Introduction
While the majority of human neurons are generated
prenatally, substantial neuronal growth, remodeling and
differentiation continue during the first 2 years of life (1-3).
Postnatal neurogenesis in humans is largely confined to the
subventricular zone; however, protracted neuronal migration occurs,
notably within the cerebral cortex and cerebellum, which are
regions crucial for learning and motor coordination (4). In preterm infants, premature exposure
to the extrauterine environment disrupts the typical trajectory of
neuronal maturation. This disruption affects all stages of neuronal
development, increasing the risk of long-term neurodevelopmental
and behavioral disorders. Individual resilience to these risks
varies and may be enhanced by early interventions (5,6).
Regardless of gestational age at birth, neonatal
hypoxic-ischemic encephalopathy (HIE) represents a severe
complication. HIE is a form of brain injury resulting from
perinatal oxygen deprivation and reduced cerebral blood flow. It is
a leading cause of neonatal brain damage, with an estimated
incidence of 2-3 per 1,000 live births in developed countries
(7-9). At
the molecular level, HIE initiates a cascade of cellular events,
including energy failure, glutamate excitotoxicity, calcium
overload and oxidative stress, collectively contributing to
neuronal injury. Secondary inflammation and apoptosis further
exacerbate this damage, highlighting the complex pathophysiology of
HIE (10).
Among the various strategies evaluated to mitigate
perinatal brain injury, including erythropoietin, stem cell
therapies and melatonin, therapeutic hypothermia has exhibited the
most consistent clinical efficacy. Cooling the body to 32-34˚C
decelerates the metabolic processes and attenuates key pathological
mechanisms, such as inflammation, oxidative stress, and apoptosis.
Consequently, therapeutic hypothermia is widely considered the
standard of care for infants with moderate to severe HIE,
significantly reducing mortality (11-13).
Furthermore, hypothermia has been shown to improve
neurodevelopmental outcomes (14-16).
While previous research has primarily focused on the
effects of hypothermia on mature neurons exposed to hypoxia, its
effects on neurons at various stages of maturation, including those
not directly affected by oxygen deprivation, are less well
understood. Given that the neonatal brain, particularly in preterm
infants, contains a substantial population of immature neural
cells, the response of these cells to both hypothermic and
hyperthermic conditions represents a critical area of
investigation. The present study investigated the effects of
temperature modulation (hypothermia and hyperthermia) on neurons at
different stages of maturation. Using a well-established neural
stem cell model at various differentiation stages, previously
validated in several publications (17-19),
the present study examined the differential responses of immature
and mature neurons to short-term hypothermia. The findings
presented herein provide novel insight into how neural cells at
different developmental stages respond to environmental stressors,
with implications for optimizing therapeutic strategies for
neonatal brain injury.
Materials and methods
Isolation and differentiation of
neural stem cells
The neural stem cells used in the present study were
obtained from the bank of the Laboratory for Stem Cells (School of
Medicine, University of Zagreb, Zagreb, Croatia). No additional
animals were sacrificed for the purposes of the present study.
Isolation procedure, performed in the Laboratory for Stem Cells and
described in some of the authors' previous publications (17-19),
included 14.5-day-old C57/BL6 albino mouse embryos. They were
obtained by mating 3-4-month-old animals. After confirming
pregnancy, on day 14, the animals were sacrificed by cervical
dislocation. After opening the abdominal cavity and isolating
embryos from the uterine wall, embryos were first separated from
the amniotic layer and their telencephalic wall was then cut into
small sections prior to exposure to the digestive enzymes. Cells
were grown in proliferation medium which contained 1% N2
(17502-048, Gibco, Thermo Fisher Scientific, Inc.), 1% Pen/Strep
(penicillin/streptomycin, 5,000 U/ml; 15070063, Gibco, Thermo
Fisher Scientific, Inc.), 2% B27 (17502, Gibco, Thermo Fisher
Scientific, Inc.), 20 ng/ml epidermal growth factor (EGF; PMG8041,
Gibco, Thermo Fisher Scientific, Inc.), 10 ng/ml basic fibroblast
growth factor (bFGF; PMG0035, Gibco, Thermo Fisher Scientific,
Inc.) and 5 mM HEPES (H0887, MilliporeSigma). After a sufficient
number of cells was obtained, which was usually in the range of 2-5
million, their differentiation was achieved in the plates covered
with 50 µg/ml poly-D-lysin (PDL; P6407, MilliporeSigma) and 10
µg/ml laminin (L2020, MilliporeSigma). Differentiation medium,
which induced the differentiation of immature cells into neurons,
was comprised of 1% N2, 1% Pen/Strep, 1% FBS (15070063, Gibco,
Thermo Fisher Scientific, Inc.), 2% B27+ (A3582801, Gibco, Thermo
Fisher Scientific, Inc.) and 5 mM HEPES. The use of these cells was
already described and validated in previous publications (17-19).
After obtaining neural stem cells by the enzymatic
dissection of the neutrospheres, cells attached on coated
coverslips were grown in an incubator up to 14 days. Of note, five
different groups of each maturity stage were formed, which
corresponded to following temperatures: 32, 30 and 28˚C (three
groups of hypothermia), 39˚C (hyperthermia, and the control group
(37˚C). These temperatures were selected on the basis of clinical
experience with applied hypothermia protocols (12-16).
The temperature of 39˚C was selected as an average hyperthermic
value in pathological conditions. After being exposed to the
specific temperature for 48 h, the cells were prepared for staining
and immunocytochemistry. In this manner, there were three groups of
cells with various levels of maturity [day 2, early progenitors
(exposed to different temperatures from day 0 to 2); day 7, mid
progenitors (exposed to different temperatures from day 5 to 7);
and day 14, late progenitors (exposed to different temperatures
from day 12 till day 14)].
Cell counting
After plating the cells at a density of 50,000 cells
per well, four wells were used for counting, with three different
fields of view counted in each well. Thus, a total of 12 counts
were made for each condition tested. Prior to counting, the cells
were examined using the LIVE/DEAD Viability/Cytotoxicity kit
(L3224, Thermo Fisher Scientific, Inc.), which enabled the
recognition of living cells only.
Immunocytochemistry
To follow up on cell differentiation and analyze the
cells using immunocytochemistry, the cells were grown on coverslips
and fixed in 4% PFA (Merck KGaA). Fixation at 4˚C lasted for 10
min. Permeabilization of the cell membranes was performed using
0.2% Triton (Merck KGaA) in PBS for 15 min. Subsequently, the cells
were washed with PBS. Blocking was performed with 3% goat serum in
PBS at room temperature for 2 h. The primary antibodies used were
anti-Nestin (1:100; cat. no. MAB353, Merck KGaA) and
anti-microtubule-associated protein 2 (Map2; 1:5,000; cat. no.
ab5392, Abcam). After the primary antibodies had attached to their
antigens, the cells were washed with PBS. The secondary antibodies
were then added and the cells were incubated at room temperature
with the secondary antibody for 1 h. The secondary antibody used
were the following: goat anti-mouse IgG (H+L), Alexa Fluor™ 488
(1:1,000; cat. no. A11001, Thermo Fisher Scientific, Inc.) and goat
anti-chicken IgG (H+L), Alexa Fluor™ 546 (1:1,000; cat. no. A11040,
Thermo Fisher Scientific, Inc.). For the counterstain procedure,
1:20,000 DAPI (Roche 10236276001, Merck KGaA) at room temperature
was used.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cells using the
commercial QIAshredder (79656, Qiagen, Inc.) and the RNeasy Mini
kit (74104, Qiagen, Inc.). The RNA concentration was quantified
using a NanoDrop ND1000 spectrophotometer (Thermo Fisher
Scientific, Inc.). The concentration of RNA for all samples was
adjusted to 25 ng/µl. The conversion from RNA to cDNA was
accomplished utilizing a high-capacity RNA-to-cDNA kit (4387406,
Applied Biosystems, Thermo Fisher Scientific, Inc.). qPCR was then
performed using TaqMan Gene Expression Assays for three genes of
interest: Neural cell adhesion molecule 1 (Ncam1; Mm01149710_m1,
Thermo Fisher Scientific, Inc.), integrin beta-1 (Itgb1;
Mm01253230_m1, Thermo Fisher Scientific, Inc.) and cadherin-2
(Cdh2; Mm01162497_m1, Thermo Fisher Scientific, Inc.). As
housekeeping genes, β-actin (Mm02619580_g1, Thermo Fisher
Scientific, Inc.) and hypoxanthine phosphoribosyltransferase 1
(Hprt1; Mm03024075_m1, Thermo Fisher Scientific, Inc.) were used.
The relative quantification was performed using the
2-ΔΔCq method (20).
Statistical analyses
Statistical analyses were conducted in R Studio
version 1.4.1717 (Posit PBC). Comparisons between groups were made
using one-way ANOVA followed by Tukey's Honest Significant
Difference (HSD) post hoc test. A value of P<0.05 was considered
to indicate a statistically significant difference.
Results
Short-term exposure to hypothermia has
more detrimental effects on early- and mid-stage than on late-stage
neural precursors
To investigate the effects of neuronal precursor
maturation stage and temperature on cell numbers, the cells were
exposed to various temperatures for 48 h on days 0, 5 and 12 of
maturation. Cell counts were then performed on days 2, 7 and 14.
This allowed for the comparison of the effects of temperature
exposure at different maturation stages on cell number.
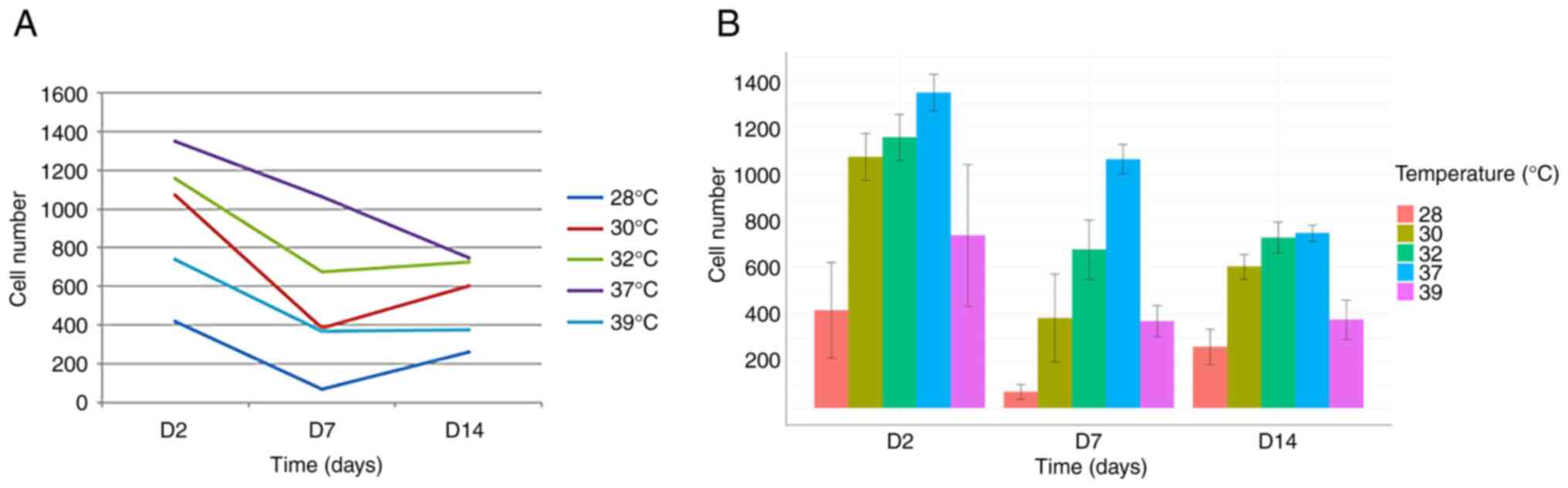
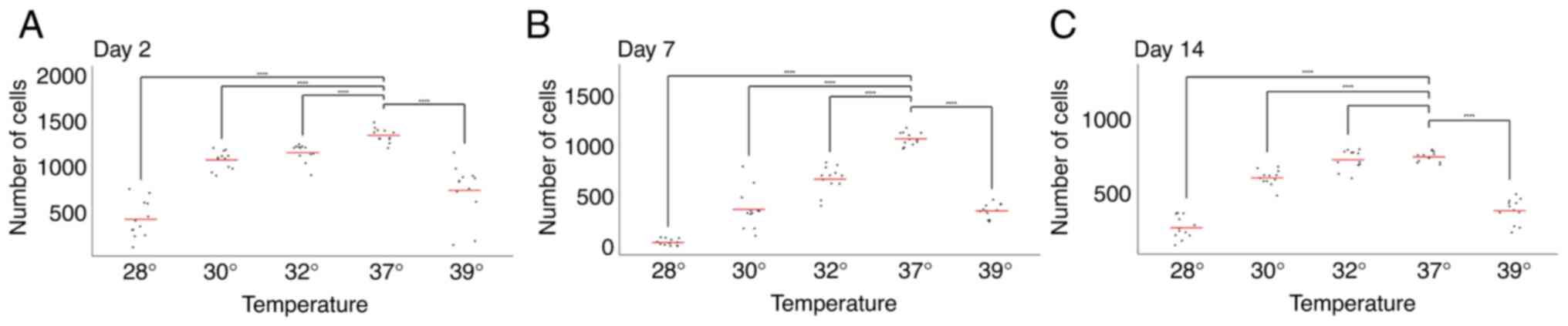
The comparison of cell numbers at a physiological
temperature (37˚C) revealed a decline in cell numbers between days
2, 7 and 14, consistent with previous observations by our group
(17-19)
(Figs. 1 and 2).
The analysis of the day 7 time point revealed an
accelerated rate of decline in cell numbers following exposure to
both hypothermia (at all temperatures tested) and hyperthermia.
This suggests that cells exposed to temperature changes between
days 5 and 7 responded negatively, resulting in a more pronounced
reduction in cell numbers compared to days 2 and 14 (Figs. 1 and 2).
Conversely, and notably, the opposite effect was
observed on day 14. Under all three hypothermic conditions (32, 30
and 28˚C), cell numbers on day 14 exceeded those on day 7 (Figs. 1 and 2). This strongly suggests a beneficial
effect of hypothermia on cell numbers when applied between days 12
and 14. Furthermore, no significant difference in cell number was
observed on day 14 between cells grown at 37˚C and those exposed to
32˚C (Fig. 2).
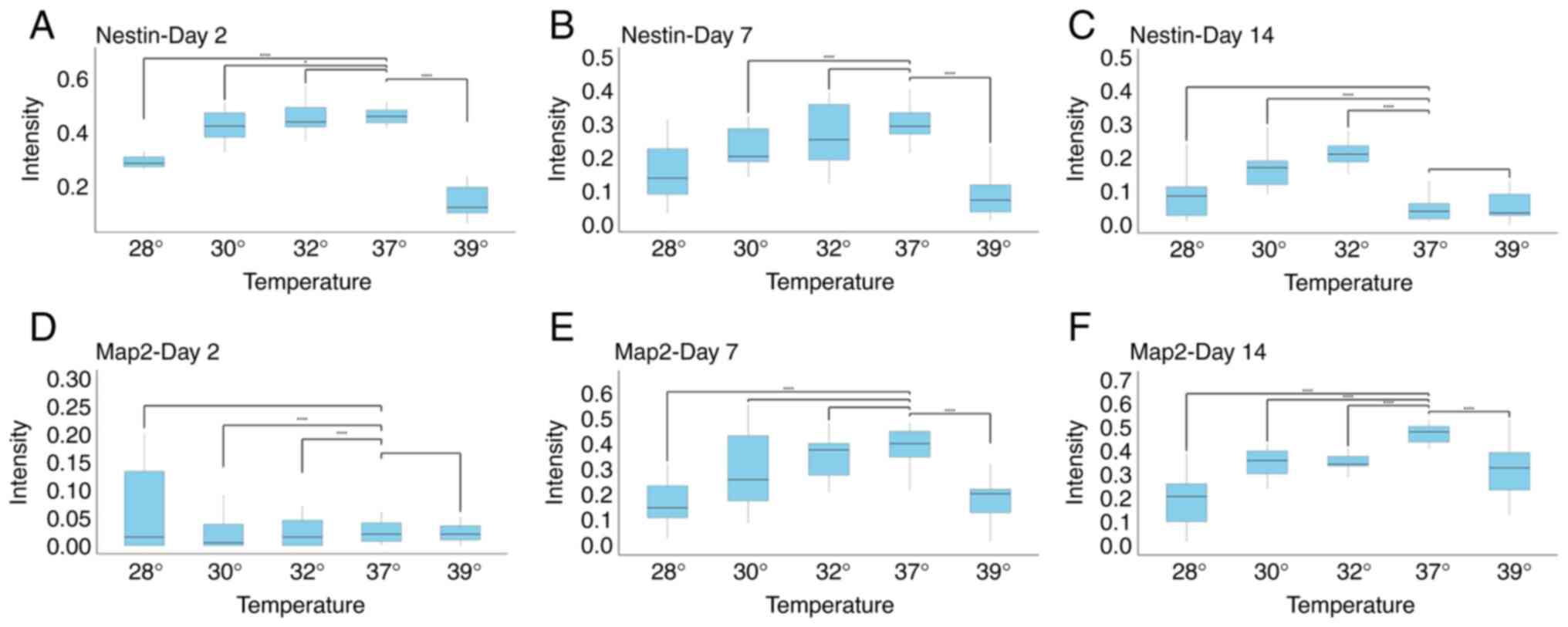
The expression of Nestin and Map2,
markers of differentiation of neurons, is dependent on the
temperature and maturity of cells following exposure to
hypothermia
To investigate whether changes in temperature
influence the expression of markers of neural precursors maturity,
the expression levels of Nestin and Map2 were analyzed. As was
expected, neural precursors at day 2, and to a lesser extent, at
day7, expressed Nestin, while this marker was almost completely
absent on day 14 (Figs. 3 and
4). When early precursors were
exposed to hypothermia, the expression of Nestin was not markedly
altered, apart from the condition of the extreme change of
temperature to 28˚C. However, given that the majority of cells at
28˚C were either dead or exhibiting signs of cell death, this
finding probably reflects a severe impairment in cell metabolism.
Notably, hyperthermia also caused a marked decrease in the
expression of Nestin. A similar finding was observed on day 7,
where all changes in temperatures decreased the expression of
Nestin. On the other hand, the opposite and surprising finding was
observed on day 14. While cells at D14 normally no longer expressed
Nestin, decreasing the temperature to 32˚C and to a lesser extent,
to 30˚C, markedly increased Nestin expression (Figs. 3 and 4).
The analysis of Map2 expression revealed the
following: As was expected, Map2 expression was not detected in the
earliest stage of neural precursors, and temperature changes did
not affect this. At day 7, decreasing the temperature to 32˚C did
not influence Map2 expression. A reduction in Map2 levels was
observed only at more extreme temperatures, consistent with the
findings obtained for Nestin expression. At day 14, both decreases
and increases in temperature reduced Map2 expression. Notably, even
a decrease to 32˚C reduced Map2 levels, coinciding with a marked
increase in Nestin expression at the same differentiation stage
(Figs. 3 and 4).
A comparison of all three stages of neuronal
maturity revealed that at days 2 and 7, either no significant
changes were observed, or a decrease in marker expression was
observed, generally being associated with extreme temperature
changes, and thus likely representing non-specific effects. At day
14, however, a temperature of 32˚C decreased Map2 levels, but
increased Nestin levels, suggesting a form of dedifferentiation
(Figs. 3 and 4). This coincides with the observation that
cell numbers at day 14 did not significantly differ between 37˚ and
32˚C (Fig. 2).
Hypothermia increases the expression
of genes linked to neuronal plasticity and migration
Having observed that temperature changes influence
both cell survival and the expression of genes associated with the
differentiation stage, the present study then investigated the
effects of hypothermia and hyperthermia on the expression of genes
related to migration, differentiation, plasticity and regeneration.
While Ncam1 was barely detectable in early progenitors, cells at
days 7 and 14 strongly expressed this gene. Of note, although
temperature changes did not significantly alter Ncam1 expression at
day 7, mild hypothermia (32 and 30˚C) significantly increased Ncam1
expression at day 14 (Fig. 5A).
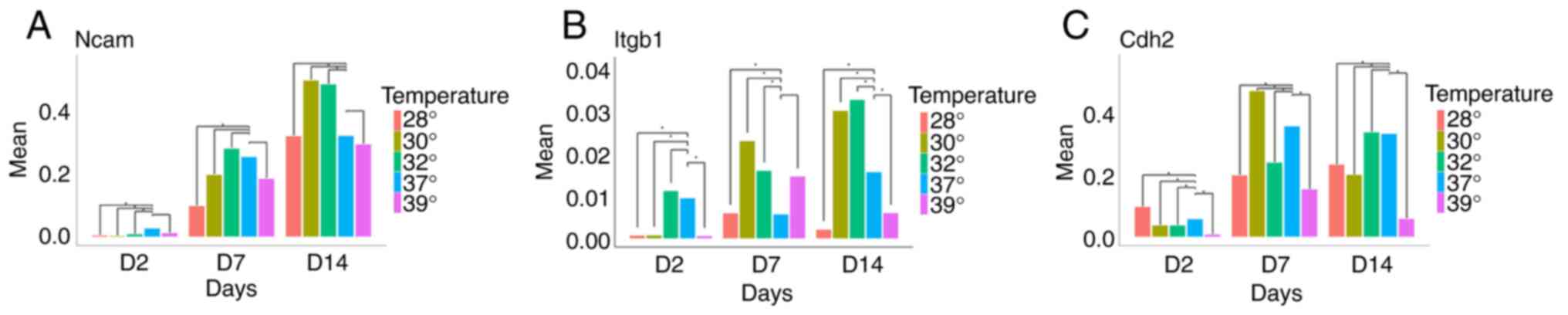 | Figure 5Expression of (A) Ncam1, (B) Itgb1
and (C) Cdh2 at days 2, 7 and 14, in five different temperature
conditions. (A) When compared to normothermia, it noteworthy that
an increase in Ncam expression was observed following exposure to
mild hypothermia on day 14. (B) A similar finding, including on day
7 as well, was found for Itgb1. (C) Cdh2 expression exhibited a
different pattern, with an increase in its expression observed only
on day 7 when the cells were exposed to 30˚C.
*P<0.01. Ncam, Neural cell adhesion molecule 1;
Itgb1, integrin beta-1; Cdh2, cadherin-2. |
Another gene examined was Itgb1. It was found that
temperatures of 32 and 30˚C increased Itgb1 expression at both days
7 and 14, whereas more extreme hypothermia (28˚C) and hyperthermia
reduced its expression (Fig.
5B).
The analysis of Cdh2 expression revealed a
significant increase only at day 7 when cells were cultured at
30˚C. In all other conditions, a decrease in Cdh2 expression was
observed (Fig. 5C).
Discussion
Therapeutic hypothermia represents a promising
intervention for mitigating neonatal brain injury resulting from
HIE. By reducing the core body temperature of infants to 32-34˚C
for 24 to 72 h, hypothermia decreases metabolic demand and
attenuates secondary injury cascades, including inflammation and
apoptosis (21). Clinical trials
have demonstrated improved survival rates and a reduced risk of
severe neurodevelopmental disabilities in term and late preterm
infants. However, its efficacy is time-dependent, with maximal
benefits observed when initiated within 6 h of birth. Current
guidelines recommend hypothermia as the standard of care for
moderate to severe HIE in term and near-term infants; however, its
use in mild HIE, preterm infants and resource-limited settings
remains a subject of ongoing investigation (22). From a molecular and
pathophysiological perspective, hypothermia has been shown to
inhibit excitotoxicity by reducing glutamate release, thereby
preventing excessive calcium influx and subsequent cell death
(21). It also downregulates
inflammatory pathways, suppressing the release of pro-inflammatory
cytokines implicated in cell damage and apoptosis. Regarding
apoptosis, or programmed cell death, hypothermia attenuates caspase
activation, the enzymatic process responsible for apoptosis
(23).
However, the neonatal nervous system, particularly
in preterm infants, comprises a heterogeneous population of cells
at various stages of maturation. It is therefore plausible that not
all cell types are equally susceptible to hypoxic injury.
Furthermore, some preterm infants may not experience hypoxia at
all, yet hypothermia may be considered a prophylactic intervention
to enhance survival prospects. Consequently, the present study
investigated the effects of temperature modulation on normoxic
cells at different developmental stages. To the best of our
knowledge, data regarding the impact of temperature changes on such
a broad spectrum of cell types are limited. Therefore, the present
study compared the responses of cells at three distinct
differentiation stages, unexposed to hypoxic insults, to
temperature variations. Notably, deviations from the physiological
temperature of 37˚C generally reduced cell numbers. However, the
experimental design used herein yielded a key observation: While
early- and mid-stage neural precursors exhibited negative responses
to both hypothermia and hyperthermia, a distinct benefit was
observed at day 14. Under all hypothermic conditions tested, the
number of viable cells at day 14 exceeded that at day 7.
Furthermore, no significant difference in viable cell numbers was
detected between cells cultured at 37 and 32˚C at day 14. This
indicates that only at day 14, and only with mild hypothermia
(32˚C), were detrimental effects on neural precursors absent. This
finding was further investigated by examining the expression of
Nestin, a marker of immature cells, and Map2, a marker of mature or
nearly mature neurons. Again, late-stage neural precursors
displayed a unique response: Mild hypothermia reduced Map2
expression, while simultaneously increasing Nestin expression. This
increase in Nestin expression was the sole increase observed in
this aspect of the study. Nestin, an intermediate filament protein,
is predominantly expressed in stem and progenitor cells during
development, particularly within the central nervous system, muscle
and other tissues. It is a widely accepted marker for neural stem
cells (NSCs) and other multipotent stem cells (24). An elevated expression of Nestin is
also observed in reactive astrocytes and other glial cells
following central nervous system injury, such as stroke or
traumatic brain injury. Notably, Nestin-expressing neurons have
been identified in both rats and humans, suggesting that even
mature neurons can, under certain conditions, express this protein
(25). Moreover, there is evidence
to indicate that increasing the expression of Nestin in both
neurons and astrocytes contributes to the improved survival of
neural tissue affected by pathological condition (26).
Map2 is a protein localized to dendrites and
interacts with microtubules, the primary structural filaments of
neurons. An increased expression of Map2 in neural precursors is a
key indicator of terminal differentiation. Consequently, it has
been demonstrated that Map2 inhibition is associated with a reduced
rate of differentiation and the maintenance of mitotic capacity
(27). The expression of Map2 is
known to be modulated by various external factors (28). Moreover, decreased expression of Map2
has been reported in certain pathological conditions (29). The dichotomy regarding the effects of
hypothermia on Map2 expression centers on whether a reduced
expression of Map2 signals neuroprotection or impaired neuronal
maturation. On the one hand, hypothermia has been shown to
attenuate the injury-induced loss of Map2, suggesting the
preservation of late-stage neural precursors and contributing to
neuroprotection after trauma (30).
On the other hand, Map2 is critical for dendritic outgrowth,
microtubule stabilization and synaptic plasticity; thus, its loss
or reduced expression can reflect impaired maturation and
pathological dendritic morphology, as observed in various
neurodegenerative and neuropsychiatric disorders (29). Thus, while the decreased expression
of Map2 under hypothermia may indicate preserved neuronal
integrity, a decrease in Map2 expression more broadly could also
signify disrupted neuronal development and function. This dual
interpretation highlights the complexity of Map2 as both a marker
and mediator of neuronal health, where context and timing of
expression changes are key to understanding its role.
Given the findings of the present study suggesting
that mild hypothermia may postpone the terminal differentiation of
neurons and enhance their regenerative potential, the present study
investigated the expression of three genes implicated in these
processes. Ncam1, widely expressed in neural tissue, is involved in
neural stem cell adhesion, migration and differentiation. It plays
a crucial role in neurogenesis and synaptic plasticity (31). Generally, Ncam1 upregulation promotes
cell adhesion, neural plasticity and migration (32-34).
The finding of the present study that mild hypothermia increases
Ncam1 expression at day 14 is consistent with the observed increase
in Nestin expression and a decrease in Map2 expression, as these
changes collectively suggest reduced terminal differentiation and
an enhanced capacity for regenerative processes. Itgb1 is a
molecule involved in guiding neurons to their appropriate locations
during brain development. Furthermore, it participates in neuronal
adhesion and migration (35-37).
Very similar to Ncam1, the finding of the present study that mild
hypothermia increases Itgb1 expression at days 7 and 14 is
consistent with the observed increase in Nestin expression and a
decrease in Map2 expression. All these changes collectively suggest
reduced terminal differentiation and an enhanced capacity for
regenerative processes. Cdh2 is a cell adhesion molecule highly
expressed in neural tissues. It plays a crucial role in maintaining
the neural stem cell niche and influencing NSC differentiation,
migration, and synapse formation (38). Unlike Ncam1 and Itgb1, hypothermia
did not exert a consistent effect on Cdh2 expression; some
temperatures increased and others decreased its expression.
Clearly, additional mechanisms are involved in this molecular
pathway, and further investigations are required to elucidate these
complex interactions.
Regardless of that, it was clearly demonstrated that
Ncam1 and Itgb1 play crucial roles in neural stem cell migration
and neurite extension. When upregulated, these molecules enhance
cellular mobility and neurite formation, while their downregulation
produces opposite effects. The mechanistic elements of these
phenomena are well-described (39).
In addition, it has been reported that hypothermia leads to an
enhanced neurite extension in mouse brain slice cultures, which is
mediated by a surge in TNF-α levels (40). Moreover, a previous in vivo
study on rat spinal cord injury found that hypothermia plus NSC
transplantation tended to promote graft migration: The combined
therapy may promote migration of the transplanted cells at the
injury site (41).
While it is known that the use of mice as an animal
model and mouse cells are still often used in neuroscience, certain
possible limitations of the present study should be mentioned.
Mouse NSCs are widely used as models of human neural tissue due to
broad genetic and cellular similarities; however, critical
species-specific differences can limit their translational
relevance (42). Moreover,
comparative transcriptomic research has found that a number of
orthologous genes exhibit divergent expression or splicing patterns
in mouse vs. human neural cells (43). Developmental timelines also markedly
differ: Human neurodevelopment is far more prolonged and complex
than that in mice; thus, equivalent developmental stages do not
align. In other words, the results of the present study are very
useful; however, for further progress, more advanced, human brain
organoid models, for example, such as the ones previously reported
(44) should be used.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Croatian Ministry
of Science, Education and Youth as part of a project entitled
‘Development of personalized tests for biological brain age and
early detection of dementia-BrainClock’ (grant reference no.
NPOO.C3.2.R3-I1.04.0089). The study was additionally supported by
the Croatian Science Foundation, project DevDown, under the project
no. IP-2022-10-4656.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LDM and DM were involved in the conceptualization of
the study and in the writing of the manuscript. LDM and EEP were
involved in the study methodology. LDM, EEP, AEH and DM were
involved in data validation, formal analysis and data
investigation, as well as in the writing, reviewing and editing of
the manuscript. DM provided resources (all chemicals, kits, plastic
and other expendables). DM also supervised the study, and was
involved in project administration and funding acquisition. LDM and
DM confirm the authenticity of all the raw data. All authors have
read and agreed to the published version of the manuscript.
Ethics approval and consent to
participate
The neural stem cells used in the present study were
obtained from the bank of the Laboratory for Stem Cells (School of
Medicine, University of Zagreb). No additional animals were
sacrificed for the purposes of the present study. The procedure by
which cells were obtained and used was approved by the Ethical
Board of the School of Medicine (approval no.
380-59-10106-17-100/27).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gilmore JH, Knickmeyer RC and Gao W:
Imaging structural and functional brain development in early
childhood. Nat Rev Neurosci. 19:123–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mrzljak L, Uylings HB, Van Eden CG and
Judás M: Neuronal development in human pre-frontal cortex in
prenatal and postnatal stages. Prog Brain Res. 85:185–222.
1990.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Landing BH, Shankle WR, Hara J, Brannock J
and Fallon JH: The development of structure and function in the
postnatal human cerebral cortex from birth to 72 months: Changes in
thickness of layers II and III correlate to the onset of new
age-specific behaviors. Pediatr Pathol Mol Med. 21:321–342.
2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z,
Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, et
al: Corridors of migrating neurons in the human brain and their
decline during infancy. Nature. 478:382–386. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
La Rosa C, Parolisi R and Bonfanti L:
Brain structural plasticity: From adult neurogenesis to immature
neurons. Front Neurosci. 14(75)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tierney AL and Nelson CA: Brain
development and the role of experience in the early years. Zero
Three. 30:9–13. 2009.PubMed/NCBI
|
|
7
|
Russ JB, Simmons R and Glass HC: Neonatal
encephalopathy: Beyond hypoxic-ischemic encephalopathy. NeoReviews.
22:e148–e162. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu L, Johnson HL, Cousens S, Perin J,
Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al:
Global, regional, and national causes of child mortality: An
updated systematic analysis for 2010 with time trends since 2000.
Lancet. 379:2151–2161. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gopagondanahalli KR, Li J, Fahey MC, Hunt
RW, Jenkin G, Miller SL and Malhotra A: Preterm hypoxic-ischemic
encephalopathy. Front Pediatr. 4(114)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sanchez RM, Koh S, Rio C, Wang C, Lamperti
ED, Sharma D, Corfas G and Jensen FE: Decreased glutamate receptor
2 expression and enhanced epileptogenesis in immature rat
hippocampus after perinatal hypoxia-induced seizures. J Neurosci.
21:8154–8163. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Babbo CCR, Mellet J, van Rensburg J,
Pillay S, Horn AR, Nakwa FL, Velaphi SC, Kali GTJ, Coetzee M,
Masemola MYK, et al: Neonatal encephalopathy due to suspected
hypoxic ischemic encephalopathy: Pathophysiology, current, and
emerging treatments. World J Pediatr. 20:1105–1114. 2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rutherford M, Ramenghi LA, Edwards AD,
Brocklehurst P, Halliday H, Levene M, Strohm B, Thoresen M,
Whitelaw A and Azzopardi D: Assessment of brain tissue injury after
moderate hypothermia in neonates with hypoxic-ischaemic
encephalopathy: A nested substudy of a randomised controlled trial.
Lancet Neurol. 9:39–45. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou T, Mo J, Xu W, Hu Q, Liu H, Fu Y and
Jiang J: Mild hypothermia alleviates oxygen-glucose
deprivation/reperfusion-induced apoptosis by inhibiting ROS
generation, improving mitochondrial dysfunction and regulating DNA
damage repair pathway in PC12 cells. Apoptosis. 28:447–457.
2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shankaran S, Laptook AR, Ehrenkranz RA,
Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright
LL, Higgins RD, et al: Whole-body hypothermia for neonates with
hypoxic-ischemic encephalopathy. N Engl J Med. 353:1574–1584.
2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Azzopardi D, Strohm B, Marlow N,
Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL,
Juszczak E, Kapellou O, et al: Effects of hypothermia for perinatal
asphyxia on childhood outcomes. N Engl J Med. 371:140–149.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jacobs SE, Berg M, Hunt R, Tarnow-Mordi
WO, Inder TE and Davis PG: Cooling for newborns with hypoxic
ischaemic encephalopathy. Cochrane Database Syst Rev.
31(CD003311)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Isaković J, Slatković F, Jagečić D,
Petrović DJ and Mitrečić D: Pulsating extremely low-frequency
electromagnetic fields influence differentiation of mouse neural
stem cells towards astrocyte-like phenotypes: In vitro pilot study.
Int J Mol Sci. 25(4038)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Petrović DJ, Jagečić D, Krasić J, Sinčić N
and Mitrečić D: Effect of fetal bovine serum or basic fibroblast
growth factor on cell survival and the proliferation of neural stem
cells: The influence of homocysteine treatment. Int J Mol Sci.
24(14161)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jagečić D, Petrović DJ, Šimunić I,
Isaković J and Mitrečić D: The oxygen and glucose deprivation of
immature cells of the nervous system exerts distinct effects on
mitochondria, mitophagy, and autophagy, depending on the cells'
differentiation stage. Brain Sci. 13(910)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cornette L: Therapeutic hypothermia in
neonatal asphyxia. Facts Views Vis Obgyn. 4:133–139.
2012.PubMed/NCBI
|
|
22
|
Lemyre B and Chau V: Hypothermia for
newborns with hypoxic-ischemic encephalopathy. Paediatr Child
Health. 23:285–291. 2018.PubMed/NCBI View Article : Google Scholar : (In English,
French).
|
|
23
|
Shankaran S: Therapeutic hypothermia for
neonatal encephalopathy. Curr Treat Options Neurol. 14:608–619.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang J, Huang Y, Cai J, Ke Q, Xiao J,
Huang W, Li H, Qiu Y, Wang Y, Zhang B, et al: A
nestin-cyclin-dependent kinase 5-dynamin-related protein 1 axis
regulates neural stem/progenitor cell stemness via a metabolic
shift. Stem Cells. 36:589–601. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hendrickson ML, Rao AJ, Demerdash ON and
Kalil RE: Expression of nestin by neural cells in the adult rat and
human brain. PLoS One. 6(e18535)2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mizuno Y, Takeuchi T, Takatama M and
Okamoto K: Expression of nestin in Purkinje cells in patients with
Creutzfeldt-Jakob disease. Neurosci Lett. 352:109–112.
2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dinsmore JH and Solomon F: Inhibition of
MAP2 expression affects both morphological and cell division
phenotypes of neuronal differentiation. Cell. 64:817–826.
1991.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Johnson GV and Jope RS: The role of
microtubule-associated protein 2 (MAP-2) in neuronal growth,
plasticity, and degeneration. J Neurosci Res. 33:505–512.
1992.PubMed/NCBI View Article : Google Scholar
|
|
29
|
DeGiosio RA, Grubisha MJ, MacDonald ML,
McKinney BC, Camacho CJ and Sweet RA: More than a marker: Potential
pathogenic functions of MAP2. Front Mol Neurosci.
15(974890)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Taft WC, Yang K, Dixon CE, Clifton GL and
Hayes RL: Hypothermia attenuates the loss of hippocampal
microtubule-associated protein 2 (MAP2) following traumatic brain
injury. J Cereb Blood Flow Metab. 13:796–802. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Amoureux MC, Cunningham BA, Edelman GM and
Crossin KL: N-CAM binding inhibits the proliferation of hippocampal
progenitor cells and promotes their differentiation to a neuronal
phenotype. J Neurosci. 20:3631–3640. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu JQ, Habegger L, Noisa P, Szekely A, Qiu
C, Hutchison S, Raha D, Egholm M, Lin H, Weissman S, et al: Dynamic
transcriptomes during neural differentiation of human embryonic
stem cells revealed by short, long, and paired-end sequencing. Proc
Natl Acad Sci USA. 107:5254–5259. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang C, Wang X, Wang W, Chen Y, Chen H,
Wang W, Ye T, Dong J, Sun C, Li X, et al: Single-cell RNA
sequencing analysis of human embryos from the late Carnegie to
fetal development. Cell Biosci. 14(118)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Huang R, Yuan DJ, Li S, Liang XS, Gao Y,
Lan XY, Qin HM, Ma YF, Xu GY, Schachner M, et al: NCAM regulates
temporal specification of neural progenitor cells via profilin2
during corticogenesis. J Cell Biol. 219(e201902164)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Blaess S, Graus-Porta D, Belvindrah R,
Radakovits R, Pons S, Littlewood-Evans A, Senften M, Guo H, Li Y,
Miner JH, et al: Beta1-integrins are critical for cerebellar
granule cell precursor proliferation. J Neurosci. 24:3402–3412.
2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Belvindrah R, Hankel S, Walker J, Patton
BL and Müller U: Beta1 integrins control the formation of cell
chains in the adult rostral migratory stream. J Neurosci.
27:2704–2717. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fujioka T, Kaneko N, Ajioka I, Nakaguchi
K, Omata T, Ohba H, Fässler R, García-Verdugo JM, Sekiguchi K,
Matsukawa N and Sawamoto K: β1 integrin signaling promotes neuronal
migration along vascular scaffolds in the post-stroke brain.
EBioMedicine. 16:195–203. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Alimperti S and Andreadis ST: CDH2 and
CDH11 act as regulators of stem cell fate decisions. Stem Cell Res.
14:270–282. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kolkova K, Novitskaya V, Pedersen N,
Berezin V and Bock E: Neural cell adhesion molecule-stimulated
neurite outgrowth depends on activation of protein kinase C and the
Ras-mitogen-activated protein kinase pathway. J Neurosci.
15:2238–2246. 2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schmitt KR, Boato F, Diestel A, Hechler D,
Kruglov A, Berger F and Hendrix S: Hypothermia-induced neurite
outgrowth is mediated by tumor necrosis factor-alpha. Brain Pathol.
20:771–779. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang D and Zhang J: Effects of hypothermia
combined with neural stem cell transplantation on recovery of
neurological function in rats with spinal cord injury. Mol Med Rep.
11:1759–1767. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Radoszkiewicz K, Jezierska-Woźniak K,
Waśniewski T and Sarnowska A: Understanding intra- and
inter-species variability in neural stem cells' biology is key to
their successful cryopreservation, culture, and propagation. Cells.
12:488–499. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Breschi A, Gingeras TR and Guigó R:
Comparative transcriptomics in human and mouse. Nat Rev Genet.
18:425–440. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Murray A, Gough G, Cindrić A, Vučković F,
Koschut D, Borelli V, Petrović DJ, Bekavac A, Plećaš A, Hribljan V,
et al: Dose imbalance of DYRK1A kinase causes systemic progeroid
status in Down syndrome by increasing the un-repaired DNA damage
and reducing LaminB1 levels. EBioMedicine.
2023(104692)2023.PubMed/NCBI View Article : Google Scholar
|















![(A and B) Immunohistochemical staining
of neural stem cells in three stages of differentiation: Days 1, 7
and 14, exposed to a normal temperature (37˚C; indicated by ‘N’)
and to hypothermia (32˚C; indicated by ‘H’). The panels on the left
represent DAPI, while those in the middle represent (A) Nestin and
(B) Map2. The panels on the right represent the merged image. It is
visible that on day 1, Nestin is strongly expressed in both
normothermic and hypothermic conditions. On day 7, the Nestin
signal is weaker, but hypothermia does not influence it in any
notable manner. The most notable finding can be seen on day 14
[compare D14N and D14H in (A)]: While in normal conditions Nestin
was almost completely absent, hypothermia re-established the
expression of Nestin. The opposite is visible with the expression
of Map2, which is reduced in hypothermia on day 14 [compare D14N
and D14H in (B)]. Scale bars, 100 µm.](/article_images/mi/5/5/mi-05-05-00252-g02.jpg)