrent chemoradiotherapy alone; iv) Three-drug
induction chemotherapy followed by anti-EGFR monoclonal antibody +
RT vs. chemoradiotherapy alone; v) induction chemotherapy followed
by RT + anti-EGFR monoclonal antibody vs. induction chemotherapy
followed by chemoradiotherapy; and vi) Anti-EGFR monoclonal
antibody in HPV+-oropharyngeal cancer (OPC).
RT + concurrent anti-EGFR monoclonal
antibody vs. RT alone (1 RCT)
In a phase III clinical trial, Bonner et al
(22) randomized patients (n=424)
with stage III or IV, non-metastatic, squamous cell carcinoma of
the oropharynx, hypopharynx, or larynx to receive either RT +
cetuximab (intervention arm) or RT alone (control arm). Cetuximab
was administered 1 week before RT at a dose of 400
mg/m2, followed by 250 mg/m2 weekly for the
duration of RT. The primary endpoint was the duration of LRC. The
secondary endpoints were OS, PFS, overall response rate (ORR) and
safety. The duration of LRC was 24.4 months in the intervention
group, which received cetuximab + RT, compared with 14.9 months in
the control group, which received RT alone [hazard ratio (HR) for
locoregional progression, 0.68; 95% CI, 0.52-0.89; P=0.005]. The
median PFS was 17.1 months for patients who received RT + cetuximab
whereas it was 12.4 months for patients receiving RT alone (HR for
disease progression/death, 0.70; 95% CI, 0.54-0.90; P=0.006). The
ORR was 74% in the intervention group, compared with 64% in the
control group (odds ratio, 0.57; 95% CI, 0.36-0.90; P=0.02). After
a median follow-up duration of 54 months, the median OS was 49
months in the intervention arm compared with 29.3 months in the
control arm (HR for mortality, 0.74; 95% CI, 0.57-0.97; P=0.03)
(38). In terms of adverse events,
apart from an increased incidence of acneiform rash and infusion
reactions, the two groups did not show any statistically
significant differences in terms of grade ≥3 toxicities. The most
frequently observed side effects of RT included mucositis,
dysphagia, pain, xerostomia, weight loss and performance status
deterioration, the incidences of which were similar in the two
groups. Severe late effects associated with RT were also similar in
the two groups. The 5-year OS was found to be 45.6% in the
cetuximab + RT group and 36.4% in the RT-alone control group
(38). In addition, the 5-year OS
was significantly higher among patients who experienced
cetuximab-induced acneiform rash of grade ≥2 severity compared with
those who had no rash or a grade1 rash (HR, 0.49; 95% CI,
0.34-0.72; P=0.002). This finding supported the possibility of
cetuximab induced acneiform rash being a biomarker of an
immunological response associated with optimal outcomes (38,39). In
conclusion, this trial demonstrated the effectiveness of cetuximab
in combination with RT for improving LRC whilst reducing mortality
compared with RT alone (22,38).
Concurrent chemoradiotherapy +
anti-EGFR monoclonal antibody vs. concurrent chemoradiotherapy
alone (3 RCTs)
In total, three phase III clinical trials (23-25)
tested the effectiveness of concurrent chemoradiotherapy + an
anti-EGFR agent compared with concurrent chemoradiotherapy alone.
In the RTOG 0522 trial, 891 patients with stage III or IV
non-metastatic squamous cell carcinoma of the oropharynx,
hypopharynx or larynx were randomly allocated to receive RT +
concurrent cisplatin with or without cetuximab (23). In this trial, PFS was the primary
outcome. Secondary outcomes included OS, locoregional failure
(LRF), distant metastasis and adverse events. The 3-year PFS was
58.9% (95% CI, 54.2-63.6%) in the intervention arm (cetuximab +
cisplatin-RT) vs. 61.2% (95% CI, 56.7-65.8%) in the control arm
(cisplatin-RT; P=0.76). The 3-year OS was 75.8% (95% CI,
71.7-79.9%) in the intervention arm vs. 72.9% (95% CI, 68.7-77.1%)
in the control arm (P=0.32). The 3-year LRF was 25.9% (95% CI,
21.7-30.1%) in the intervention arm compared with 19.9% (95% CI,
16.2-23.7%) in the control arm (P=0.97). The rates of distant
metastasis in the two arms were not significantly different (13.0
vs. 9.7%; P=0.08). Updated results after a median follow-up
duration of >10 years suggested that the addition of cetuximab
to RT and cisplatin did not improve the PFS or OS or prevented
distant metastasis (40). No
significant differences were found between the intervention and
control arms in terms of the 30-day mortality rate (1.8 vs. 2.0%;
P=0.81). In terms of adverse events, patients who received
cetuximab + cisplatin and RT experienced more treatment-related
grade 5 adverse events (10 vs. 3; P=0.05) compared with patients in
the control group. The incidence of grade 3-4 radiation mucositis
was found to be higher in the cetuximab arm compared with the
control arm (43.2 vs. 33.3%; P=0.003). Patients in the intervention
group exhibited higher rates of grade 3 to grade 4 skin reactions,
fatigue, anorexia, and hypokalemia during the first 90 days of
therapy compared with those of the control group. Therefore, the
findings of this trial suggested the absence of benefits of adding
cetuximab to cisplatin + RT in terms of PFS or OS.
A single institution study (n=536) by Patil et
al (24) included patients with
non-metastatic, stage III or IV squamous cell carcinoma of the
oropharynx, larynx, hypopharynx or oral cavity who were randomly
allocated to receive RT + cisplatin (30 mg/m2) with or
without nimotuzumab (24). PFS was
the primary outcome in this trial and the median follow-up duration
was 39.13 months. Secondary outcomes included LRC, disease-free
survival (DFS), OS, and adverse events. The 2-year PFS was 61.8%
(95% CI, 55.2-67.7) in the intervention arm, compared with 50.1%
(95% CI, 43.7-56.2) in the control arm (HR, 0.69; 95% CI,
0.53-0.89; P=0.004). The 2-year LRC was 67.5% (95% CI, 60.9-73.3%)
in the intervention arm vs. 57.6% (95% CI, 50.9-63.6%) in the
control arm (HR, 0.67; 95% CI, 0.50-0.89; P=0.006). The 2-year OS
was 63.8% (95% CI, 57.3-69.6%) in the intervention arm vs. 57.7%
(95% CI, 50.9-63.6%) in the control arm (HR, 0.84; 95% CI,
0.65-1.08; P=0.163). DFS was also improved with the addition of
nimotuzumab (HR, 0.71; 95% CI, 0.55-0.92; P=0.008). In terms of
adverse events, a higher incidence of grade 3-5 mucositis occurred
in the nimotuzumab + cisplatin + RT arm (66.7 vs. 55.8%; P=0.01).
However, the incidence of other grade 3-5 adverse effects was
similar between the two groups. Therefore, the results of this
trial suggested that nimotuzumab improved both LRC and DFS when it
was added to the RT + weekly 30 mg/m2 cisplatin regimen,
albeit without improvements in OS.
The DAHANCA 19 study included patients (n=619) with
biopsy-verified HNSCC of the oral cavity, oropharynx, hypopharynx,
and larynx in which the investigators tested the efficacy of the
anti-EGFR agent zalutumumab (25).
Patients in the intervention group received accelerated RT +
nimorazole with zalutumumab, whilst the control group received
accelerated RT + nimorazole only. Patients in both arms of the
study received 40 mg/m2 cisplatin weekly. The 3-year LRC
rates were similar between the intervention (accelerated RT +
nimorazole + zalutumumab) and control (accelerated RT + nimorazole)
arms (78 vs. 79%; HR, 0.8; 95% CI, 0.6-1.2). Disease-specific
survival (DSS; HR, 1.0; 95% CI, 0.7-1.7) and OS (HR, 0.9; 95% CI,
0.6-1.3) were also similar for the intervention and control arms.
The outcomes were not influenced by p16 positivity (HR, 1; 95% CI,
0.6-1.8) or p16 negativity (HR, 0.8; 95% CI, 0.5-1.4). The 5-year
LRC rates were also similar between the intervention arm and the
control arm (70 vs. 74%; HR, 1.10; 95% CI, 0.81-1.50). There was no
significant impact on the 5-year DSS (HR, 1.12; 95% CI, 0.79-1.60)
or OS (HR, 1.17; 95% CI, 0.89-1.52) (41). In addition, 94% of the patients in
the zalutumumab arm experienced a skin rash. Grade 3-4 skin rash
occurred in 29% of patients, where 13% of patients had to terminate
the zalutumumab treatment due to the rash. Patients in the
zalutumumab arm experienced significant rates of confluent
mucositis (70% vs. 56%, P=0.001) and grade 3-4 in-field skin
reaction (27% vs. 4% P<0.0001) (41).
Therefore, this trial concluded that zalutumumab,
when added to chemoradiotherapy, did not improve outcomes like LRC
or OS and therefore not an effective treatment option for patients
with LAHNSCC.
RT + anti-EGFR monoclonal antibody vs.
concurrent chemoradiotherapy alone (2 RCTs)
There were two phase III RCTs that compared the
efficacy of RT + anti-EGFR monoclonal antibodies with that of
chemoradiotherapy alone (26,27). The
ARTSCAN III trial included patients with stage III and IV SCC of
the oropharynx, hypopharynx, or larynx without distant metastases
(26). Patients were randomly
allocated to receive either cetuximab + RT or cisplatin + RT. The
primary endpoint set for the ARTSCAN III trial was OS, whereas the
secondary endpoints included LRC, local control with dose-escalated
RT, distant failure and adverse events. After 3 years, the OS was
78% (95% CI, 71-85%) in the cetuximab RT arm, whereas it was 88%
(95% CI, 83-94%) in the cisplatin + RT group (HR, 1.63; 95% CI,
0.93-2.86; P=0.086). The cumulative incidence of locoregional
failures at 3 years was 23% (95% CI, 16-31%) in the intervention
arm vs. 9% (95% CI, 4-14%) in the control arm (adjusted
cause-specific HR, 2.49; 95% CI, 1.33-4.66; P=0.0045) There was no
difference in the cumulative incidence of distant failures. Post
hoc subgroup analyses revealed that the HR for OS was 5.70 (95% CI,
1.67-19.5) for patients with p16+-OPC treated with RT +
cetuximab compared with those treated with RT + cisplatin (P=0.03).
By contrast, the acute toxicity profiles differed between the
intervention and control arms. The incidence of acute mucositis
(P=0.035), skin reactions (P=0.001) and acneiform rashes
(P<0.001) was higher in the cetuximab group compared with the
cisplatin group whereas the incidence of nausea (P=0.001), vomiting
(P=0.015), acute kidney injury (P<0.001), tinnitus (P=0.002),
dysphagia (P=0.033) and neutropenia (P<0.001) was significantly
higher in the cisplatin arm. Late toxicities, such as taste
alteration and hearing impairments, were significantly more common
in the cisplatin arm, whilst late pain and mucosal toxicities were
more common in the cetuximab arm. Based on the findings of this
trial, cetuximab + RT was deemed to be inferior to cisplatin + RT
for the treatment of LAHNSCC.
In the HN.6 trial, 320 patients with locoregionally
advanced squamous cell carcinoma of the oral cavity, oropharynx,
larynx, or hypopharynx were randomly allocated to receive standard
fractionation RT and concurrent cisplatin (arm A) or accelerated RT
and concurrent panitumumab (arm B) (27). In this trial, the primary endpoint
was PFS. It was designed as a non-inferiority trial (a test of
non-inferiority of arm B to arm A was to be done if superiority of
arm B was not detected in the primary analysis and non-inferiority
would be claimed if the upper limit of a two-sided 95% CI for HR
was ≤1.15). There were no statistically significant differences in
the PFS between the two arms (HR, 0.95; 95% CI, 0.60-1.50;
stratified log-rank test, P=0.83). The 2-year PFS was 73% (95% CI,
65-79%) for arm A and 76% (95% CI, 68-82%) for arm B. Regarding OS,
there was no statistically significant difference between the two
arms (HR, 0.89; 95% CI, 0.54-1.48; stratified log-rank test,
P=0.66). The 2-year OS was 85% (95% CI, 78-90%) for arm A, compared
with 88% (95% CI, 82-92%) for arm B. There were no statistically
significant differences between the two arms in terms of the 2-year
cumulative incidence of local recurrence, regional recurrence, or
distant recurrence. Sub-group analyses based on performance status,
T category, N status, primary site, p16 expression status and
smoking history did not reveal any differences in PFS between the
two arms. There was also no significant difference in the quality
of life (QoL) parameters (measured using Functional Assessment
Cancer Therapy-Head &Neck) or swallowing outcomes between the
two arms (27,42). At 1 year post treatment no difference
was found between the arms in terms of FACT-H&N change from
baseline: -1.70 (control arm) and-4.81 (intervention arm), P=0.194.
Swallowing related QOL (measured using SWAL-QOL and MDADI) declined
from baseline to every subsequent time point (42).
Regarding adverse events, ototoxic effects (such as
hearing loss and tinnitus), gastrointestinal tract symptoms (such
as nausea, vomiting and dehydration), nephrotoxic effects and
weight loss were more common in the cisplatin + standard RT arm. By
contrast, skin toxicity and grade ≥3 mucositis were more common in
the accelerated RT + panitumumab group. The incidence of
non-hematological grade ≥3 adverse events was similar between the
two arms (88% in arm A vs. 92% in arm B; P=0.25). These results
suggested that the trial failed to prove the non-inferiority of
panitumumab + accelerated RT in terms of PFS or OS compared with
standard chemoradiotherapy for the treatment of LAHNSCC.
Three-drug induction chemotherapy
followed by anti-EGFR monoclonal antibody + RT vs.
chemoradiotherapy alone (2 RCTs)
GORTEC 2007-02(28)
and INTERCEPTOR-GONO (29) are two
phase III clinical trials that investigated the effectiveness of a
three-drug induction chemotherapy followed by RT + an anti-EGFR
agent compared with that of chemoradiotherapy alone. GORTEC 2007-02
included patients with stage III-IV non-metastatic SCC of the oral
cavity, oropharynx, hypopharynx, or larynx, with heavy nodal
disease (N2b, N2c or N3), which are known to develop distant
metastasis (28,43). The intervention arm received 3 cycles
of TPF followed by cetuximab + RT (n=181). The control arm received
3 cycles of concurrent chemotherapy (carboplatin 70
mg/m2/day on days 1-4; and fluorouracil 600
mg/m2/day on days 1-4, continuous infusion every 3
weeks). The RT dose was 70 Gy in 35 fractions delivered by the
conformal or IMRT technique. PFS was designated as the primary
endpoint. Secondary endpoints included OS, LRC, Rate of distant
metastasis (RDM) and acute and late toxicities. The median
follow-up time was 2.8 years for the intervention arm and 2.6 years
for the control arm. The PFS rate for 2 years was 36% in the
intervention arm and 38% in the control arm, with a HR of 0.93 (95%
CI, 0.73-1.20; P=0.58). LRC (HR, 0.98; 95% CI, 0.74-1.3; P=0.90)
and OS (HR, 1.12; 95% CI, 0.86-1.46; P=0.39) also did not differ
between the two arms. Distant metastasis was less frequent in the
TPF arm (if first event, HR, 0.54, 95% CI, 0.30-0.99; P=0.05 in
favor of TPF + cetuximab-RT arm; if first or later event, HR, 0.62
95% CI, 0.40-0.95; P=0.03 in favor of TPF + cetuximab-RT arm).
These observations were found to be independent of p16 status
(P-value for interaction, 0.35) (44). The PFS benefit of TPF + cetuximab +
RT was found to be absent in both p16+ (HR, 0.78; 95%
CI, 0.28-2.20; P=0.64) and p16- (HR 1.28; 95% CI,
0.84-1.93; P=0.25) OPC. There was no difference in the rates of
distant metastasis between p16+- and
p16--OPC. By contrast, a significant improvement in PFS
was found in p16+-OPC compared with p16--OPC
(P<0.001), regardless of the treatment received. This is in
accordance with the findings from previous research (45). In terms of adverse events, the TPF +
cetuximab + RT arm experienced higher rates of grades 3 and 4 fever
(9 vs. 0.6%; P<0.001), grade 3 and 4 neutropenia (26 vs. 6%;
P<0.001) and febrile neutropenia (17 vs. 0%; P<0.001).
However, the incidence of grade 3 and 4 skin reactions was higher
in the cetuximab + RT arm (P<0.001). Based on these findings,
TPF + cetuximab + RT was proposed to not be effective for improving
the outcomes of patients with LAHNSCC compared with concurrent
chemoradiotherapy.
The INTERCEPTOR-GONO study compared the
effectiveness of induction chemotherapy followed by bio-RT (IBRT)
against cisplatin based concurrent chemo-RT (CRT) in locally
advanced head and neck cancers (29). The induction regimen was TPF, similar
to that applied in the GORTEC 2007-02 study. The Bio-RT (BRT)
consisted of cetuximab at a loading dose of 400 mg/m2 1
week before RT, followed bs a reduced dose of 250 mg/m2
weekly for 7 weeks during RT. The RT dose was 70 Gy in 35 fractions
delivered using IMRT or the three-dimensional conformal technique.
The primary endpoint was OS. Secondary endpoints included PFS,
objective response rate (ORR) and toxicities. The median OS was 59
months in both arms (HR, 1.05; 95% CI, 0.71-1.54; P=0.8). The
median PFS was 31.6 months in the IBRT arm and 40.3 months in the
chemoradiotherapy arm (HR, 1.03; 95% CI, 0.72-1.48; P=0.48). The
ORR was 79% (95% CI, 0.55-0.72) in the intervention arm and 76%
(95% CI, 0.55-0.73) in the control arm (P=0.47). Regarding adverse
events, severe neutropenia (P=0.04) and skin toxicity (P=0.017)
were significantly more common in the IBRT arm. Weight loss was
significantly more frequent in the chemoradiotherapy arm (P=0.017).
The findings of the INTERCEPTOR-GONO study supported those of the
GORTEC 2007-02 study. Although patients in the GORTEC study were of
advanced nodal disease resulting in worse outcomes, the findings
from these two trials suggested the lack of effectiveness of
three-drug induction chemotherapy followed by RT + anti-EGFR
therapy compared to chemoradiotherapy alone in LAHNSCC (28,29,44).
Induction chemotherapy followed by RT
+ anti-EGFR therapy vs. induction chemotherapy followed by
chemoradiotherapy (1 RCT)
A previous open-label phase III trial conducted by
The Spanish Cooperative Group for the Treatment of Head and Neck
Cancer recruited patients with unresectable head and neck cancer
who achieved stable disease following induction chemotherapy and
randomized them into the cetuximab + RT (intervention arm) and
cisplatin + RT (control arm) groups (30). Both arms received induction
chemotherapy with the TPF regime. The RT dose was 70 Gy in 35
fractions delivered using the conformal technique. The concurrent
agent was 100 mg/m2 cisplatin on days 1, 22 and 43 in
the control arm, compared with 400 mg/m2 cetuximab on
day 1 followed by 250 mg/m2 cetuximab weekly during RT.
The primary endpoint assessed was the non-inferiority of the
intervention arm compared with the control arm in terms of OS.
Secondary endpoints included PFS, safety and QoL. The median
follow-up duration was 43.9 months in the control arm and 41.1
months in the intervention arm. The median OS was 63.6 months in
the control arm and 42.9 months in the intervention arm (HR, 1.106;
90% CI, 0.888-1.378; P=0.4492). The median PFS was 39.9 months in
the control arm and 20.2 months in the intervention arm (HR, 1.190;
95% CI, 0.925-1.530; P=0.1759). A complete response or partial
response was seen in 76.1% of the patients in the control arm and
79.7% of the patients in the intervention arm (P=0.3809). Acute AEs
of special interest occurring in >10% of patients were mucosal
inflammation (74.2%), radiation dermatitis (43.4%), dysphagia
(28.3%), neutropenia (22.9%), anemia (18.5%), vomiting (17.6%) and
ototoxicity (10.7%) in the control arm. In the intervention arm
they included mucosal inflammation (79.7%), radiation dermatitis
(46.5%), dysphagia (26.7%) and skin toxicity due to cetuximab
(21.8%). Late AEs included neurotoxicity (11.2% cisplatin + RT arm
vs. 4.0% in the cetuximab + RT arm; P=0.0058), xerostomia (22.0% in
the cisplatin + RT arm vs. 27.7% in the cetuximab + RT arm;
P=0.1777), and asthenia (5.9% in the cisplatin + RT arm vs. 5.9% in
the cetuximab + RT arm; P=0.9703). Improvement of QoL dimensions
was demonstrated in the cetuximab + RT arm compared with cisplatin
+ RT arm in terms of physical functioning (P=0.0287), appetite loss
(P=0.0248), and social contact (P=0.0153).
Although there were positive findings in the
cetuximab + RT arm in terms of QoL and AEs of special interest, the
non-inferiority of cetuximab + RT over the standard treatment
regimen of chemoradiotherapy (cisplatin + RT) in terms of OS could
not be proven in this trial on patients with LAHNSCC who received
prior induction chemotherapy.
Anti-EGFR therapy in
HPV+-OPCs (3 RCTs)
There were three randomized phase III trials for
patients with HPV+-OPC that compared the efficacy of
cisplatin + RT and cetuximab + RT. These were the De-ESCALaTE
(31), NRG RTOG 1016(32) and TROG12.01(33) trials. The De-ESCALaTE trial included
patients with advanced low-risk p16+ oropharyngeal SCC
(31). Patients were randomly
allocated to receive either cisplatin-based chemoradiotherapy or
cetuximab bio-RT. The primary endpoint was overall (acute and late)
severe toxicity (grades 3-4) and all grade toxicities. The
incidence of overall grade 3-5 toxicities was similar between the
two groups. The mean number of events per patient was 4.81 (95% CI,
4.23-5.40) for cisplatin and 4.82 (95% CI, 4.22-5.43) for cetuximab
(P=0.98). The incidence of overall toxicity of all grades was also
similar (mean number of events per patient, 30.1,95% CI,28.3-31.9
for the intervention arm and 29.2, 95% CI,27.3-31.0 in the control
arm; P=0.49).
Secondary outcomes were OS, time to recurrence and
QoL. The 2-year OS rate was 89.4% in the cetuximab group and 97.5%
in the cisplatin group (HR, 5.0; 95% CI, 1.7-14.7; log-rank,
P=0.0012). There was a significant improvement in the 2-year OS of
patients at stage III (T4 or N3) who received cisplatin compared
with that of patients who received cetuximab (93.3 vs. 67.1%;
log-rank, P=0.0304). There was also a significant difference (HR,
4.4; 95% CI, 1.5-13.1; log-rank, P=0.0035) in the 2-year OS between
the cisplatin (97.2%) and cetuximab (89.7%) groups in p16 and HPV
DNA dual-positive patients. The 2-year recurrence rate was 16.1% in
the cetuximab group, compared with 6% in the cisplatin group (HR,
3.4; 95% CI, 1.6-7.2; log-rank, P=0.0007). The mean global QoL
scores on the European Organization for Research and Treatment of
Cancer (EORTC) core QoL questionnaire (QLQ-C30) did not differ
significantly between the two groups at any of the timepoints, with
the mean difference at 24 months being 1.51 (in favor of cisplatin;
P=0.09976) (46). There was a
significant difference in social functioning (in favor of
cetuximab; mean difference, 8.67; P=0.0374) at the end of
treatment, although this difference disappeared 6 months later. In
terms of swallowing, there was no significant difference between
the two groups at 24 months (mean difference, 6.90 points in favor
of cisplatin; P=0.1279).
Based on the findings of this trial the
investigators concluded that cisplatin +RT should be considered as
the standard of care in HPV+-OPC patients who are able to tolerate
cisplatin (31,46).
The NRG RTOG 1016 trial was a non-inferiority trial
in patients with low- and intermediate-risk HPV+-OPC at
stages T1-T2, N2a-N3 M0 or T3-T4, N0-N3 M0(32). Patients were randomly allocated to
either the RT + cetuximab or the RT + cisplatin groups. In this
trial, the primary endpoint was OS, whilst secondary endpoints
included PFS, LRF, DM, adverse events and QoL. The RT + cetuximab
group did not reach the non-inferiority criteria for OS (HR, 1.45;
one-sided 95% upper CI, 1.94; P-value for non-inferiority, 0.5056;
one-sided log-rank, P=0.0163). The estimated OS at 5 years was
77.9% (95% CI, 73.4-82.5%) in the cetuximab group, compared with
84.6% (95% CI, 80.6-88.6%) in the cisplatin group. The PFS was
significantly lower in the cetuximab group compared with the
cisplatin group (HR, 1.72; 95% CI, 1.29-2.29; P=0.0002). The
estimated 5-year PFS was 67.3% (95% CI, 62.4-72.2%) in the
cetuximab group and 78.4% (95% CI, 73.8-83.0%) in the cisplatin
group. LRF was found to be significantly higher in the cetuximab
group (HR, 2.05; 95% CI, 1.35-3.10; P=0.0005). The estimated 5-year
rates of locoregional failure were 17.3% (95% CI, 13.7-21.4%) in
the cetuximab group and 9.9% (95% CI, 6.9-13.6%) in the cisplatin
group. Regarding distant metastasis, there was no significant
difference between the two groups (HR, 1.49; 95% CI, 0.94-2.36;
P=0.09). The estimated 5-year rates of distant metastasis were
11.7% for the cetuximab group vs. 8.6% for cisplatin group. As
regards QoL measurements, EORTC QLQ-H&N35 (European
Organization for Research and Treatment of Cancer Quality of Life
Questionnaire Head and Neck Module) completion patterns were
similar between the two groups. Patient-reported severity scores of
swallowing issues were found to be increased in both groups at the
end of treatment compared with the pre-treatment scores. However,
there was no statistically significant difference in the mean
change of scores from baseline between the two groups (47.4±24.52
vs. 48.0±27.79; P=0.86). The proportions of grade 3-4 acute adverse
events were similar in the cetuximab (77.4; 95% CI, 73.0-81.5%) and
cisplatin groups (81.7; 95% CI,77.5-85.3%; P=0.1586). The late
moderate to severe toxicity rate was 16.5% (95% CI, 12.9-20.7%) in
the cetuximab group and 20.4% (95% CI, 16.4-24.8%) in the cisplatin
group (P=0.1904). Acneiform rash was significantly more common in
the cetuximab group, whereas the cisplatin arm experienced higher
rates of myelosuppression, anemia, nausea, vomiting, anorexia,
dehydration, hyponatremia, kidney injury and hearing impairment.
Late hearing impairment was significantly more frequent in the
cisplatin group. At treatment completion, 57.3% of patients (95%
CI, 52.2-62.2%) in the cetuximab group and 61.5% of patients (95%
CI, 56.5-66.3%) in the cisplatin group had a feeding tube. At 1
year after treatment, this proportion dropped to 8.4% (95% CI,
5.8-11.8%) in the cetuximab group and 9.2% (95% CI, 6.5-12.7%) in
the cisplatin group (P=0.79). These findings suggested that
cetuximab + RT was not superior to cisplatin + RT for improving the
outcomes (in terms of OS or PFS) of patients with
HPV+-OPC.
The TROG12.01 trial (33) included patients with low-risk
p16+-OPC at stage III (excluding T1-2N1) or stage IV
(excluding T4 and/or N3 and/or N2b-c if smoking history >10 pack
years and/or distant metastases) according to the AJCC 7th edition
guidelines. Patients were randomly allocated to receive IMRT (70 Gy
in 35 fractions over 7 weeks) combined with either weekly cisplatin
(40 mg/m2 for 7 cycles) or cetuximab (loading dose of
400 mg/m2 in the week before RT, followed by 250
mg/m2 weekly for 7 weeks along with RT). The primary
endpoint of the study was symptom severity as assessed using the
MDASI-HN from baseline to 13 weeks after RT completion. Secondary
endpoints included other MDASI-HN scores, failure-free survival
(FFS), OS, time to locoregional failure (TTLRF), pattern of
failure, F-18 fluorodeoxyglucose (FDG)-positron emission tomography
(PET) complete response rates at 13 weeks after RT,
clinician-assessed acute and late toxicities, and rates of enteral
feeding at 12 months. The median follow-up duration was 4.1 years
(range 0.4-5.3 years). There was no statistically significant
difference in the MDASI-HN symptom severity Area Under the Curve
(AUC) from baseline to week 20 between the cetuximab and cisplatin
arms (difference in AUC: 0.05 (95% CI, -0.19-0.30; P=0.66). There
were no significant differences in other MDASI-HN scores, including
modified symptom severity (P=0.97), symptom interference score
(P=0.071), mucositis symptoms (P=0.91) or common symptoms (P=0.92).
The 3-year FFS was superior in the cisplatin arm (93 vs. 80%; HR,
3.0; 95% CI, 1.2-7.7; P=0.015). There was no significant difference
in 3-year OS between the groups (98% in the cisplatin group vs. 96%
in the cetuximab group; HR, 2.3; 95% CI, 0.4-12.7; P=32) a The
FDG-PET complete response rate at 20 weeks (13 weeks after the
completion of RT) was 79% (95% CI, 69-87%) in the cisplatin arm and
69% (95% CI, 58-78%) in the cetuximab arm (P=0.16). Regarding acute
toxicities, radiation dermatitis and acneiform rash were more
common in the cetuximab arm, whereas febrile neutropenia, emesis,
dry mouth, and fatigue were more common in the cisplatin arm. There
was no significant difference in the incidence of late toxicities.
No patient was on enteral feeding at 12 months after RT completion
in either of the two arms. Freedom from distant failure was better
in the cisplatin +RT arm compared to the cetuximab + RT arm (3-year
freedom from distant failure rates were 97% vs. 88%, HR, 4.1; 95%
CI, 1.2-14.9, P=0.018). These findings suggested that cetuximab +
RT should remain as the SOC for patients with low-risk HPV-positive
OPC.
Discussion
A total of 12 phase III clinical trials in which
patients received an anti-EGFR agent as treatment, either alone or
in combination with RT and/or chemotherapy were reviewed in the
present study. Only two studies (22,24)
reported improvements in their respective primary outcomes.
However, these two studies differed in terms of the nature of
treatment combinations received and the primary outcomes evaluated.
Bonner et al (22) revealed
improved LRC (HR for locoregional progression/death, 0.68; P=0.005)
when the intervention arm received cetuximab + RT, while the
control group received RT alone (22,38). By
contrast, in studies in which control patients received cisplatin +
RT, the cetuximab group did not show any similar improvements in
outcomes (23,26,28-33),
where cetuximab + chemoradiotherapy (23) and cetuximab + RT (26,28-33)
were not reported to be superior compared with standard
chemoradiotherapy alone. Therefore, it was concluded that adding
the anti-EGFR agent cetuximab to chemoradiotherapy confers no
advantage over chemoradiotherapy alone for improving outcomes,
where replacing cisplatin with cetuximab may even result in
inferior survival outcomes for patients with LAHNSCC, including
patients with HPV+-OPC (23,26,31-33).
Patil et al (24) reported significant improvements in
PFS after the addition of nimotuzumab to chemoradiotherapy. The
2-year PFS was 61.8% (95% CI, 55.2-67.7) in the intervention arm
and 50.1% (95% CI, 43.7-56.2) in the control arm (HR,0.69; 95%
CI,0.53-0.89; P=0.004). with additional effectiveness in terms of
LRC and DFS but not in terms of OS. The investigators who evaluated
nimotuzumab have discussed the probable reasons for the positive
outcomes based on the results of a subgroup analysis of the RTOG
0522 trial (23,24). This subgroup analysis of the RTOG
0522 trial revealed a trend towards improved outcomes in younger
patients, patients with HPV negative tumors of the oropharynx or
hypopharynx and patients in the T4 tumor subgroup (23). While these subgroups represented a
small minority of the study population in the RTOG 0522 trial, the
nimotuzumab trial had higher proportions of such patients (23,24).
Moreover, a subgroup analysis of the Patil et al study also
revealed that the addition of nimotuzumab led to a decrease in
progression by 50% for p16 negative patients, strengthening their
hypothesis (24). The weekly
‘lighter chemoradiotherapy’ regime, which resulted in fewer
radiation interruptions compared with the RTOG 0522 trial, was also
stated to be a probable reason for the positive outcomes in the
nimotuzumab trial. Another possible reason is that nimotuzumab is
biologically and structurally different from cetuximab and
panitumumab in that it can interrupt both ligand-dependent and
ligand-independent signaling of the EGFR pathway (24,47,48).
However, before concluding that the combination of nimotuzumab +
cisplatin + RT is superior to cisplatin + RT alone, it is
imperative to consider that cisplatin was administered at a reduced
dose (30 mg/m2 weekly) instead of 100 mg/m2
every 3 weeks or 40 mg/m2 weekly. Previous studies
comparing cisplatin given at two different dose levels (30
mg/m2 weekly + RT vs. 100 mg/m2 every 3 weeks
+ RT) for the treatment of LAHNSCC have reported that 100
mg/m2 provided every 3 weeks is superior to 30
mg/m2 weekly in terms of LRC (49,50).
Since it remains unclear if the efficacy of the chemoradiotherapy
regimen was optimal in the study by Patil et al (24), it is currently inappropriate to make
the assumption that adding nimotuzumab to the standard
chemoradiotherapy procedure can confer any advantages over
chemoradiotherapy alone (24).
Larger clinical studies are required to test this hypothesis more
adequately.
Phase III trials that tested the effectiveness of
zalutumumab + chemoradiotherapy compared with chemoradiotherapy
(5-year LRC, 70 vs. 74%; HR, 1.10; 95% CI, 0.81-1.50) or the
effectiveness of panitumab + RT compared with chemoradiotherapy
(2-year PFS: 76% (95% CI, 68-82) vs. 73% (95% CI, 65-79); HR, 0.95;
95% CI, 0.60-1.50; stratified log-rank test, P=0.83P=0.83 did not
demonstrate any advantages of these anti-EGFR agents (25,27).
Induction chemotherapy followed by bio-RT (cetuximab-RT) also did
not confer any improvements in outcomes compared with concurrent
chemoradiotherapy alone (28,29). In
total, 6 of the 12 included studies reported QoL data (24,27,30-33).
The trial by Hitt et al (30)
found improvements in several QoL metrics, including physical
functioning (P=0.0287), appetite loss (P=0.0248) and social contact
(P=0.0153) in the cetuximab + RT group compared with the cisplatin
+ RT group. In the study by Patil et al (24), there was no difference the global
health status QoL scores over time (P=0.396) between the
nimotuzumab +chemoRT arm and the control arm (chemoRT) (51). Similarly, in the HN.6 trial (27,42),
there was no difference in QoL between the panitumumab + RT and
cisplatin + RT groups. The De-ESCALate (31,46), NRG
RTOG 1016(32) and TROG
12.01(33) trials also found no
difference in the QoL scores between the intervention and control
groups.
Overall severe toxicity was assessed as the primary
outcome in the De-ESCALaTE trial by Mehanna et al (31), revealing no significant difference
between the cetuximab + RT arm and the cisplatin + RT arm. Symptom
severity assessed using the MDASI-HN was set as the primary
endpoint in the TROG 12.01 trial, but the trial did not reveal any
significant differences between the cisplatin + RT and cetuximab +
RT arms (33).
A number of trials included in the present review
reported increased rates of mucositis (23-30)
and skin reactions (22,23,25-31)
following treatment with anti-EGFR agents. Three of the trials that
used cetuximab have reported increased rates of acneiform rashes
(22,26,33).
Increased rates of neutropenia were reported in trials that used
TPF + cetuximab + RT (28,29). By contrast, the incidence of nausea,
vomiting, renal toxicity, and ototoxicity was higher following
cisplatin administration (26,27,30,33).
Novel combination therapies, involving
immunotherapy agents: the NRG-HN-004 trial tested the effectiveness
of the immune checkpoint inhibitor durvalumab (an anti-programmed
death-ligand 1 antibody) in combination with RT in
cisplatin-ineligible patients compared to cetuximab +RT (52). The phase II results of this trial
showed no improvement in PFS and significantly higher rates of
locoregional failure with durvalumab +RT compared with cetuximab
+RT (53). Furthermore, PembroRad is
a phase II trial testing the efficacy of pembrolizumab (an
anti-programmed cell death protein-1 antibody) compared with
cetuximab concurrent with RT in patients with LAHNSCC who are
refractory to cisplatin. However, the results of the study
demonstrated that pembrolizumab concurrent with RT did not improve
the tumor control rate or survival compared with cetuximab + RT,
although there were less cases of toxicity in the pembrolizumab arm
(54). REACH, a phase III trial,
examined whether the combination of avelumab (an anti-programmed
death-ligand 1 antibody) + cetuximab + RT is superior to cisplatin
+ RT, or cetuximab + RT, in cisplatin ineligible patients in terms
of PFS. According to preliminary results, the avelumab + cetuximab
+ RT combination was tolerable for patients with LAHNSCC. However,
further analysis showed no improvement in 1-year PFS with this
combination compared to the control arm (55-57).
Of note, EGFR gene copy number alterations and high
EGFR expression have been reported to be associated with poor
prognosis in patients with HNSCC (58-60).
However, to the best of our knowledge, at present, there are no
validated molecular markers for the prediction of the response to
anti-EGFR therapy (13) except for
the finding that in patients who had skin rash when cetuximab was
added to RT showed a positive trend in survival outcomes (13,38,39).
This systematic review had several limitations.
Firstly, heterogeneity was found in the treatment combinations and
the outcomes examined in the studies included in this review.
Therefore, it was not possible to perform a meta-analysis. An
analysis to test if any of the studies biased the results was not
performed, which is a potential limitation of our review. Finally,
in this review we included only phase III clinical trials and only
12 studies were eligible for inclusion. Therefore, evidence was
synthesized based on the findings from these 12 studies published
between 2006 and 2022.
In conclusion, cetuximab added to radical RT
improved LRC, OS and PFS compared with RT alone, suggesting that
cetuximab + RT is a treatment option for patients with LAHNSCC who
cannot tolerate platinum-based chemotherapy (22,38,61).
However, anti-EGFR monoclonal antibodies cetuximab, nimotuzumab,
and zalutumumab when added to chemoradiotherapy did not improve OS
compared to chemoradiotherapy alone (23-25).
Induction chemotherapy followed by cetuximab + RT was not
associated with any difference in outcomes compared with concurrent
chemoradiotherapy (28,29). Phase III trials among p16+
patients with OPC reported inferior outcomes with cetuximab + RT
compared with cisplatin + RT (31-33).
Based on the findings of those studies, it is difficult to suggest
that anti-EGFR therapy in any form confers any advantage over
conventional chemoradiotherapy for p16+ patients with
OPC. Results of the recent clinical trials evaluating novel
combinations involving immunotherapy, against chemoradiotherapy for
the treatment of patients diagnosed with LAHNSCC are not very
encouraging (52-57).
Additional studies in the field of translational biomarker research
are required to build biomarker-based, novel treatment combinations
of anti-EGFR agents for LAHNSCC.
Supplementary Material
PubMed search results.
SCOPUS search on January 31, 2024
Embase search results (January 31,
2024)
Cochrane search results.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
KCT, RR, LMN, MR, KP and NJ conceptualized the
study. JVP, KCT and LMN designed the study methodology. JVP
performed the literature search and the screening of titles. JVP
and LMN performed the review of the abstracts to identify
potentially eligible studies. KCT, JVP and LMN conducted the review
of the full texts and selected the studies eligible for inclusion.
LMN wrote the first draft of the manuscript. LMN and JVP prepared
the table and figures. RR, MR, KP, NJ, JVP, LMN and KCT edited and
critically revised the manuscript. LMN and KCT can confirm the
authenticity of the raw data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barsouk A, Aluru JS, Rawla P, Saginala K
and Barsouk A: Epidemiology, risk factors, and prevention of head
and neck squamous cell carcinoma. Med Sci (Basel).
11(42)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marur S and Forastiere AA: Head and neck
squamous cell carcinoma: Update on epidemiology, diagnosis, and
treatment. Mayo Clin Proc. 91:386–396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Adelstein DJ, Li Y, Adams GL, Wagner H,
Kish JA, Ensley JF, Schuller DE and Forastiere AA: An intergroup
phase III comparison of standard radiation therapy and two
schedules of concurrent chemoradiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098.
2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pignon JP, Bourhis J, Domenge C and
Designé L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: Three meta-analyses of updated
individual data. MACH-NC collaborative group. meta-analysis of
chemotherapy on head and neck cancer. Lancet. 355:949–955.
2000.PubMed/NCBI
|
|
6
|
Pignon JP, le Maître A, Maillard E and
Bourhis J: MACH-NC Collaborative Group. Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lacas B, Carmel A, Landais C, Wong SJ,
Licitra L, Tobias JS, Burtness B, Ghi MG, Cohen EEW, Grau C, et al:
Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An
update on 107 randomized trials and 19,805 patients, on behalf of
MACH-NC group. Radiother Oncol. 156:281–293. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Trotti A, Bellm LA, Epstein JB, Frame D,
Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L and Zilberberg MD:
Mucositis incidence, severity and associated outcomes in patients
with head and neck cancer receiving radiotherapy with or without
chemotherapy: A systematic literature review. Radiother Oncol.
66:253–262. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bossi P and Platini F: Radiotherapy plus
EGFR inhibitors: Synergistic modalities. Cancers Head Neck.
2(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Harari PM and Huang SM: Head and neck
cancer as a clinical model for molecular targeting of therapy:
Combining EGFR blockade with radiation. Int J Radiat Oncol Biol
Phys. 49:427–433. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Iberri DJ and Colevas AD: Balancing safety
and efficacy of epidermal growth factor receptor inhibitors in
patients with squamous cell carcinoma of the head and neck.
Oncologist. 21(391)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fasano M, Della Corte CM, Viscardi G, Di
Liello R, Paragliola F, Sparano F, Iacovino ML, Castrichino A,
Doria F, Sica A, et al: Head and neck cancer: The role of anti-EGFR
agents in the era of immunotherapy. Ther Adv Med Oncol.
13(1758835920949418)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kalyankrishna S and Grandis JR: Epidermal
growth factor receptor biology in head and neck cancer. J Clin
Oncol. 24:2666–2672. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang SM and Harari PM: Epidermal growth
factor receptor inhibition in cancer therapy: Biology, rationale
and preliminary clinical results. Invest New Drugs. 17:259–269.
1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bai J, Guo XG and Bai XP: Epidermal growth
factor receptor-related DNA repair and radiation-resistance
regulatory mechanisms: A mini-review. Asian Pac J Cancer Prev.
13:4879–4881. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dassonville O, Formento JL, Francoual M,
Ramaioli A, Santini J, Schneider M, Demard F and Milano G:
Expression of epidermal growth factor receptor and survival in
upper aerodigestive tract cancer. J Clin Oncol. 11:1873–1878.
1993.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ang KK, Andratschke NH and Milas L:
Epidermal growth factor receptor and response of head-and-neck
carcinoma to therapy. Int J Radiat Oncol Biol Phys. 58:959–965.
2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liang K, Ang KK, Milas L, Hunter N and Fan
Z: The epidermal growth factor receptor mediates radioresistance.
Int J Radiat Oncol Biol Phys. 57:246–254. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bonner JA, Maihle NJ, Folven BR,
Christianson TJ and Spain K: The interaction of epidermal growth
factor and radiation in human head and neck squamous cell carcinoma
cell lines with vastly different radiosensitivities. Int J Radiat
Oncol Biol Phys. 29:243–247. 1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Moher D, Shamseer L, Clarke M, Ghersi D,
Liberati A, Petticrew M, Shekelle P and Stewart LA: PRISMA-P Group.
Preferred reporting items for systematic review and meta-analysis
protocols (PRISMA-P) 2015 statement. Syst Rev. 4(1)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan
PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar
AK, et al: Randomized phase III trial of concurrent accelerated
radiation plus cisplatin with or without cetuximab for stage III to
IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 32:2940–2950.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Patil VM, Noronha V, Joshi A, Agarwal J,
Ghosh-Laskar S, Budrukkar A, Murthy V, Gupta T, Mahimkar M, Juvekar
S, et al: A randomized phase 3 trial comparing nimotuzumab plus
cisplatin chemoradiotherapy versus cisplatin chemoradiotherapy
alone in locally advanced head and neck cancer. Cancer.
125:3184–3197. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eriksen JG, Maare C, Johansen J, Primdahl
H, Evensen JF, Kristensen CA, Andersen LJ and Overgaard J:
Evaluation of the EGFR-inhibitor zalutumumab given with primary
curative (CHEMO) radiation therapy to patients with squamous cell
carcinoma of the head and neck: Results of the DAHANCA 19
randomized phase 3 trial. Int J Radiat Oncol Biol Phys.
88(465)2014.
|
|
26
|
Gebre-Medhin M, Brun E, Engström P, Cange
HH, Hammarstedt-Nordenvall L, Reizenstein J, Nyman J, Abel E,
Friesland S, Sjödin H, et al: ARTSCAN III: A randomized phase III
study comparing chemoradiotherapy with cisplatin versus cetuximab
in patients with locoregionally advanced head and neck squamous
cell cancer. J Clin Oncol. 39:38–47. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Siu LL, Waldron JN, Chen BE, Winquist E,
Wright JR, Nabid A, Hay JH, Ringash J, Liu G, Johnson A, et al:
Effect of standard radiotherapy with cisplatin vs accelerated
radiotherapy with panitumumab in locoregionally advanced squamous
cell head and neck carcinoma: A randomized clinical trial. JAMA
Oncol. 3:220–226. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Geoffrois L, Martin L, De Raucourt D, Sun
XS, Tao Y, Maingon P, Buffet J, Pointreau Y, Sire C, Tuchais C, et
al: Induction chemotherapy followed by cetuximab radiotherapy is
not superior to concurrent chemoradiotherapy for head and neck
carcinomas: Results of the GORTEC 2007-02 phase III randomized
trial. J Clin Oncol. 36:3077–3083. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Merlano MC, Denaro N, Vecchio S, Licitra
L, Curcio P, Benasso M, Bagicalupo A, Numico G, Russi E, Corvo' R,
et al: Phase III randomized study of induction chemotherapy
followed by definitive radiotherapy + cetuximab versus
chemoradiotherapy in squamous cell carcinoma of head and neck: the
INTERCEPTOR-GONO study (NCT00999700). Oncology. 98:763–770.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hitt R, Mesía R, Lozano A, Iglesias
Docampo L, Grau JJ, Taberna M, Rubió-Casadevall J, Martínez-Trufero
J, Morillo EDB, García Girón C, et al: Randomized phase 3
noninferiority trial of radiotherapy and cisplatin vs radiotherapy
and cetuximab after docetaxel-cisplatin-fluorouracil induction
chemotherapy in patients with locally advanced unresectable head
and neck cancer. Oral Oncol. 134(106087)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mehanna H, Robinson M, Hartley A, Kong A,
Foran B, Fulton-Lieuw T, Dalby M, Mistry P, Sen M, O'Toole L, et
al: Radiotherapy plus cisplatin or cetuximab in low-risk human
papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An
open-label randomised controlled phase 3 trial. Lancet. 393:51–60.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gillison ML, Trotti AM, Harris J, Eisbruch
A, Harari PM, Adelstein DJ, Jordan RCK, Zhao W, Sturgis EM,
Burtness B, et al: Radiotherapy plus cetuximab or cisplatin in
human papillomavirus-positive oropharyngeal cancer (NRG Oncology
RTOG 1016): A randomised, multicentre, non-inferiority trial.
Lancet. 393:40–50. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rischin D, King M, Kenny L, Porceddu S,
Wratten C, Macann A, Jackson JE, Bressel M, Herschtal A, Fisher R,
et al: Randomized trial of radiation therapy with weekly cisplatin
or cetuximab in low-risk HPV-associated oropharyngeal cancer (TROG
12.01)-a trans-tasman radiation oncology group study. Int J Radiat
Oncol Biol Phys. 111:876–886. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sobin LH, Gospodarowicz MK and Christian
Wittekind C (eds): International Union Against cancer (UICC): TNM
classification of malignant tumours. 7th edition. Oxford,
Wiley-Blackwell, 2009.
|
|
35
|
Fleming ID, Cooper JS, Henson DE, Hutter
RVP, Kennedy BJ and Murphy GP (eds): American Joint Committee on
Cancer (AJCC). AJCC cancer staging manual. 5th edition.
Philadelphia: J. B. Lippincott, 1997.
|
|
36
|
Greene FL, Page DL, Fleming ID, Fritz A
and Balch CM: American Joint Committee on Cancer. AJCC cancer
staging manual. 6th edition. New York, NY: Springer-Verlag,
2002.
|
|
37
|
Rosenthal DI, Mendoza TR, Chambers MS,
Asper JA, Gning I, Kies MS, Weber RS, Lewin JS, Garden AS, Ang KK,
et al: Measuring head and neck cancer symptom burden: The
development and validation of the M. D. Anderson symptom inventory,
head and neck module. Head Neck. 29:923–931. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bonner JA, Harari PM, Giralt J, Cohen RB,
Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-Year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash and survival. Lancet
Oncol. 11:21–28. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Roman J, Dissaux G, Gouillou M, Gobel Y,
Potard G, Leclere JC, Conan-Charlet V, Gujral D, Abgral R, Guibourg
B, et al: Prolonged overall treatment time and lack of skin rash
negatively impact overall survival in locally advanced head and
neck cancer patients treated with radiotherapy and concomitant
cetuximab. Target Oncol. 12:505–512. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Caudell JJ, Torres-Saavedra P, Rosenthal
DI, Axelrod R, Nguyen-Tan PF, Sherman E, Weber RS, Galvin JM,
El-Naggar AK, Konski AA, et al: Long-term update of NRG/RTOG 0522:
A randomized phase 3 trial of concurrent radiation and cisplatin
with or without cetuximab in locoregionally advanced head and neck
cancer. Int J Radiat Oncol Biol Phys. 116:533–543. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Eriksen JG, Maare C, Johansen J, Primdahl
H, Evensen J, Kristensen CA, Andersen LJ and Overgaard J: 5-Y
update of the randomized phase III trial DAHANCA19: Primary (Chemo)
RT +/- zalutumumab in HNSCC. Radiother Oncol. 127:S137–S138.
2018.
|
|
42
|
Ringash J, Waldron JN, Siu LL, Martino R,
Winquist E, Wright JR, Nabid A, Hay JH, Hammond A, Sultanem K, et
al: Quality of life and swallowing with standard chemoradiotherapy
versus accelerated radiotherapy and panitumumab in locoregionally
advanced carcinoma of the head and neck: A phase III randomised
trial from the Canadian cancer trials group (HN.6). Eur J Cancer.
72:192–199. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cohen EE, Karrison TG, Kocherginsky M,
Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB,
Yunus F, et al: Phase III randomized trial of induction
chemotherapy in patients with N2 or N3 locally advanced head and
neck cancer. J Clin Oncol. 32:2735–2743. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tao Y, Geoffrois L, Martin L, De Raucourt
D, Miny J, Maingon P, Lafond C, Tuchais C, Sire C, Babin E, et al:
Impact of p16 expression on induction taxotere-cisplatin-5 FU (TPF)
followed by cetuximab-radiotherapy in N2b-N3 head and neck squamous
cell carcinoma (HNSCC): Results of GORTEC 2007-02 phase III
randomized trial. J Clin Oncol. 35 (15 Suppl)(S6070)2017.
|
|
45
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jones DA, Mistry P, Dalby M, Fulton-Lieuw
T, Kong AH, Dunn J, Mehanna HM and Gray AM: Concurrent cisplatin or
cetuximab with radiotherapy for HPV-positive oropharyngeal cancer:
Medical resource use, costs, and quality-adjusted survival from the
De-ESCALaTE HPV trial. Eur J Cancer. 124:178–185. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Garrido G, Tikhomirov IA, Rabasa A, Yang
E, Gracia E, Iznaga N, Fernández LE, Crombet T, Kerbel RS and Pérez
R: Bivalent binding by intermediate affinity of nimotuzumab: A
contribution to explain antibody clinical profile. Cancer Biol
Ther. 11:373–382. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Berger C, Krengel U, Stang E, Moreno E and
Madshus IH: Nimotuzumab and cetuximab block ligand-independent EGF
receptor signaling efficiently at different concentrations. J
Immunother. 34:550–555. 2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Noronha V, Joshi A, Patil VM, Agarwal J,
Ghosh-Laskar S, Budrukkar A, Murthy V, Gupta T, D'Cruz AK, Banavali
S, et al: Once-a-week versus once-every-3-weeks cisplatin
chemoradiation for locally advanced head and neck cancer: A phase
III randomized noninferiority trial. J Clin Oncol. 36:1064–1072.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mashhour K and Hashem W: Cisplatin weekly
versus every 3 weeks concurrently with radiotherapy in the
treatment of locally advanced head and neck squamous cell
carcinomas: What is the best dosing and schedule? Asian Pac J
Cancer Prev. 21:799–807. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Menon N, Patil V, Noronha V, Joshi A,
Bhattacharjee A, Satam BJ, Mathrudev V, Ghosh Laskar S and Prabhash
K: Quality of life in patients with locally advanced head and neck
cancer treated with concurrent chemoradiation with cisplatin and
nimotuzumab versus cisplatin alone-additional data from a phase 3
trial. Oral Oncol. 122(105517)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Radiation Therapy With Durvalumab or
Cetuximab in Treating Patients With Locoregionally Advanced Head
and Neck Cancer Who Cannot Take Cisplatin-Full Text View-ClinicalTrials.gov, 2022. Available from:
https://clinicaltrials.gov/ct2/show/NCT03258554.
|
|
53
|
Mell LK, Torres-Saavedra P, Wong S, Chang
S, Kish JA, Minn AJ III, Jordan R, Liu T, Truong MT, Winquist E, et
al: Radiotherapy with durvalumab vs cetuximab in patients with
locoregionally advanced head and neck cancer and a contraindication
to cisplatin: Phase II results of NRG-HN004. Int J Radiat Oncol
Biol Phys. 114(1058)2022.
|
|
54
|
Tao Y, Biau J, Sun X, Sire C, Martin L,
Alfonsi M, Prevost JB, Modesto A, Lafond C, Tourani JM, et al:
Pembrolizumab versus cetuximab concurrent with radiotherapy in
patients with locally advanced squamous cell carcinoma of head and
neck unfit for cisplatin (GORTEC 2015-01 PembroRad): A multicenter,
randomized, phase II trial. Ann Oncol. 34:101–110. 2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Randomized Trial of
Avelumab-cetuximab-radiotherapy Versus SOCs in LA SCCHN
(REACH)-Full Text View-ClinicalTrials.gov, 2022. Available from:
https://clinicaltrials.gov/ct2/show/NCT02999087.
|
|
56
|
Tao Y, Aupérin A, Sun X, Sire C, Martin L,
Coutte A, Lafond C, Miroir J, Liem X, Rolland F, et al:
Avelumab-cetuximab-radiotherapy versus standards of care in locally
advanced squamous-cell carcinoma of the head and neck: The safety
phase of a randomised phase III trial GORTEC 2017-01 (REACH). Eur J
Cancer. 141:21–29. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Bourhis J, Tao Y, Sun X, Sire C, Martin L,
Liem X, Coutte A, Pointreau Y, Thariat J, Miroir J, et al: LBA35
Avelumab-cetuximab-radiotherapy versus standards of care in
patients with locally advanced squamous cell carcinoma of head and
neck (LA-SCCHN): Randomized phase III GORTEC-REACH trial. Ann
Oncol. 32 (Suppl 5)(S1310)2021.
|
|
58
|
Temam S, Kawaguchi H, El-Naggar AK,
Jelinek J, Tang H, Liu DD, Lang W, Issa JP, Lee JJ and Mao L:
Epidermal growth factor receptor copy number alterations correlate
with poor clinical outcome in patients with head and neck squamous
cancer. J Clin Oncol. 25:2164–2170. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Chung CH, Ely K, McGavran L,
Varella-Garcia M, Parker J, Parker N, Jarrett C, Carter J, Murphy
BA, Netterville J, et al: Increased epidermal growth factor
receptor gene copy number is associated with poor prognosis in head
and neck squamous cell carcinomas. J Clin Oncol. 24:4170–4176.
2006.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bossi P, Resteghini C, Paielli N, Licitra
L, Pilotti S and Perrone F: Prognostic and predictive value of EGFR
in head and neck squamous cell carcinoma. Oncotarget.
7:74362–74379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Posner MR and Wirth LJ: Cetuximab and
radiotherapy for head and neck cancer. N Engl J Med. 354:634–636.
2006.PubMed/NCBI View Article : Google Scholar
|