Introduction
Chronic disease, including inflammatory bowel
disease (IBD) is associated with significant costs. Although a
number of studies have evaluated the costs of care (including
medication, hospitalisation and surgery ambulatory visits) in the
high-income regions, the data are relatively limited in the lower-
and middle-income countries (LMICs) (1). The care of patients with IBD in LMICs
has certain additional caveats; the use of advanced therapies
including biologicals and small molecules is limited by a lack of
access, availability and comfort with their use (2,3). There
may also be an additional role of risk of infections, including
tuberculosis and the higher uptake of complementary and alternative
medication that may reduce the compliance with standard therapies,
resulting in increased flares (4).
The treatment paradigm for ulcerative colitis (UC),
a chronic relapsing-remitting disease, continues to evolve and the
data for a top-down approach for UC are limited, as are those
against Crohn's disease (5).
Therefore, standard therapies, including 5-aminosalicylates and
thiopurines remain the major tools in the treatment of UC,
particularly in LMICs (6). IBD,
including UC results in not only in a physical, mental and social
burden, but is also associated with a marked financial burden to
patients, as well as their caregivers (7). Any flare of the disease increases this
burden. The need for rescue therapy in the form of advanced
therapies (anti-TNF agents, anti-integrins, anti-IL-12/23 and small
molecules) or surgery increases both the short-, as well as
long-term financial burden. However, the selection of therapy in
numerous regions of the globe is dictated by cost considerations,
and 5-aminosalicylic acid (5-ASA) compounds and thiopurines are
often used as a first-line therapy; surgery appears fairly early in
the treatment paradigm. It is unclear whether the cost of flares is
substantially higher than ongoing therapy during periods of
remission in LMICs (8). The present
study aimed to analyse the monthly cost of care of patients with UC
in remission during their regular follow-up and compare this to the
expenses born during an episode of acute exacerbation, in order to
estimate the costs of such episodes of flares. Cost-of-illness
analysis for patients with UC in remission, as well as for those
with acute exacerbations was performed; this included direct costs
(medical and non-medical), as well as indirect costs during
out-patient department (OPD) visits and in-patient admissions.
Patients and methods
Study setting
The present study was a prospective observational,
cross-sectional, single-centre study conducted between January,
2022 to June, 2024; the study included 25 patients with UC who were
in remission [simple clinical colitis activity index (SCCAI) score
<3] and 51 patients with UC who presented with flares and
required hospitalisation for acute severe ulcerative colitis (ASUC)
at the Post Graduate Institute of Medical Education and Research
(PGIMER) in Chandigarh, India. The present study was approved by
the Institute Ethics Committee of the Post Graduate Institute of
Medical Education and Research (INT/IEC/2022/000488). A written
informed consent/assent was obtained from all subjects or their
legal guardians.
Study population. UC cases in
remission
UC cases in remission were managed in the OPD.
Patients were deemed to be in disease remission with both i) a
SCCAI score <3 with a stable bowel frequency over the past 6
months; and ii) a normal faecal calprotectin level (<100 mcg/g)
(4). The evaluation of patients
included an investigation of clinical history and an examination,
periodic biochemical testing, faecal calprotectin level assessment
and an unprepared sigmoidoscopic examination. Any treatment
modification was determined as per the disease activity during OPD
visits. The total duration for OPD follow-up for UC cases in
remission was 6 months.
Patients with flares requiring
hospitalisation. Those patients who had severe flares requiring
hospital admission were included and the majority of these were
patients had ASUC. Patients who were admitted for non-flare
conditions, such as intercurrent infection were excluded. The
evaluation of cases with flares included an investigation of
clinical history and an examination, biochemical testing, stool
analysis for routine examination, faecal calprotectin level
assessment, Clostridioides difficile toxin assay and
unprepared sigmoidoscopic examination. Standard treatment for ASUC
was with intravenous steroid and venous thromboprophylaxis, and
5-aminosalicylate optimisation was performed, followed by a
response assessment using the Oxford criteria and those deemed to
be non-responsive were offered second-line therapy (intravenous
cyclosporine or intravenous infliximab or colectomy) (9).
Cost estimation
The data on expenses were categorised as direct
medical, direct non-medical and indirect costs, which were summated
as total expenditures. The cost of treatment was defined as
follows: i) Direct medical costs: These included the cost of drugs,
as well as any investigations, including the cost of surgery if
required. The cost of drugs was calculated by taking the average
cost of five commonly used drug brands used for treatment which
were available locally, while the cost of investigations included
the standard cost of tests, as fixed by the institution. ii) Direct
non-medical costs: These included the cost of stay and travel. The
cost of stay included the cost of in-hospital admission, as well as
the per-day-cost of admission during the admitted period for
patients, as well as the stay of lodging for 1 patient attendant.
Travelling costs included standard railway ticket costs for sleeper
class, as fixed by the government railway agency (IRCTC) from
Chandigarh to the nearest railway station of the hometown of the
patient. iii) Indirect costs: These included total job earnings
lost during the treatment period. These included daily wages lost
for patients, as well as the attendant of each patient.
Statistical analysis
All descriptive and inferential statistics were
generated using SPSS software version 23 (IBM Corp.). Cost data are
expressed as the average per capita monthly cost in Indian rupees
(INR). Wherever appropriate, data in percentages and numbers are
presented. The Chi-squared test was used to analyse the differences
between categorical data. Fisher's exact test was used if the
expected frequency was ≤5 in any cell. An independent samples
t-test or the Mann Whitney U test were used to compare continuous
variables depending on the normality of the distribution (as per
the one-sample Kolmogorov-Smirnov test). A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients enrolled and patient
characteristics
The present study enrolled 25 patients with UC in
remission and 51 patients with ASUC.
UC cases in remission. Among the 25 patients
with UC in remission, 12 (48%) were male and the median age of the
patients was 35 years (range, 22-60 years). The majority of the
patients were in socioeconomic upper lower class (n=12) and lower
middle class (n=8), as per the modified Kuppuswamy socieoeconomic
scale (10). All the patients were
in clinical remission for varying periods of time (3-96 months;
mean, 19±24 months). E3 disease was the most common (10 patients,
40%) and no patient had any extraintestinal manifestations. The
majority of the patients were on a combination of 5-ASA with
thiopurine (16 patients, 64%) while the remainder were controlled
on 5-ASA compounds alone (Table
I).
 | Table IDemographic and clinical features of
patients with UC in remission vs. those with flares (with
ASUC). |
Table I
Demographic and clinical features of
patients with UC in remission vs. those with flares (with
ASUC).
| Baseline clinical
characteristics | Patients with UC in
remission (n=25) | Patients with ASUC
(n=51) | P-value |
|---|
| Age, median; (range)
years | 35 (22-60) | 51 (16-65) | 0.521 |
| Male/female | 12 (48%)/13
(52%) | 27 (53%)/24
(47%) | 0.684 |
| SCCAI score; median
(range) | 0 (0-2) | 11 (6-14) | <0.001 |
| Disease free
period | 19±24 months | NA | |
| Extent of
diseasea | | | |
|
E1(Y/N) | 3 (12%)/22 (88%) | 1 (2%)/50 (98%) | 0.101 |
|
E2
(Y/N) | 8 (32%)/17 (68%) | 8 (16%)/43 (84%) | 0.112 |
|
E3
(Y/N) | 10 (40%)/15
(60%) | 21 (41%)/30
(59%) | 0.934 |
|
Unknown
(Y/N) | 4 (16%)/21 (84%) | 21 (41%)/30
(59%) | 0.038 |
|
Extraintestinal
manifestations (Y/N) | 0 (0%)/25 (100%) | 11 (22%)/40
(78%) | 0.013 |
|
Musculoskeletal | - | 7 (14%) | |
|
Ankylosing
spondylitis | - | 1 (2%) | |
|
Aphthous
ulcer | - | 1 (2%) | |
|
Pyoderma
gangrenosum | - | 1 (2%) | |
|
Uveitis | - | 1 (2%) | |
| Current
therapya | | | |
|
None
(Y/N) | 0 (0%)/25 (100%) | 12 (24%)/39
(76%) | 0.007 |
|
5-ASA only
(Y/N) | 9 (36%)/16 (64%) | 31 (61%)/20
(39%) | 0.042 |
|
5-ASA with
thiopurine (Y/N) | 16 (64%)/9 (36%) | 8 (16%)/43 (84%) | <0.001 |
|
Advanced
therapy (Y/N) | 0 (0%)/25 (100%) | 0 (0%)/51 (100%) | - |
| Management | | | |
|
Medical
only | 25 (100%) | 44 (86%) | 0.050 |
|
Surgical
intervention | - | 7 (14%) | |
| Outcome | | | |
|
Recovered on
medical therapy alone Y/N | 25 (100%)/0(0%) | 44 (86%)/7 (14%) | 0.050 |
|
Underwent
surgery and recovered | - | 4 (8%) | |
|
Underwent
surgery but did not survive | - | 3 (6%) | |
| Duration of hospital
stay; median (range) days | NA | 8 (2-46) | |
|
<4
days | - | 2 (4%) | |
|
4-5
days | - | 44 (86%) | |
|
>15
days | - | 5 (10%) | |
Patients with flares. Among the 51 patients
with ASUC included in the present study, 27 (53%) patients were
male and the median age of the patients was 38 years (range, 16-65
years). The majority of the patients belonged to class II (upper
lower) of the modified Kuppuswamy socioeconomic scale. The majority
of the patient had extensive colitis (E3) (21 patients, 41%),
whereas extra-intestinal manifestations (EIM) were observed in 11
(22%) patients, the most common being musculoskeletal
manifestations (7 patients, 14%) (Table
I). Of note, 31 (61%) of the patients were on 5-ASA compounds
before the episode of flares, while 8 (16%) patients were on a
combination of 5-ASA and azathioprine, and remainder of the
patients were not on any form of therapy or had terminated
treatment. While the condition of the majority of the treated
patients improved with medical management (86%), 14% of the
patients (7/51 patients) required surgery.
Cost analysis. UC cases in
remission
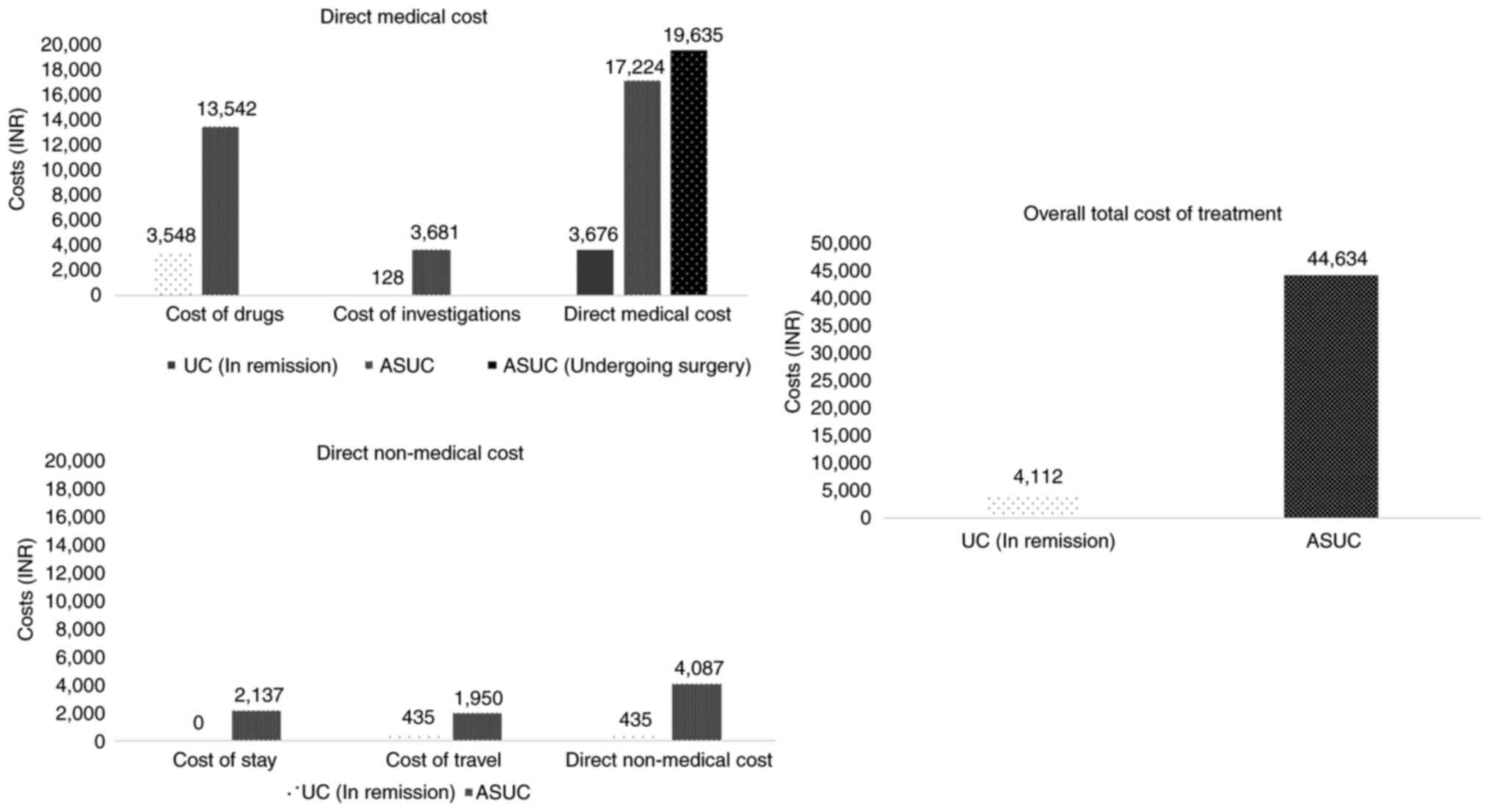
Cost analysis of the patients with UC in remission
revealed the average monthly expense due to direct medical costs to
be 3,676/- INR, which included the cost of drugs (3,548/- INR), as
well as investigations (128/- INR). The average direct non-medical
cost was 435/- INR, which included only the travelling cost (435/-
INR), as none of the patients in remission incurred additional
expenses on accommodation during their OPD visits (Fig. 1). The average additional indirect
cost incurred by the patients due to lost job earnings during the
treatment period was negligible, as the majority of the patients
could adjust their leave periods for planned hospital visits and
did not lose any daily wages. Overall, the total per capita average
monthly cost of treatment for patients with UC in remission in a
tertiary government setup was calculated to be 4,112/- INR
(Table II).
 | Table IICost of care for patients with UC in
remission compared to patients with ASUC. |
Table II
Cost of care for patients with UC in
remission compared to patients with ASUC.
| Cost-of-illness | Patients with UC in
remission (n=25) | Patients with ASUC
(n=51) |
|---|
| Direct medical
cost | 3,676/- (89.40%) | 20,038/-
(44.89%) |
| Cost of drugs | 3,548/- (86.28%) | 16,357/-
(36.65%) |
| Cost of
investigations | 128/- (3.12%) | 3,681/- (8.24%) |
| Direct non-medical
cost | 435/- (10.58%) | 4,087/- (9.16%) |
| Cost of stay | 0/- (0%) | 2,137/- (4.79%) |
| Cost of travel | 435/- (10.58%) | 1,950/- (4.37%) |
| Indirect cost | 1/- (0.02%) | 20,509/-
(45.95%) |
| Total cost | 4,112/- | 44,634/- |
Patients with flares. For the management of
an episode of ASUC, the average direct medical cost was 20,038/-
INR, which included the cost of medical and surgical management
(16,357/- INR), as well as cost of investigations (3,681/- INR). An
additional per patient cost of 20,500/- INR incurred among patients
undergoing surgery for the management of cases of ASUC. The average
direct non-medical cost was 4,087/- INR, including the cost of stay
during hospitalisation (2,137/- INR) and travelling costs (1,950/-
INR). The average additional indirect costs incurred by the
patients and their attendants due to lost job earnings during the
treatment period was 20,509/- INR. Overall, the total per capita
average cost of treatment for an episode of ASUC in a tertiary
government setup was calculated to be 44,634/- INR (Table II). There was a significant
difference (P<0.001) in the total cost of therapy among those
who were managed conservatively with medical therapy alone vs.
those who underwent surgical management. The total cost of therapy
for the medically managed cases (n=44/51 patients, 86%) was
estimated to be 19,615/- INR (15,150.5-27,034.5 INR), while the
cost for those managed with surgical resection (n=7/51 patients,
14%) was 1,62,705/- INR (78,296-2,03,663 INR) (Table III).
 | Table IIIDuration of hospital stay and
cost-of-treatment among patients with ASUC (n=51) managed medically
vs. surgically. |
Table III
Duration of hospital stay and
cost-of-treatment among patients with ASUC (n=51) managed medically
vs. surgically.
| Hospital
stay/costs | Patients with ASUC
managed medically (n=44) | Patients with ASUC
managed surgically (n=7) | P-value |
|---|
| Duration of
hospital stay, days (IQR) | 7 (5.5-10.5) | 14 (10.5-26) | 0.030 |
| Cost of treatment
(IQR) | 19,615/-
(15,150.50-27,034.50) | 162,705/-
(78,296-203,663) | <0.001 |
The majority of the patients were admitted for 4-15
days in the hospital (86%), while those who underwent surgery had a
significantly longer hospitalisation period (P=0.030). The median
duration of patient admission was 8 days (IQR, 6-11 days). The
median duration of hospital stays for patients managed with
surgical resection (n=7/51 patients, 14%) was 14 days (IQR,
10.5-26), which was significantly longer (P=0.030) than the median
duration of hospital stays for the patients who were managed only
medical therapy alone (n=44/51 patients, 86%; median days of
hospital stay, 7 days; IQR, 5.5-10.5) (Table III).
Discussion
The present study demonstrated that the cost of
managing patients with UC during flares is almost 11-fold the cost
of managing patients with UC in remission, which highlights the
importance of stringent disease control not only to decrease
overall patient morbidity and improve quality of life, but also to
lessen the overall financial burden. The cost of flares increases
further if there is a need for surgical treatment. For patients
with UC in remission, the direct medical cost (mostly contributed
by the cost of drug) was responsible for almost 90% of the total
cost of care, while the direct non-medical cost due to stay and
travel during patient OPD follow-up was almost 10%; the indirect
cost was negligible. In patients with acute flares with ASUC, the
direct non-medical cost was ~10%. However, the direct medical cost
due to drugs, surgery and investigations and the indirect cost due
to the loss of wages almost equally contributed (~45% each) to the
cost of illness.
Previous studies from Asia highlight similar trends
of cost-of-illness in patients with UC. Kamat et al
(11) computed the annual median
cost of patients with UC in remission and relapse in southern India
to be 43,140/- and 52,436.5/- INR, roughly translating into monthly
cost of 3,595/- and 4,370/- INR, respectively. Direct costs of UC
management were 84% compared to almost 100% observed in the
patients with UC in remission in the present study (9). The recent study from Iran by Pakdin
et al (12) estimated the
mean annual costs for patients with UC to be US$ 1,077/- (~7,514/-
INR per month). In direct medical costs, the cost of medication
contributed to the majority of expenses (32%), while overall
indirect costs due to both short-term and long-term disability
contributed to major costs per patient (58%) among UC cases
(12). The high financial impact
resulting from short-term disability due to the temporary
absenteeism of patients with ASUC and their caregivers was also a
major determinant of cost-of-illness in the present study
(45.95%).
The annual health care costs for patients with UC in
remission in India are still lower than those estimated in western
countries. The cost analysis of European IBD Inception cohort's 10
years follow-up evaluation revealed the annual per patient
expenditure for patients with UC to be €1524/- (~11,878/- INR per
month); among which the most costly contributions were due to
medical and surgical hospitalisation (45%) (13). In the USA, the mean annual cost per
patient with UC was calculated to be US$ 5,066/- (~35,343/- INR per
month) (14). However, in the
present study, none of the included subjects among the UC with
remission group were maintained on newer advanced therapies, which
could have underestimated the financial impact of maintenance
therapy.
Flares of IBD negatively affect the quality of life
of patients with UC. The severity of the flares has a direct
bearing on the financial burden inflicted by the disease. Bassi
(15), in a study conducted in the
UK, demonstrated that when compared with quiescent cases of IBD,
disease relapse was associated with a 2-3-fold increase in costs
for non-hospitalised cases and a 20-fold increase in costs for
hospitalised cases (15). In the
present study as well, disease flares increased the cost of care of
treatment by 11-fold and highlighted role of hospitalisation in
major direct cost of care. The cost of flare requiring
hospitalisation was estimated in a recent systematic review and
meta-analysis across various continents for patients with UC
(16). This ranges from US$ 187/-
(~15,655/- INR) in Asia to US$ 3,874/- (~324,326/- INR) in the USA.
In Europe, the mean cost of in-hospital care is US$ 1,236/-
(~103,476/- INR) compared to 44,634/- INR in the present study
(16).
The presents study demonstrated that the maximum
financial burden in the care of patients with UC during periods of
exacerbation was due to the lost productivity and absenteeism of
patients and their caregivers, which translates into an indirect
cost of care. Studies from western countries have highlighted
similar results; for patients with UC belonging to the working-age
population, the indirect cost burden may be greater than the direct
cost burden to the patient (17,18).
These findings highlight the need for the financial security system
to tide over the state of acute financial crises during the period
of hospitalisation for patients with acute UC flares. Other than
stringent measures to control disease activity, social awareness of
the disease and the collective effort of society to support for
patients with IBD is required; this also emphasizes the need for
health insurance, IBD patient support groups and financial support
schemes for patients with IBD. Country-specific costs of medication
differ and the inclusion of specific drugs in government or private
insurance schemes empowers patients with their right to
healthcare.
The are some limitations to the present study which
should be mentioned. There is a possibility of a selection bias due
to the inclusion of only a government tertiary referral centre in
north India; this may have led to the underestimation of the costs
due to the disproportionate representation of different social
classes when compared to a private setup. Furthermore, none of the
patients in remission was on advanced therapy or had undergone
surgery in the past, which thus limits cost analysis to these
subsets of patients. The costs of travel were standardised as per
the distance from the hospital for public transport and could have
underestimated the actual costs if the patients used private
transport for their comfort and ease. In addition, the study may
not have addressed the issues and costs to the caregivers who
participate in the care of patients with flares.
However, the findings of the present study may prove
helpful to treating physicians and may aid in the identification of
the cost of managing flares. The findings presented herein are also
a reminder that the cost of flares is not only limited to morbidity
and mortality, but is also financial. To be on continuous treatment
with maintenance therapy is also a financially prudent choice. It
should also be noted that a large number of patients with flares
were not on therapy, having terminated therapy with the improvement
of symptoms.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
VS, VSR, UD and ANP developed the study protocol. VS
and ANP conceptualised the study. AIY, VSR and PP were involved in
data collection. AIY was involved in data analysis. AIY, VS and AP
were involved in data interpretation. VS and UD provided resources
(stationary and data collection tools). AIY and VS were involved in
the writing of the manuscript. VSR, VS and AP confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
Ethics Committee of the Postgraduate Institute of Medical Education
and Research, Chandigarh, India (INT/IEC/2022/000488) and written
informed consent was obtained from participants/legal guardians
prior to inclusion.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Burisch J, Claytor J, Hernandez I, Hou JK
and Kaplan GG: The cost of inflammatory bowel disease care: How to
make it sustainable. Clin Gastroenterol Hepatol. 23:386–395.
2025.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Aswani-Omprakash T, Sharma V, Bishu S,
Balasubramaniam M, Bhatia S, Nandi N, Shah ND, Deepak P and
Sebastian S: Addressing unmet needs from a new frontier of IBD: The
South Asian IBD Alliance. Lancet Gastroenterol Hepatol. 6:884–885.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Balasubramaniam M, Nandi N,
Aswani-Omprakash T, Sebastian S, Sharma V and Deepak P: South Asian
Ibd Alliance Board Of Directors. Identifying care challenges as
opportunities for research and education in inflammatory bowel
disease in South Asia. Gastroenterology. 163:1145–1150.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rana VS, Mahajan G, Patil AN, Singh AK,
Jearth V, Sekar A, Singh H, Saroch A, Dutta U and Sharma V: Factors
contributing to flares of ulcerative colitis in North India-a
case-control study. BMC Gastroenterol. 23(336)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Noor NM, Lee JC, Bond S, Dowling F,
Brezina B, Patel KV, Ahmad T, Banim PJ, Berrill JW, Cooney R, et
al: A biomarker-stratified comparison of top-down versus
accelerated step-up treatment strategies for patients with newly
diagnosed Crohn's disease (PROFILE): A multicentre, open-label
randomised controlled trial. Lancet Gastroenterol Hepatol.
9:415–427. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bayoumy AB, Mulder CJJ, Ansari AR, Barclay
ML, Florin T, Kiszka-Kanowitz M, Derijks L, Sharma V and de Boer
NKH: Uphill battle: Innovation of thiopurine therapy in global
inflammatory bowel disease care. Indian J Gastroenterol. 43:36–47.
2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pal P, Banerjee R, Vijayalaxmi P, Reddy DN
and Tandan M: Depression and active disease are the major risk
factors for fatigue and sleep disturbance in inflammatory bowel
disease with consequent poor quality of life: Analysis of the
interplay between psychosocial factors from the developing world.
Indian J Gastroenterol. 43:226–236. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sharma V, Shukla J, Suri V, Jena A,
Mukerjee A, Mandavdhare HS, Bhalla A and Dutta U: Cost concerns,
not the guidelines, drive clinical care of IBD during COVID
pandemic in a resource limited setting. Expert Rev Gastroenterol
Hepatol. 15:465–466. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Travis SP, Farrant JM, Ricketts C, Nolan
DJ, Mortensen NM, Kettlewell MG and Jewell DP: Predicting outcome
in severe ulcerative colitis. Gut. 38:905–910. 1996.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ain SN, Khan ZA and Gilani MA: Revised
kuppuswamy scale for 2021 based on new consumer price index and use
of conversion factors. Indian J Public Health. 65:418–421.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kamat N, Ganesh Pai C, Surulivel Rajan M
and Kamath A: Cost of illness in inflammatory bowel disease. Dig
Dis Sci. 62:2318–2326. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pakdin M, Zarei L, Bagheri Lankarani K and
Ghahramani S: The cost of illness analysis of inflammatory bowel
disease. BMC Gastroenterol. 23(21)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Odes S, Vardi H, Friger M, Wolters F,
Russel MG, Riis L, Munkholm P, Politi P, Tsianos E, Clofent J, et
al: Cost analysis and cost determinants in a european inflammatory
bowel disease inception cohort with 10 years of follow-up
evaluation. Gastroenterology. 131:719–728. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kappelman MD, Rifas-Shiman SL, Porter CQ,
Ollendorf DA, Sandler RS, Galanko JA and Finkelstein JA: Direct
health care costs of crohn's disease and ulcerative colitis in US
children and adults. Gastroenterology. 135:1907–1913.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bassi A, Dodd S, Williamson P and Bodger
K: Cost of illness of inflammatory bowel disease in the UK: A
single centre retrospective study. Gut. 53:1471–1478.
2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Van Linschoten RCA, Visser E, Niehot CD,
Van Der Woude CJ, Hazelzet JA, Van Noord D and West RL: Systematic
review: Societal cost of illness of inflammatory bowel disease is
increasing due to biologics and varies between continents. Aliment
Pharmacol Ther. 54:234–248. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM
and Chao J: Systematic review: The costs of ulcerative colitis in
Western countries. Aliment Pharmacol Ther. 31:693–707.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Khalili H, Everhov ÅH, Halfvarson J,
Ludvigsson JF, Askling J, Myrelid P, Söderling J, Olen O and
Neovius M: SWIBREG Group. Healthcare use, work loss and total costs
in incident and prevalent Crohn's disease and ulcerative colitis:
Results from a nationwide study in Sweden. Aliment Pharmacol Ther.
52:655–668. 2020.PubMed/NCBI View Article : Google Scholar
|