Introduction
Liver cancer was the seventh leading cause of
cancer-related mortality in 2022, contributing to over a quarter
million fatalities worldwide (1).
Hepatocellular carcinoma (HCC) is its most common form, accounting
for 75-85% of all cases. However, >50% of HCC cases are
diagnosed at an advanced stage, where treatment options are limited
(2).
As the first-line treatment for advanced-stage HCC,
sorafenib is an oral multikinase inhibitor that suppresses tumor
proliferation and induces apoptosis. Despite its initial efficacy,
numerous patients develop resistance or experience severe
side-effects, leading to the discontinuation of treatment (2-4). As
a well-known treatment for psychiatric disorders, sertraline has
exhibited promising results in cancer therapy due to its antitumor
properties via apoptosis- and autophagy-related effects, and
synergistic effects with other drugs (5-7).
The antitumor properties of sertraline were first identified by
Telerman et al in 1993(8).
As a mechanism characterized by the lysosomal
degradation of intracellular proteins and organelles, autophagy has
attracted significant attention regarding its role in human
diseases and physiology. Autophagy can promote or inhibit cancer
development, as well as the progression and response to therapy
(9). During the early stages of
tumorigenesis, autophagy functions as a protective mechanism for
the body, limiting cancer development. However, in the advanced
stages, autophagy enables malignant cells to survive under stress
conditions, such as the hypoxic tumor microenvironment and
therapy-induced starvation (10).
Autophagy is regulated by proteins, such as
Beclin-1, p62/sequestosome1 (SQSTM1) and autophagy-related protein
(ATG)8/LC3, which are involved in the formation and maturation of
autophagosomes. These autophagosomes fuse with lysosomes for the
degradation of cellular debris (11). Recent observations suggest that
sorafenib induces autophagy in liver cancer cells by modulating
several signaling pathways, including the mammalian target of
rapamycin (mTOR) and SHP-1/STAT3/MCL-1/Beclin-1 pathways, as well
as by modulating endoplasmic reticulum stress induction,
sphingolipid metabolism imbalance and microRNA transcription
alteration (12). The dysregulation
of autophagy has also been linked to sertraline in several cell
lines (4-7).
Sertraline has been shown to induce autophagy via the activation of
AMP-activated protein kinase (AMPK), which inhibits the mechanistic
target of the mTOR-ribosomal protein S6 kinase B1 signaling pathway
(13). On the other hand,
contradictory results have been reported in lung cancer, where
sertraline inhibits autophagy and facilitates TRAIL-induced
apoptosis (14).
The authors have previously reported that sorafenib
and sertraline exert a synergistic anticancer effect on HepG2
cells, significantly reducing cell viability and inducing apoptosis
at lower doses compared to each drug used alone (15). To further investigate the effects of
sorafenib and sertraline, and investigate a possible link between
autophagy and combined treatment with both agents in HepG2 cells,
the present study examined the mRNA expression levels and cellular
localizations of the key autophagy markers and the formation of
acidic vesicular organelles.
Materials and methods
Cells, cell culture and
treatments
HepG2 cells (cat. no. HB-8065, American Type Culture
Collection). were maintained in Dulbecco's modified Eagle's medium
(DMEM, MilliporeSigma) containing 1% antibiotics (10 mg/ml
streptomycin and 10,000 U/ml penicillin, PAN-Biotech GmbH) and 10%
fetal bovine serum (FBS, Biosera) in an incubator with 5%
CO2 at 37˚C. The monitoring of cultured HepG2 cells was
performed using the Zeiss PrimoVert inverted phase contrast
microscope (Zeiss AG). For starvation, cells were incubated in
Dulbecco's phosphate-buffered saline (DPBS, Gibco; Thermo Fisher
Scientific, Inc.) for 2 h in an incubator at 37˚C (16).
Sorafenib (LC Laboratories) and sertraline
(MilliporeSigma) were dissolved in dimethyl sulfoxide (DMSO) and
distilled water, respectively, to a concentration of 10 mmol/l. The
drugs were treated as previously reported with IC50/2
doses for 24 h (17.8 µl sorafenib and 8.9 µl sertraline) (15).
Total RNA extraction
Following a 24-h incubation period at 37˚C with the
drugs in question, whether alone or in combination, the cells were
trypsinized and washed with PBS. RNA extraction was then performed
using a Thermo Scientific GeneJET RNA Purification kit (cat. no.
K0731, Thermo Fisher Scientific, Inc.), as per the manufacturer's
instructions. The concentration and purity of the extracted RNA
samples were subsequently assessed with a BioTek Synergy Microplate
Reader (Agilent Technologies, Inc.), utilizing UV absorbance.
Complementary DNA (cDNA) synthesis and
reverse transcription-quantitative PCR (RT-qPCR)
The Thermo Scientific RevertAid First Strand cDNA
Synthesis kit (cat. no. K1621, Thermo Fisher Scientific, Inc.) was
employed to synthesize cDNA from total RNA, following the
instructions provided by the manufacturer. qPCR was conducted on a
LightCycler® 96 instrument (Roche Diagnostics) using the
Ampliqon RealQ Plus Master Mix Green Without ROX kit (cat. no.
A323402, Ampliqon A/S) and gene-specific primers for
Beclin-1 (NM_003766; forward sequence,
5'-CTGGACACTCAGCTCAACGTCA-3' and reverse sequence,
5'-CTCTAGTGCCAGCTCCTTTAGC-3'); LC3 (NM_022818; forward
sequence, 5'-GAGAAGCAGCTTCCTGTTCTGG-3' and reverse sequence,
5'-GTGTCCGTTCACCAACAGGAAG-3') and GAPDH (forward sequence,
5'-ATGGGTGTGAACCATGAGAA-3' and reverse sequence,
5'-GTGCTAAGCAGTTGGTGGTG-3'). qPCR was performed using with the
following thermocycling conditions: initial denaturation at 95˚C
for 10 min, followed by 40 cycles of denaturation at 95˚C for 15
sec, annealing at 60˚C for 30 sec, and extension at 72˚C for 30
sec. qPCR primer sequences were obtained from OriGene Technologies,
Inc. The 2-ΔΔCq was employed for
the relative quantification of gene expression, with GAPDH
serving as the internal control (17). The untreated control was used as a
calibrator to determine whether DMSO influences the expression of
related genes.
Confocal laser scanning
microscopy
Autoclaved coverslips were introduced into the wells
of a 12-well plate, with 100,000 cells seeded per well. On the
subsequent day, the cells were treated with sertraline and
sorafenib, either individually or in combination, and incubated for
24 h. The medium of the starvation control group was washed twice,
replaced with DPBS, and incubated at 37˚C for 2 h. A stock solution
of acridine orange (Thermo Fisher Scientific, Inc.) was prepared in
water at a concentration of 1 mg/ml and stored at 4˚C. The staining
was conducted with acridine orange (cat. no. A6014,
MilliporeSigma). at a final concentration of 1 µg/ml for 20 min at
37˚C following fixation of the cells with 4% paraformaldehyde (cat.
no. P6148, MilliporeSigma) for 15 min at room temperature. The
cells were then washed three times with PBS and imaged using a
Zeiss LSM 700 confocal microscope (Zeiss AG). The excitation laser
for green fluorescence was 473 nm, and for red fluorescence, it was
559 nm. The emission filters were 520 and 572 nm, respectively.
Following the treatments and subsequent fixation of
the cells, they were also labeled with p62 (1:200; cat. no. A19700,
ABclonal Biotech Co., Ltd.) and LC3 (1:50; cat. no. A5618, ABclonal
Biotech Co., Ltd.) antibodies. Incubation was performed at 4˚C
overnight. The cells were incubated with a 0.1% Triton X-100/PBS
solution for permeabilization for 10 min. The cells were then
incubated at 37˚C with PBS containing 1% BSA for 30 min to block
non-specific binding. Specific primary antibodies were prepared at
a dilution of 1:50 for p62 and 1:200 for LC3, and the cells were
incubated overnight at 4˚C. The following day, the cells were
washed three times with PBS and then incubated for 1 h at room
temperature in the dark with Alexa Fluor 488-conjugated (1:200;
cat. no. A-11008, Invitrogen; Thermo Fisher Scientific, Inc.) and
Alexa Fluor 555-conjugated (1:200; cat. no. A-21428, Invitrogen;
Thermo Fisher Scientific, Inc.) anti-rabbit secondary antibodies.
After staining, the cells were washed three times with PBS and
incubated with DAPI (cat. no. D1306, Thermo Fisher Scientific,
Inc.) solution (1 µg/ml) for 10 min for nuclear staining. The
preparations were mounted with a mounting medium and imaged using a
Zeiss LSM700 confocal microscope (Zeiss AG).
Statistical analysis
The bar graphs were generated, and the statistical
analysis was performed using GraphPad Prism 9 Software (Dotmatics).
A one-way analysis of variance (ANOVA) was conducted to determine
the difference between the means of the groups, followed by Tukey's
multiple comparisons test to assess pairwise differences. The data
are presented as the mean ± standard error of mean (SEM). A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
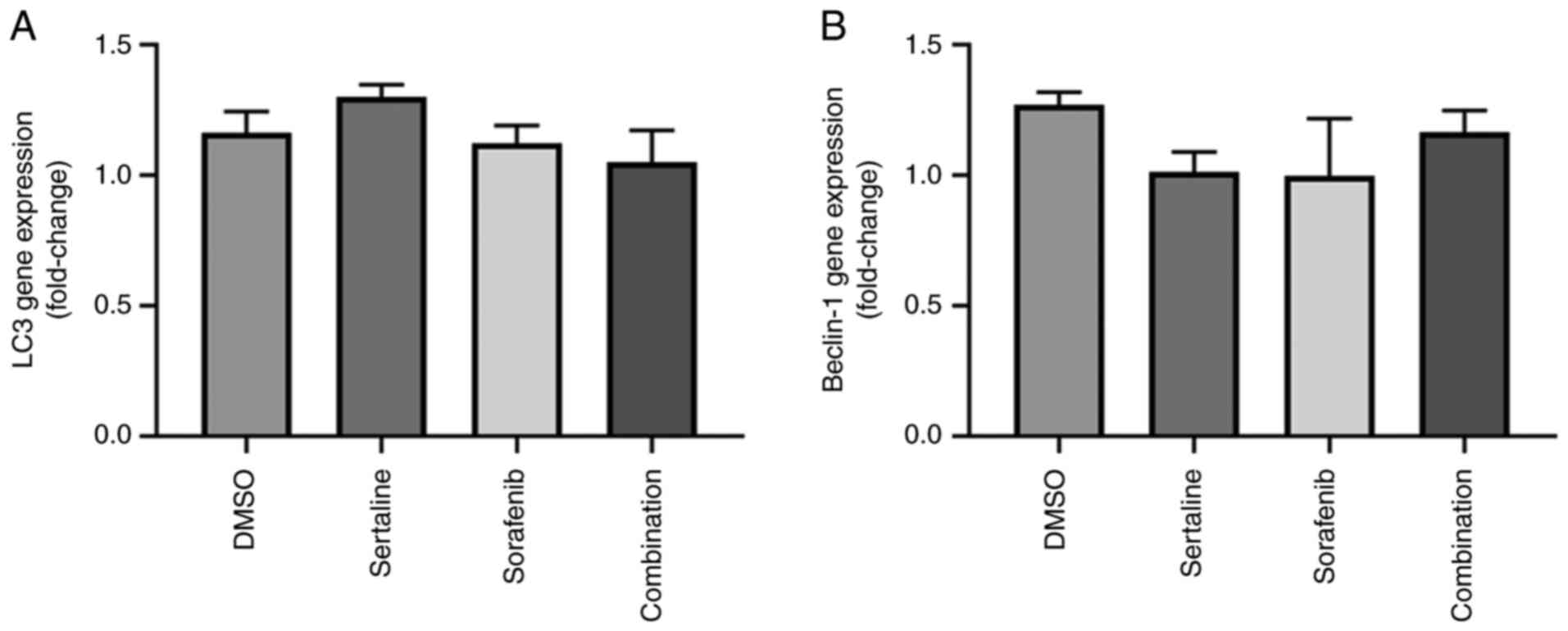
Effects of sertraline and sorafenib on
LC3 and Beclin-1 gene expression levels
The effects of sertraline and sorafenib on
LC3 and Beclin-1 gene expression levels are
demonstrated in Fig. 1. No
significant differences were found between the groups for both
genes. The drugs did not induce a significant change in the
LC3 and Beclin-1 gene expression levels (P=0.0916 for
LC3 and P=0.6022 for Beclin-1).
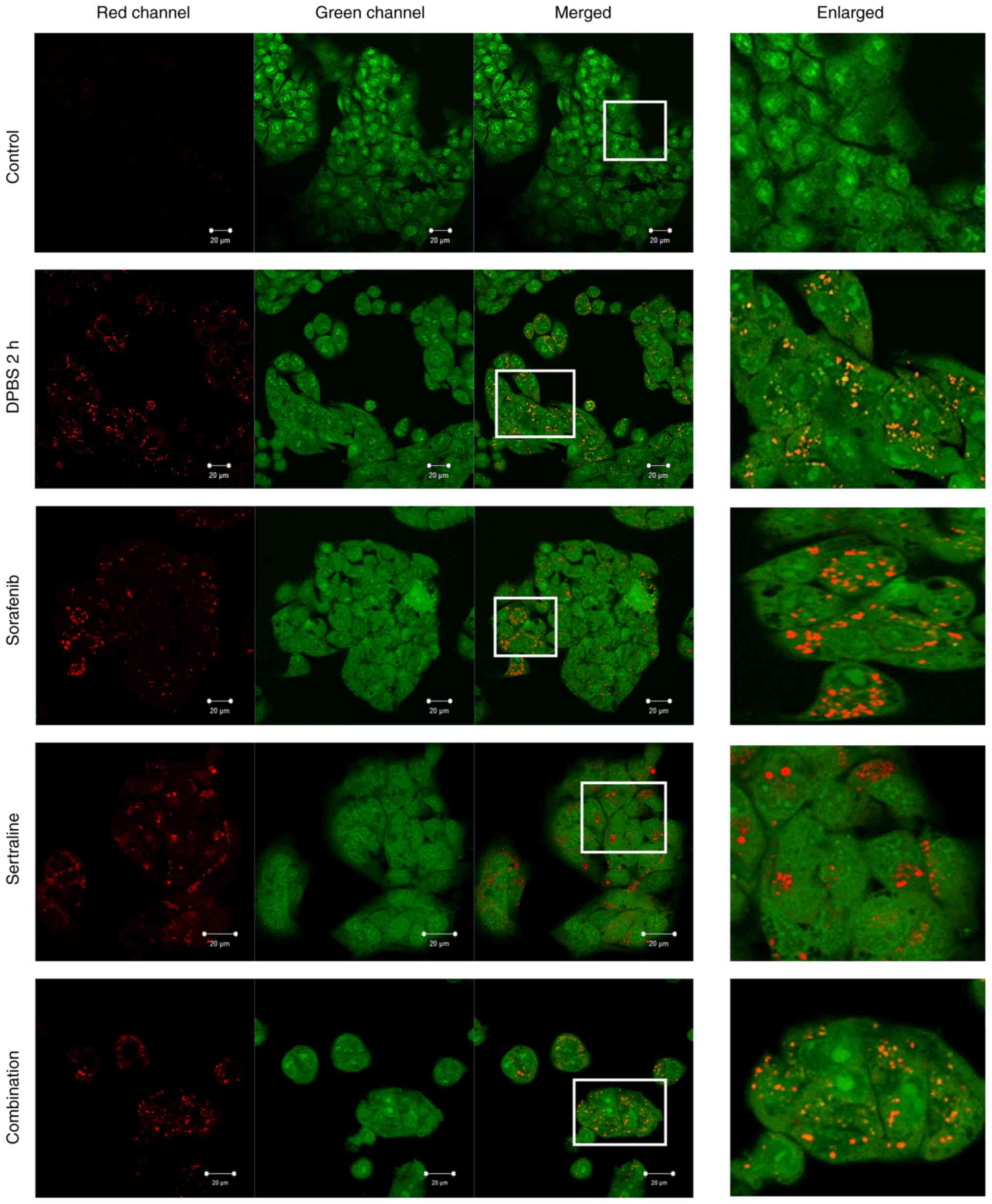
Effects of sertraline and sorafenib on
the formation of acidic vesicular organelles
The formation of acidic vesicular organelles
following treatment with sertraline, sorafenib, or a combination of
both was evaluated using acridine orange staining and compared to a
starvation control group that was incubated in DPBS for 2 h, as
demonstrated in Fig. 2. In the
starvation control, acridine orange formed aggregates that emitted
bright red fluorescence, indicating the acidic compartments.
Aggregate formation was also evident following sertraline,
sorafenib and combination treatments. Conversely, such aggregates
were not observed in the control cells (Fig. 2).
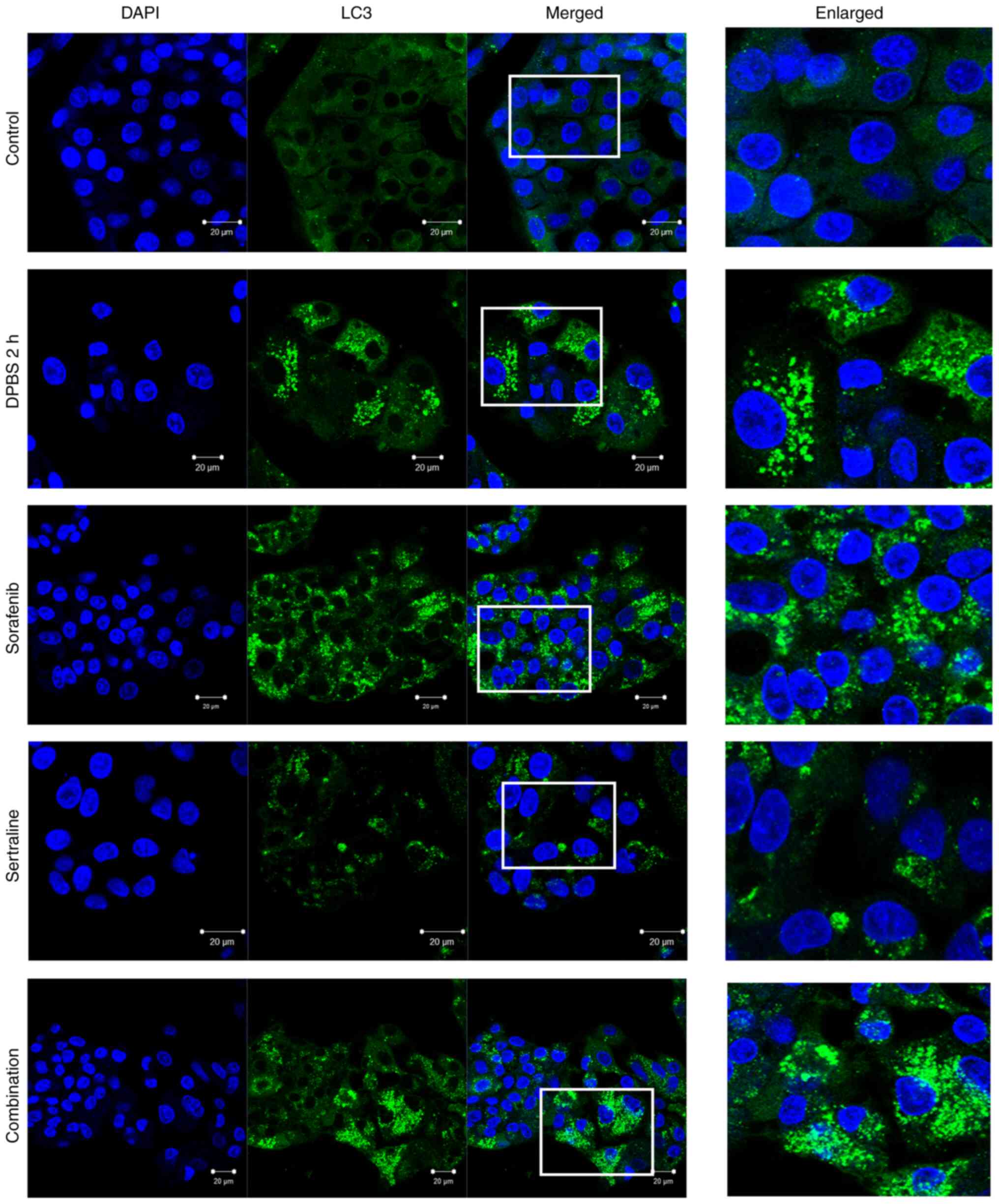
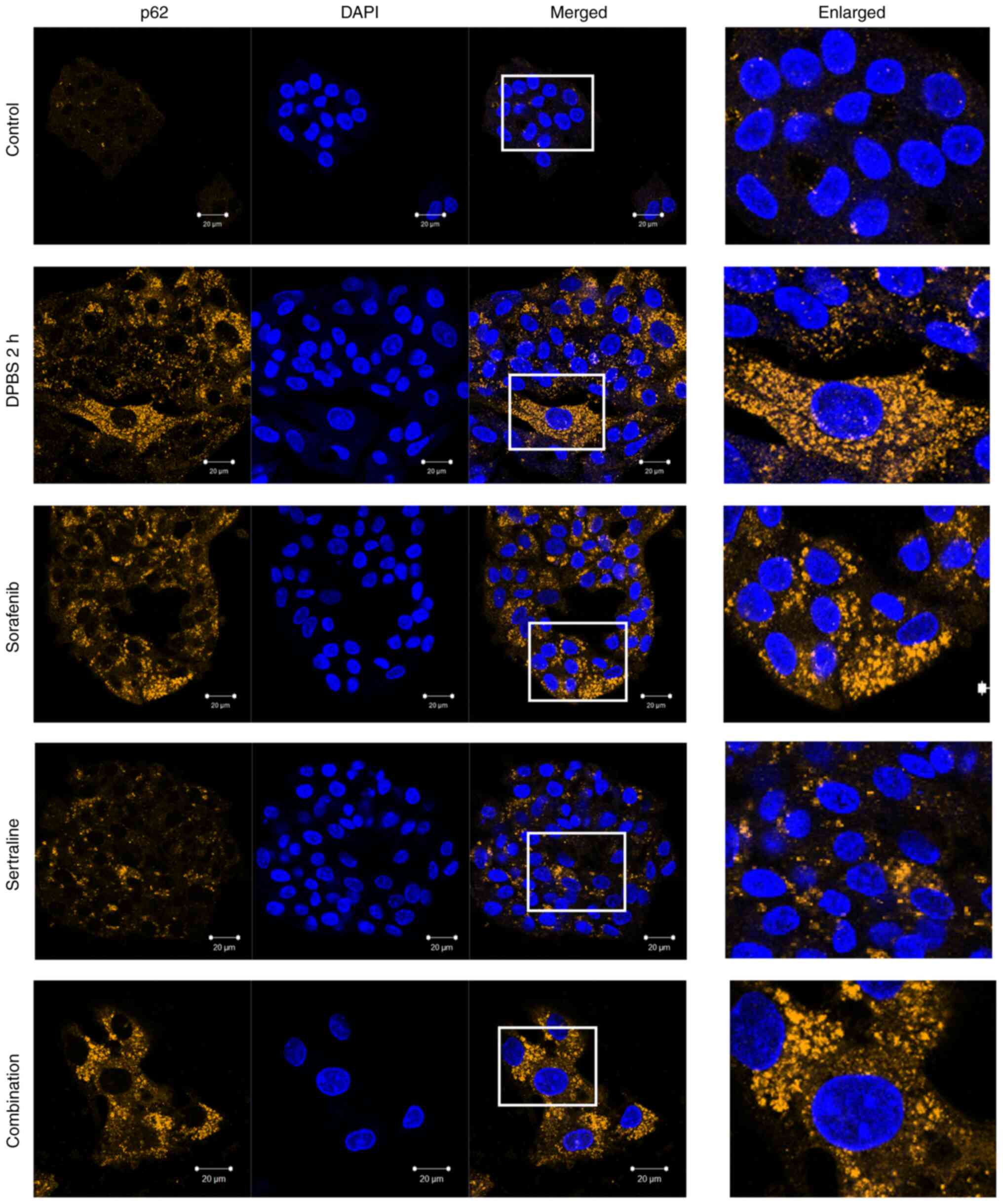
Effects of sertraline and sorafenib on
the localization of LC3 and p62
The autophagic activity was further evaluated by
detecting LC3 and p62 localization using immunofluorescence
staining. As illustrated in Fig. 3,
clear LC3 puncta were observed, indicating autophagosome formation
in the starvation control cells and drug-treated cells. A plurality
of spots was particularly observed in cells treated with sorafenib
or sertraline-sorafenib combination therapy. A similar pattern was
obtained for the appearance of p62 puncta in starvation-induced
cells and drug-treated cells (Fig.
4). By contrast, LC3 and p62 puncta formations were not evident
in the untreated control group.
Discussion
A significant number of patients are diagnosed with
advanced liver cancer and, regrettably, do not derive long-term
benefit from systemic therapy due to the adverse effects and
development of drug resistance through a number of different
mechanisms (18). The mechanisms
contributing to a decreased response to sorafenib include the
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and Janus
kinase-signal transducer and activator of transcription (JAK-STAT)
pathways, the inhibition of pro-apoptotic signals, the presence of
cancer stem cells, epithelial-mesenchymal transition, and
hypoxia-driven responses (19). The
administration of combination treatments within the context of drug
repurposing may facilitate the attainment of superior outcomes for
cancer patients. The antidepressant, sertraline, was previously
demonstrated to exhibit promising anticancer effects, both when
administered alone and in combination with other agents, across a
range of cancer cell lines (20).
Previously, the authors also demonstrated that sorafenib and
sertraline exerted a synergistic anticancer effect in HepG2 cells,
resulting in a significant reduction in cell viability and
increased apoptosis at lower concentrations compared to treatment
with each drug separately (15). The
present study aimed to further investigate the effects of sorafenib
and sertraline with a possible link to autophagy in HepG2
cells.
The regulation of autophagy is considered a viable
strategy in cancer therapy. Autophagy is an essential process for
maintaining cellular homeostasis, and it involves the degradation
and recycling of cellular components (21). Various conditions can trigger
autophagy, including nutrient deprivation, growth factor
withdrawal, hypoxia, or drug treatment (22). Accordingly, in the present study,
autophagy was induced by nutrient deprivation by incubating the
cells in DPBS for 2 h,, which served as a positive control
alongside drug treatment.
The autophagy process begins with the formation of
the isolation membrane, known as the phagophore, which elongates to
engulf cytoplasmic components. The assembly and formation of
autophagosomes rely on the coordinated action of multiple
functional units, including the ULK1 complex, the PI3K complex, the
ATG9A system, and the ATG12- and LC3-conjugation systems (23). Initially, LC3 exists in the precursor
form, which is cleaved by the enzyme ATG4 to produce its cytosolic
form LC3-I. Subsequently, ATG7 and ATG3 attach
phosphatidylethanolamine (PE) to LC3-I, converting it into LC3-II,
which is then directed to the developing autophagosome (24). This conversion is critical for
autophagy advancement and membrane structure stability, rendering
LC3 a prominent autophagic marker. When the autophagosome is fully
formed, PE is removed by ATG4, and LC3 is then released back into
the cytosol (25).
p62/SQSTM1 is a cargo receptor that plays a pivotal
role in the intersection between the ubiquitin-proteasome system
and autophagy by recognizing ubiquitinated proteins destined for
autophagic destruction (26). As p62
accumulates in autophagosomes, it can serve as an indicator of
autophagic activity. A direct interaction between p62 and LC3
facilitates the degradation of ubiquitinated protein aggregates by
autophagy (27). The present study
demonstrated clear LC3 puncta in the cytoplasm of sertraline,
sorafenib and combination treatmetn groups, indicating an increase
in the number of autophagosomes following the respective
treatments, similar to that in the starvation group compared to the
control group.
However, while a common approach to assessing
autophagy involves counting LC3 puncta or autophagosomes, an
increase in the number of autophagosomes does not necessarily
indicate enhanced autophagy, as it could also suggest a blockage in
the process. The majority of assays utilize LC3 as a model
substrate to measure autophagic flux. It is important to determine
the extent to which LC3-II is degraded in a lysosome-dependent
manner over a specified period (28). Moreover, given that autophagy is a
multistep process, the measurement of LC3 or p62 alone is
insufficient to provide a comprehensive understanding of the
cellular events that occur (29).
This is regarded as a limitation of the present study.
Acridine orange is a cell-permeable green
fluorophore that accumulates in acidic vesicular organelles by
protonation. Depending on the concentration, it exhibits a
metachromatic shift to red fluorescence (30). Consequently, red fluorescence can be
observed in acidic vesicular organelles such as autolysosomes. For
the purpose of leveraging this property, acridine orange was used
to measure the increase in acidic vesicular organelle volume during
autophagy induction. The findings of the present study regarding
the use of acridine orange were in accordance with the results for
LC3 and p62. This demonstrated that changes related to autophagic
activity were induced in the sertraline, sorafenib and combination
treatment groups.
Although sorafenib was demonstrated to induce
autophagy in liver cancer, it was also shown that autophagy
triggered by liver cancer cells that have developed resistance to
sorafenib could contribute to the emergence of further drug
resistance. The effect of sertraline on autophagy has been found to
vary depending on the cell type (31). While some studies have shown that it
induces the autophagic flux (6,7,13,32),
others have reported that it inhibits autophagy (13). To the best of our knowledge, the
present study is the first to demonstrate the LC3 and p62 puncta,
indicating an increase in the number of autophagosomes in
sertraline-treated HepG2 cells. Furthermore, when applied in
combination with sorafenib, it appears to introduce changes related
to the autophagic flux effectively. These findings suggest that
sertraline may play a dual role in cancer therapy, acting as a
supportive agent due to its antidepressant effects, while having
the ability to modulate autophagy in addition to its other
anticancer activities (20). Jiang
et al (6) reported that
sertraline induced autophagy and inhibited cell growth, leading
exclusively to autophagic cell death without triggering
caspase-mediated apoptosis. In non-small cell lung cancer cells,
the combination of sertraline and erlotinib enhanced autophagy
activation and tumor cell death through the mutual regulation of
the AMPK/mTOR/S6K pathways. However, when autophagy was inhibited,
sertraline alone or in combination with erlotinib was less
effective (6). Hwang et al
(13) reported the role of
sertraline in AMPK-MTOR signaling-mediated autophagy (12). Moreover, recent research suggests
that sertraline targets prostate cancer stem cells by regulating
redox balance and activating apoptotic and autophagic signaling
pathways (32). Contradictory
results were observed in TRAIL-resistant lung cancer cells. Zinnah
et al (14) reported that, by
inhibiting autophagy, sertraline reduced AMPK phosphorylation and
increased death receptor 5 expression, facilitating TRAIL-induced
apoptosis (14).
In conclusion, the present study demonstrates that
the combination of sorafenib and sertraline induces changes related
to autophagic activity in HepG2 cells. While the autophagy-inducing
effects of sorafenib are well-documented, variable effects of
sertraline on autophagy in different types of cancer have been
demonstrated. The present study revealed that sorafenib and the
antidepressant, sertraline, when applied alone or in combination,
resulted in an increase in the number of autophagosomes compared to
the control group, thereby introducing changes related to
autophagic activity in HepG2 cells. Future studies are required to
explore the molecular mechanisms underlying the effects of
sorafenib and sertraline in order to obtain a more in-depth
understanding of the clinical applicability of this combination in
addressing treatment resistance and improving patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: The present study was partially supported by the
Scientific and Technological Research Council of Türkiye under
grant no. 1919B012211158.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YDC and ZGO were involved in the conceptualization
of the study. GD, YDC and ZGO were involved in the formal analysis.
GD, NP, MEE, ZS, CB, ZGO and YDC were involved in the investigative
aspects of the study. GD, NP, MEE, ZS, CB and YDC were involved in
the study methodology. GD, NP, MEE, ZS, CB and YDC provided
resources. The resources provided include laboratory reagents,
equipment and technical support essential for conducting the
experiments. YDC and ZGO supervised the study. GD and YDC were
involved in visualization (preparation of the figures) and in data
validation. GD, NP, MEE, ZS, CB, ZGO and YDC were involved in the
writing of the original draft, and in the writing, review and
editing of the manuscript. All authors (GD, NP, MEE, ZS, CB, ZGO
and YDC) confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B,
Wu F, Wang Q, Wang S, Rong D, Reiter FP, et al: The mechanisms of
sorafenib resistance in hepatocellular carcinoma: theoretical basis
and therapeutic aspects. Signal Transduct Target Ther.
5(87)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu J, Xia S, Zhang B, Mohammed DM, Yang
X, Zhu Y and Jiang X: Small molecule tyrosine kinase inhibitors
approved for systemic therapy of advanced hepatocellular carcinoma:
Recent advances and future perspectives. Discov Oncol.
15(259)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zarlashat Y, Abbas S and Ghaffar A:
Hepatocellular carcinoma: Beyond the border of advanced stage
therapy. Cancers (Basel). 16(2034)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Baú-Carneiro JL, Akemi Guirao Sumida I,
Gallon M, Zaleski T, Boia-Ferreira M and Bridi Cavassin F:
Sertraline repositioning: An overview of its potential use as a
chemotherapeutic agent after four decades of tumor reversal
studies. Transl Oncol. 16(101303)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jiang X, Lu W, Shen X, Wang Q, Lv J, Liu
M, Cheng F, Zhao Z and Pang X: Repurposing sertraline sensitizes
non-small cell lung cancer cells to erlotinib by inducing
autophagy. JCI Insight. 3(e98921)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xia D, Zhang YT, Xu GP, Yan WW, Pan XR and
Tong JH: Sertraline exerts its antitumor functions through both
apoptosis and autophagy pathways in acute myeloid leukemia cells.
Leuk Lymphoma. 58:1–10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Telerman A, Tuynder M, Dupressoir T,
Robaye B, Sigaux F, Shaulian E, Oren M, Rommelaere J and Amson R: A
model for tumor suppression using H-1 parvovirus. Proc Natl Acad
Sci USA. 90:8702–8706. 1993.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sun T, Liu H and Ming L: Multiple roles of
autophagy in the sorafenib resistance of hepatocellular carcinoma.
Cell Physiol Biochem. 44:716–727. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Elleithi Y, El-Gayar A and Amin MN:
Autophagy modulation attenuates sorafenib resistance in HCC induced
in rats. Cell Death Dis. 15(595)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gómez-Virgilio L, Silva-Lucero MD,
Flores-Morelos DS, Gallardo-Nieto J, Lopez-Toledo G,
Abarca-Fernandez AM, Zacapala-Gómez AE, Luna-Muñoz J, Montiel-Sosa
F, Soto-Rojas LO, et al: Autophagy: A key regulator of homeostasis
and disease: An overview of molecular mechanisms and modulators.
Cells. 11(2262)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang K, Zhang Q, Jia R, Xiang S and Xu L:
A comprehensive review of the relationship between autophagy and
sorafenib-resistance in hepatocellular carcinoma: Ferroptosis is
noteworthy. Front Cell Dev Biol. 11(1156383)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hwang HY, Shim JS, Kim D and Kwon HJ:
Antidepressant drug sertraline modulates AMPK-MTOR
signaling-mediated autophagy via targeting mitochondrial VDAC1
protein. Autophagy. 17:2783–2799. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zinnah KMA, Seol JW and Park SY:
Inhibition of autophagy flux by sertraline attenuates TRAIL
resistance in lung cancer via death receptor 5 upregulation. Int J
Mol Med. 46:795–805. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ozunal ZG, Cakil YD, Isan H, Saglam E and
Aktas RG: Sertraline in combination with sorafenib: A promising
pharmacotherapy to target both depressive disorders and
hepatocellular cancer. Biol Futur. 70:341–348. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen R, Zou Y, Mao D, Sun D, Gao G, Shi J,
Liu X, Zhu C, Yang M, Ye W, et al: The general amino acid control
pathway regulates mTOR and autophagy during serum/glutamine
starvation. J Cell Biol. 206:173–182. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ladd AD, Duarte S, Sahin I and Zarrinpar
A: Mechanisms of drug resistance in HCC. Hepatology. 79:926–940.
2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhu YJ, Zheng B, Wang HY and Chen L: New
knowledge of the mechanisms of sorafenib resistance in liver
cancer. Acta Pharmacol Sin. 38:614–622. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Duarte D and Vale N: Antidepressant drug
sertraline against human cancer cells. Biomolecules.
12(1513)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Parkhitko AA, Favorova OO and Henske EP:
Autophagy: Mechanisms, regulation, and its role in tumorigenesis.
Biochemistry (Mosc). 78:355–367. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer.
19(12)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Runwal G, Stamatakou E, Siddiqi FH, Puri
C, Zhu Y and Rubinsztein DC: LC3-positive structures are prominent
in autophagy-deficient cells. Sci Rep. 9(10147)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cohen-Kaplan V, Ciechanover A and Livneh
I: p62 at the crossroad of the ubiquitin-proteasome system and
autophagy. Oncotarget. 7:83833–83834. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yoshii SR and Mizushima N: Monitoring and
measuring autophagy. Int J Mol Sci. 18(1865)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pugsley HR: Assessing autophagic flux by
measuring LC3, p62, and LAMP1 Co-localization using multispectral
imaging flow cytometry. J Vis Exp. 21(55637)2017.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
He L, Fu Y, Tian Y, Wang X, Zhou X, Ding
RB, Qi X and Bao J: Antidepressants as autophagy modulators for
cancer therapy. Molecules. 28(7594)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Thomé MP, Filippi-Chiela EC, Villodre ES,
Migliavaca CB, Onzi GR, Felipe KB and Lenz G: Ratiometric analysis
of acridine orange staining in the study of acidic organelles and
autophagy. J Cell Sci. 129:4622–4632. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chinnapaka S, Bakthavachalam V and
Munirathinam G: Repurposing antidepressant sertraline as a
pharmacological drug to target prostate cancer stem cells: Dual
activation of apoptosis and autophagy signaling by deregulating
redox balance. Am J Cancer Res. 10:2043–2065. 2020.PubMed/NCBI
|