The endothelium lines the interior of blood vessels
and functions as a barrier to maintain the movement of fluids,
nutrients and immune cells between the bloodstream and surrounding
tissues (1,2). It is involved in homeostasis, vascular
contraction and cell growth regulation (3). Compromised endothelial function leads
to vasoconstriction, thrombosis and increased permeability; which
in turn contributes to the development of cardiovascular, metabolic
and inflammatory lung disease (4,5).
In the lung, the endothelium maintains the blood-air
barrier required for gas exchange and controls the vascular tone
through the release of vasodilators and vasoconstrictors.
Furthermore, it modulates immune responses by adhesion molecule and
cytokine regulation. In the eye, the endothelium forms the
blood-retinal barrier, and regulates fluid and solute transport
between the aqueous humor and the corneal stroma. Damage to corneal
endothelial cells may lead to impaired fluid regulation, corneal
clouding, vision loss and Fuchs endothelial corneal dystrophy
(6-9).
The dysfunctional endothelium becomes pro-thrombotic
by releasing von Willebrand factor, which contributes to thrombosis
and vascular disease (17,18). Under normal physiological conditions,
endothelial nitric oxide (NO) synthase (eNOS) produces NO from
L-arginine in the presence of tetrahydrobiopterin. NO is often
decreased due to an increase in the levels of oxidative stress
(19,20). This disruption in the electron
transport process of the enzyme leads to the generation of
superoxide (21,22). NO maintains vascular homeostasis,
promotes vasodilation, inhibits platelet aggregation and reduces
inflammation (23). Inflammatory
cytokines (TNF-α and IL-1β) activate nuclear factor
kappa-light-chain-enhancer of activated B cells (NF-κB) (24) and triggers inflammation, which in
severe cases may lead to endothelial apoptosis or necrosis
(25).
Inflammation is a complex biological mechanism which
involves immune cells, blood vessels, and molecular mediators
(e.g., microRNAs, adipokines and inflammasomes); to eliminate cell
injury, remove necrotic cells, and initiate tissue repair (26). Acute inflammation is an immediate,
adaptive response with limited specificity caused by noxious
stimuli (e.g., infection and tissue damage) and protects against
infection (27,28). Chronic inflammation has been
associated with cancer, diabetes, neurodegenerative disease,
pulmonary and autoimmune disease (29,30). It
can potentially damage healthy cells, tissues and organs; since it
promotes fibrosis, vascular damage, and immune dysregulation
(31). Growth hormone (GH)-releasing
hormone (GHRH), which regulates the release of GH from the anterior
pituitary gland, promotes inflammation (32).
Targeted pharmacological interventions are required
to restore endothelial function in cardiovascular, renal, metabolic
and respiratory disorders (33).
Angiotensin-converting enzyme inhibitors, β blockers,
dihydropyridine Ca2+ channel blockers, anti-oxidative
agents (e.g., vitamins C and E, and N-acetylcysteine),
phosphodiesterase inhibitors, statins, angiotensin, bradykinin and
eNOS transcription enhancer exhibit strong potential to ameliorate
endothelial dysfunction (24).
Emerging evidence suggests that growth
hormone-releasing hormone antagonists (GHRHAnts), synthetic
somatostatin analogues (SSAs) and never in mitosis A-related kinase
2 inhibition may enhance endothelial function in in vitro
and in vivo models of lung endothelial injury (34-37).
They can inhibit the the release of pro-inflammatory cytokines and
counteract endothelial hyper-permeability (38). Furthermore, SSAs downregulate VEGF
(39) and modulate tight junctions
(40,41).
GH, also known as somatotropin, is secreted by
somatotropic cells which are located in the anterior lobe of the
pituitary gland (42). This peptide
hormone is involved in growth and metabolic regulation, affecting
various physiological systems (e.g., neural, reproductive, immune,
cardiovascular and pulmonary) (32).
The GH level increases throughout childhood, reaching their peak
during adolescence, stimulating the development of bone and
cartilage (43). Following puberty,
GH continues to maintain body composition, fat balance, muscle,
bone tissue, insulin and blood sugar levels (44).
GH stimulates lipolysis, protein synthesis, free
fatty acid and glycerol levels, which contribute to decrease fat
body mass (48). It also contributes
to insulin resistance, particularly in type 1 diabetes (49). Furthermore, GH regulates fluid
homeostasis by activating the renin-angiotensin system and
stimulating distal renal tubular reabsorption, which leads to
sodium and fluid retention (50).
The multifaceted actions of GH highlight its indispensable role in
health (51).
GHD is a condition characterized by inadequate
secretion of GH from the anterior pituitary gland. In children, GHD
manifests as short stature and growth failure, often accompanied by
midface hypoplasia and increased truncal adiposity (54). Severe GHD may be apparent early in
life with a significant reduction in height velocity (43) and can be congenital or acquired due
to brain tumor, head trauma, or radiation therapy. Isolated GHD
(IGHD) is linked to gene mutations [e.g., GH1 and GHRH receptor
(GHRHR) gene] (55). IGHD type II is
an autosomal dominant disorder, caused by GH1 mutations that affect
the splicing of exon 3(56). This
leads to the production of an abnormal GH (17.5 kDa) isoform, which
disrupts hormone trafficking (57).
In adults, GHD results from structural pituitary or
hypothalamic disorder or cranial irradiation (58). Adult-onset GHD is associated with a
cluster of cardiovascular risk factors, including increased
adiposity (particularly visceral fat), reduced muscle strength,
impaired psychological well-being, insulin resistance, adverse
lipid profiles and reduced bone mineral density (59). Patients may experience depression,
difficulty concentrating, memory issues and anxiety or emotional
distress. GH replacement therapy has been shown to reverse a number
of these biological changes, improving body composition and overall
health status (60).
Gigantism is characterized by abnormally high linear
growth due to excessive GH and IGF-1 levels prior to the epiphyseal
growth plates fuse during childhood (61). In the majority of cases, gigantism is
caused by a benign (non-cancerous) pituitary tumor, known as an
adenoma, that hyper-secretes GH (62). Genetic mutations are also associated
with the formation of pituitary tumors, leading to gigantism
(63). Excessive hypothalamic GHRH
levels may activate mutations in hypothalamic GHRH-neurons
(64). The symptoms of gigantism
include accelerated growth velocity, tall stature, enlarged hands
and feet, soft-tissue thickening, prognathism (protruding jaw),
coarse facial features, muscle weakness, cardiovascular issues,
joint issues and headaches (65).
In the event that excessive GH secretion occurs
following epiphyseal closure, the condition is referred to as
acromegaly, which presents with similar features to gigantism but
without increased linear growth. When the body produces excessive
GH levels due to a non-cancerous tumor in the pituitary gland, it
can lead to acromegaly (61). This
hormone imbalance causes bones and tissues to grow to a greater
extent than usual and has been associated with high blood pressure,
diabetes and heart disease (66).
Excessive GH secretion increases the risk of
developing colon, thyroid, gastric, breast, and urinary tract
cancers (67). Specific genetic
polymorphisms within the GH/IGF-1 pathway may increase
susceptibility to malignancies (e.g., breast cancer) (68). Conversely, GH and IGF-1 deficits are
associated with a diminished incidence of tumor promotion (69), associated to IGF-1/IGF-1R (70). IGF-1 promotes cell proliferation,
differentiation and growth (71).
GHRHAnts were developed by Dr A.V. Schally
(1926-2024) (Nobel Prize in Physiology or Medicine, 1977) to
suppress malignancies (72,73); however, they have the potential to be
used in a broader variety of disorders (74,75).
These synthetic peptides reduce pituitary GH release and hepatic
IGF-1 levels, and act directly on peripheral tissues by blocking
both GHRHR and its splice variant SV1 (72,76-79).
The activation of the cyclic adenosine monophosphate (cAMP)/protein
kinase A (PKA) response element-binding protein signaling pathway
leads to GH gene transcription and downstream IGF-1 production.
GHRHAnts modulate key cellular processes involved in inflammation,
oxidative stress, fibrosis and barrier integrity (80).
In peripheral tissues, GHRHAnts exert their
protective effects by suppressing the activation of NF-κB (76) and inhibiting mitogen-activated
protein kinases (MAPKs), such as ERK1/2 and p38 (81,82).
Moreover, GHRHAnts suppress JAK/STAT signaling, particularly STAT3
activation, which is implicated in cytokine-driven inflammation and
fibrosis (83). Those peptides can
also mitigate ER stress through the induction of the unfolded
protein response (UPR) (84),
promote antioxidant defenses, and preserve barrier integrity
(85).
GHRHAnts prevent angiogenesis by reducing matrix
metalloproteinases (MMPs; MMP-2 and MMP-9) (97) and VEGF, which are vital for tumor
invasion and metastasis (98).
Moreover, they exhibit minimal systemic toxicity and do not
significantly suppress basal GH or IGF-1 levels at therapeutic
doses (73). It has been suggested
that GHRHAnts may serve as promising candidates for the treatment
of cancer, either as monotherapy or in combination with
chemotherapy, targeted agents, or immunotherapy, due to their
capacity to target multiple tumor-promoting pathways (99).
Barrier integrity is essential for maintaining
homeostasis in the alveolar-capillary barrier (100), which is involved in the
pathogenesis of complex disorders, such as ALI and ARDS (101). Those are severe and
life-threatening pulmonary disorders characterized by uncontrolled
pulmonary inflammation, increased vascular permeability,
alveolar-capillary barrier disruption and impaired gas exchange
(102). These conditions are
frequently caused by infections, sepsis, trauma, or inhalation of
toxic substances. Despite advances in supportive care,
pharmacological options for ALI/ARDS remain limited. Recent
research suggests that GHRHAnts protect against ALI and sepsis by
simultaneously modulating key inflammatory processes and supporting
the preservation of lung barrier integrity (103). MIA-602 has been demonstrated to
exert protective effects in various ALI experimental models,
including lipopolysaccharide (LPS)-induced endotoxemia and
bleomycin-induced lung damage (104). GHRHAnts maintain vascular integrity
by enhancing junctional stability and reducing pulmonary edema
(105). Treatment with GHRHAnts has
been shown to result in enhanced arterial oxygenation, lung injury
amelioration and improved survival rates in animal experiments
(103).
Pulmonary fibrosis is a progressive life-threatening
condition, which is characterized by the deposition of excessive
extracellular matrix and alveolar architecture destruction, which
result in loss of lung function (106). The chronic activation of
fibroblasts and myofibroblasts is stimulated by profibrotic
cytokines, such as transforming growth factor-β1 (TGF-β1) (107). Experimental evidence demonstrates
that GHRHAnts exert anti-fibrotic effects (108). GHRHAnts (e.g., MIA-602 and MIA-690)
have demonstrated a marked ability to inhibit key fibrotic
processes by suppressing TGF-β1 signaling, preventing
epithelial-mesenchymal transition, and decreasing fibroblast
proliferation and differentiation into myofibroblasts (72,109,110).
These peptides can also counteract the expression of profibrotic
markers such as α-smooth muscle actin, fibronectin, type I
collagen, and they can limit the deposition of collagen in lung
tissue (76). In a previous study
using a bleomycin-induced mouse model of lung fibrosis, GHRHAnts
were shown to ameliorate fibrosis, improve lung compliance and
preserve alveolar architecture (108).
The BBB is a highly selective and specialized
structure that regulates the exchange of molecules between the
bloodstream and the central nervous system (111). The disruption of this barrier can
lead to neuroinflammatory and neurodegenerative disorders,
including multiple sclerosis, traumatic brain injury, ischemic
stroke and Alzheimer's disease (112). The increased permeability of the
BBB permits the entry of immune cells, cytokines and other harmful
substances into the brain, triggering chronic inflammation,
impairing neuronal function and exacerbating vascular damage
(113).
There is experimental evidence to indicate that
GHRHAnts can protect BBB integrity and reduce neuroinflammatory
damage (114). In models of central
nervous system injury, these peptides reduce the expression of
inflammatory cytokines (e.g., IL-6 and TNF-α), suppress microglial
and astrocyte activation, and block NF-κB (32). GHRHAnts stabilize the BBB and protect
paracellular barrier integrity by maintaining tight junction
protein (such as occludin and claudin-5) expression (115).
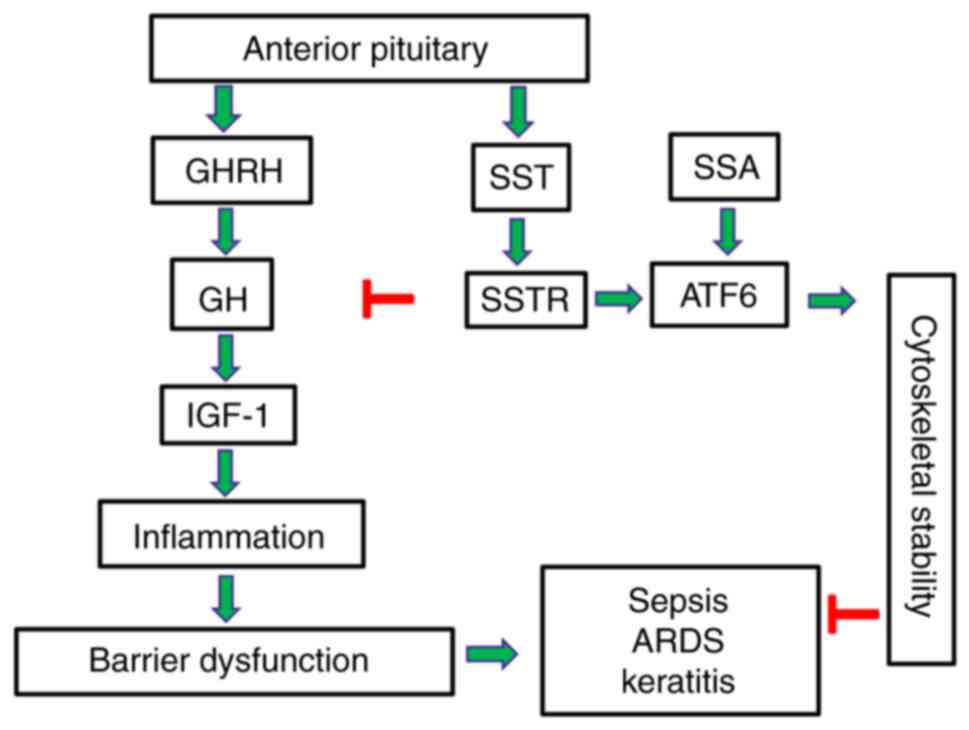
SST, also known as GH-inhibiting hormone, exerts
potent regulatory effects throughout the body (116). It functions as a neurotransmitter
in the central nervous system (117), and exerts effects, at least
partially, via the GH/IGF-1 axis (118). SST, which is affected by glucose,
inhibits GH and thyroid-stimulating hormone secretion (119).
SSAs inhibit the production of GH and serotonin,
which are used in managing GH-related disorders. Excessive GH
secretion from pituitary adenomas leads to elevated levels of
IGF-1(120). SSAs bind to SST
receptors (SSTRs) on the surface of tumors in acromegaly and
gigantism to inhibit the release of GH and reduce IGF-1 production
(121).
Neuroendocrine tumors and GH-dependent cancers
express SSTRs and respond to octreotide, which exerts
anti-proliferative effects through receptor-mediated apoptosis, the
inhibition of angiogenesis and the suppression of GH (126). It has been demonstrated that
octreotide preserves endothelial barrier function, reduces reactive
oxygen species (ROS) generation, and attenuates inflammatory
responses in a murine model of LPS-induced ALI (34).
Octreotide may exert protective effects on vascular
endothelial cells through the activation of the UPR and enhances
the expression of ER chaperone proteins (e.g., binding
immunoglobulin protein and glucose-regulated protein 94), leading
to the stabilization of cytoskeletal components (127). When activating transcription factor
6 (ATF6) is pharmacologically inhibited, the beneficial effects of
octreotide on endothelial permeability, ROS generation and improved
cytoskeletal organization are virtually diminished (128). These findings suggest that
octreotide confers protective effects on the vascular endothelium
and may be useful towards inflammation-related vascular
disorders.
Lanreotide is a long-acting SSA that exerts
pharmacological effects by binding predominantly to SSTR2 and
SSTR5(129), which are
overexpressed in GH-secreting pituitary adenomas. Lanreotide has
emerged as a valuable non-surgical therapy in acromegaly or in
patients with residual disease following pituitary surgery
(130) and may provide improved
visual symptoms due to decreased tumor mass (131). The long half-life of lanreotide
(23-30 days) and subcutaneous depot formulation offer convenient
dosing, rendering it suitable for chronic conditions requiring
long-term management (132).
Recent research suggests that lanreotide exerts
protective effects against LPS-induced endothelial dysfunction both
in vitro and in vivo. LPS typically induces
endothelial hyperpermeability, leading to increased vascular
leakage (133). Lanreotide reduces
ROS generation in endothelial cells exposed to LPS, highlighting
its role in mitigating oxidative stress. Furthermore, this
FDA-approved analog ameliorated lung inflammatory disease (134).
Pasireotide, a second-generation SST analog, is
distinguished by its high affinity for multiple SSRT subtypes
(SSTR1-5) (135,136), and it is used in patients resistant
to first-generation SSA (octreotide or lanreotide) (137,138).
Pasireotide has been approved for use in patients with active
acromegaly and Cushing's disease (139). Safety assessments reveal that
pasireotide is generally well tolerated. The most frequently
observed adverse event is hyperglycemia, a known effect of SSAs due
to reduced insulin secretion; this can be managed with standard
antidiabetic therapies. The tumor expression of SSTRs and dopamine
receptors may predict the responsiveness to pasireotide in
corticotropinomas (140).
Recent research suggests that pasireotide exerts
potent protective effects on endothelial barrier integrity
(35) and ALI, since it mitigates
LPS-induced inflammation by preserving barrier function, reducing
cytotoxicity, reinforcing antioxidant defenses, and dampening
inflammatory responses. Mechanistically, pasireotide attenuates
MAPK and JAK/STAT inflammatory signaling. These antioxidative and
anti-inflammatory actions may synergistically stabilize endothelial
junctions and suppress tissue injury (34,35,134,141).
SSAs are available in both short-acting and
long-acting formulations. Octreotide is available in subcutaneous
and intramuscular forms (122),
while lanreotide is delivered via deep subcutaneous injection
(132). Pasireotide is administered
either subcutaneously or as a long-acting depot injection (142,143)
to modulate GH-related abnormalities (144).
Targeted GH modulation presents an exciting
therapeutic intervention to alleviate human disease, and the
development of synthetic analogs to achieve normal GH levels has
been proven to be an effective strategy for the treatment of
cancers and acromegaly in clinical practice (Table I). Recent preclinical studies suggest
that SSAs and GHRHAnts counteract disorders related to endothelial
barrier dysfunction utilizing ATF6 (127,128).
Based on this information, experimental models of targeted ATF6
modulation will be developed to assist in designing GH-related
pharmacotherapies. Those advanced approaches will be able to
utilize the beneficial properties of UPR activation to
strategically ameliorate endothelial-dependent disorders which may
affect the brain, lungs and eyes (Fig.
1).
Not applicable.
Funding: The present study was supported by the National
Institute of Allergy and Infectious Diseases of the National
Institutes of Health under Award no. R03AI176433; and an
Institutional Development Award (IDeA) from the National Institute
of General Medical Sciences of the National Institutes of Health
under Award Number P20 GM103424-21.
Not applicable.
SF, MMRS and MS were involved in the writing and
preparation of the original draft of the manuscript. NB was
involved in the conceptualization and supervision of the study, in
the reviewing and editing of the manuscript, in funding acquisition
and in project administration. All authors have read and approved
the final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hennigs JK, Matuszcak C, Trepel M and
Körbelin J: Vascular endothelial cells: Heterogeneity and targeting
approaches. Cells. 10(2712)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Saravi B, Goebel U, Hassenzahl LO, Jung C,
David S, Feldheiser A, Stopfkuchen-Evans M and Wollborn J:
Capillary leak and endothelial permeability in critically ill
patients: A current overview. Intensive Care Med Exp.
11(96)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rubanyi GM: The role of endothelium in
cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 22
(Suppl 4):S1–S14. 1993.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Endothelial barrier and its abnormalities in cardiovascular
disease. Front Physiol. 6(365)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kopaliani I, Elsaid B, Speier S and
Deussen A: Immune and metabolic mechanisms of endothelial
dysfunction. Int J Mol Sci. 25(13337)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vassiliou AG, Kotanidou A, Dimopoulou I
and Orfanos SE: Endothelial damage in acute respiratory distress
syndrome. Int J Mol Sci. 21(8793)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cunha-Vaz J, Bernardes R and Lobo C:
Blood-retinal barrier. Eur J Ophthalmol. 21 (Suppl 6):S3–S9.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goncalves A and Antonetti DA: Transgenic
animal models to explore and modulate the blood brain and blood
retinal barriers of the CNS. Fluids Barriers CNS.
19(86)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu X, Zheng T, Zhao C, Zhang Y, Liu H,
Wang L and Liu P: Genetic mutations and molecular mechanisms of
Fuchs endothelial corneal dystrophy. Eye Vis (Lond).
8(24)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang X and He B: Endothelial dysfunction:
Molecular mechanisms and clinical implications. MedComm (2020).
5(e651)2024.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Siasos G, Gouliopoulos N, Moschos MM,
Oikonomou E, Kollia C, Konsola T, Athanasiou D, Siasou G, Mourouzis
K, Zisimos K, et al: Role of endothelial dysfunction and arterial
stiffness in the development of diabetic retinopathy. Diabetes
Care. 38:e9–e10. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Steyers CM III and Miller FJ Jr:
Endothelial dysfunction in chronic inflammatory diseases. Int J Mol
Sci. 15:11324–11349. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Poredos P, Poredos AV and Gregoric I:
Endothelial dysfunction and its clinical implications. Angiology.
72:604–615. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sarelius IH and Glading AJ: Control of
vascular permeability by adhesion molecules. Tissue Barriers.
3(e985954)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aghajanian A, Wittchen ES, Allingham MJ,
Garrett TA and Burridge K: Endothelial cell junctions and the
regulation of vascular permeability and leukocyte transmigration. J
Thromb Haemost. 6:1453–1460. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Peng Z, Shu B, Zhang Y and Wang M:
Endothelial response to pathophysiological stress. Arterioscler
Thromb Vasc Biol. 39:e233–e243. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Goshua G, Pine AB, Meizlish ML, Chang CH,
Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, et al:
Endotheliopathy in COVID-19-associated coagulopathy: Evidence from
a single-centre, cross-sectional study. Lancet Haematol.
7:e575–e582. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
van Hinsbergh VW: Endothelium-role in
regulation of coagulation and inflammation. Semin Immunopathol.
34:93–106. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu S, Ilyas I, Little PJ, Li H, Kamato D,
Zheng X, Luo S, Li Z, Liu P, Han J, et al: Endothelial dysfunction
in atherosclerotic cardiovascular diseases and beyond: from
mechanism to pharmacotherapies. Pharmacol Rev. 73:924–967.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shaito A, Aramouni K, Assaf R, Parenti A,
Orekhov A, Yazbi AE, Pintus G and Eid AH: Oxidative stress-induced
endothelial dysfunction in cardiovascular diseases. Front Biosci
(Landmark Ed). 27(105)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Janaszak-Jasiecka A, Płoska A, Wierońska
JM, Dobrucki LW and Kalinowski L: Endothelial dysfunction due to
eNOS uncoupling: molecular mechanisms as potential therapeutic
targets. Cell Mol Biol Lett. 28(21)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alp NJ and Channon KM: Regulation of
endothelial nitric oxide synthase by tetrahydrobiopterin in
vascular disease. Arterioscler Thromb Vasc Biol. 24:413–420.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jin RC and Loscalzo J: Vascular nitric
oxide: Formation and function. J Blood Med. 2010:147–162.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Su JB: Vascular endothelial dysfunction
and pharmacological treatment. World J Cardiol. 7:719–741.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang W, Jiang L, Tong X, He H, Zheng Y
and Xia Z: Sepsis-induced endothelial dysfunction: Permeability and
regulated cell death. J Inflamm Res. 17:9953–9973. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liehn EA and Cabrera-Fuentes HA:
Inflammation between defense and disease: Impact on tissue repair
and chronic sickness. Discoveries (Craiova). 3(e42)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tripathi S, Sharma Y and Kumar D:
Unveiling the link between chronic inflammation and cancer. Metabol
Open. 25(100347)2025.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chavda VP, Feehan J and Apostolopoulos V:
Inflammation: The cause of all diseases. Cells.
13(1906)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Furman D, Campisi J, Verdin E,
Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW,
Fasano A, Miller GW, et al: Chronic inflammation in the etiology of
disease across the life span. Nat Med. 25:1822–1832.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Castellon X and Bogdanova V: Chronic
inflammatory diseases and endothelial dysfunction. Aging Dis.
7:81–89. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Du L, Ho BM, Zhou L, Yip YWY, He JN, Wei
Y, Tham CC, Chan SO, Schally AV, Pang CP, et al: Growth hormone
releasing hormone signaling promotes Th17 cell differentiation and
autoimmune inflammation. Nat Commun. 14(3298)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chee YJ, Dalan R and Cheung C: The
interplay between immunity, inflammation and endothelial
dysfunction. Int J Mol Sci. 26(1708)2025.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fakir S, Kubra KT and Barabutis N:
Octreotide protects against LPS-induced endothelial cell and lung
injury. Cell Signal. 124(111455)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fakir S, Sigdel M, Sarker MMR and
Barabutis N: Pasireotide exerts anti-inflammatory effects in the
endothelium. J Biochem Mol Toxicol. 39(e70306)2025.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Clemens A, Klevesath MS, Hofmann M, Raulf
F, Henkels M, Amiral J, Seibel MJ, Zimmermann J, Ziegler R, Wahl P
and Nawroth PP: Octreotide (somatostatin analog) treatment reduces
endothelial cell dysfunction in patients with diabetes mellitus.
Metabolism. 48:1236–1240. 1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Barabutis N and Akhter MS: Involvement of
NEK2 and NEK9 in LPS-induced endothelial barrier dysfunction.
Microvasc Res. 152(104651)2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Karalis K, Mastorakos G, Chrousos GP and
Tolis G: Somatostatin analogues suppress the inflammatory reaction
in vivo. J Clin Invest. 93:2000–2006. 1994.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sall JW, Klisovic DD, O'Dorisio MS and
Katz SE: Somatostatin inhibits IGF-1 mediated induction of VEGF in
human retinal pigment epithelial cells. Exp Eye Res. 79:465–476.
2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Basivireddy J, Somvanshi RK, Romero IA,
Weksler BB, Couraud PO, Oger J and Kumar U: Somatostatin preserved
blood brain barrier against cytokine induced alterations: Possible
role in multiple sclerosis. Biochem Pharmacol. 86:497–507.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mentlein R, Eichler O, Forstreuter F and
Held-Feindt J: Somatostatin inhibits the production of vascular
endothelial growth factor in human glioma cells. Int J Cancer.
92:545–550. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ayuk J and Sheppard MC: Growth hormone and
its disorders. Postgrad Med J. 82:24–30. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ranke MB: Short and long-term effects of
growth hormone in children and adolescents with GH deficiency.
Front Endocrinol (Lausanne). 12(720419)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sharma R, Kopchick JJ, Puri V and Sharma
VM: Effect of growth hormone on insulin signaling. Mol Cell
Endocrinol. 518(111038)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pech-Pool S, Berumen LC, Martínez-Moreno
CG, García-Alcocer G, Carranza M, Luna M and Arámburo C:
Thyrotropin-releasing hormone (TRH) and somatostatin (SST), but not
growth hormone-releasing hormone (GHRH) nor Ghrelin (GHRL),
regulate expression and release of immune growth hormone (GH) from
chicken bursal B-lymphocyte cultures. Int J Mol Sci.
21(1436)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Dehkhoda F, Lee CMM, Medina J and Brooks
AJ: The growth hormone receptor: Mechanism of receptor activation,
cell signaling, and physiological aspects. Front Endocrinol
(Lausanne). 9(35)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ohlsson C, Mohan S, Sjögren K, Tivesten A,
Isgaard J, Isaksson O, Jansson JO and Svensson J: The role of
liver-derived insulin-like growth factor-I. Endocr Rev. 30:494–535.
2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kopchick JJ, Berryman DE, Puri V, Lee KY
and Jorgensen JOL: The effects of growth hormone on adipose tissue:
Old observations, new mechanisms. Nat Rev Endocrinol. 16:135–146.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kim SH and Park MJ: Effects of growth
hormone on glucose metabolism and insulin resistance in human. Ann
Pediatr Endocrinol Metab. 22:145–152. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hansen TK, Møller J, Thomsen K, Frandsen
E, Dall R, Jørgensen JO and Christiansen JS: Effects of growth
hormone on renal tubular handling of sodium in healthy humans. Am J
Physiol Endocrinol Metab. 281:E1326–E1332. 2001.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ricci Bitti S, Franco M, Albertelli M,
Gatto F, Vera L, Ferone D and Boschetti M: GH replacement in the
elderly: Is it worth it? Front Endocrinol (Lausanne).
12(680579)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Reed ML, Merriam GR and Kargi AY: Adult
growth hormone deficiency-benefits, side effects, and risks of
growth hormone replacement. Front Endocrinol (Lausanne).
4(64)2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kim JH, Chae HW, Chin SO, Ku CR, Park KH,
Lim DJ, Kim KJ, Lim JS, Kim G, Choi YM, et al: Diagnosis and
treatment of growth hormone deficiency: A position statement from
Korean endocrine society and Korean Society of pediatric
endocrinology. Endocrinol Metab (Seoul). 35:272–287.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Alatzoglou KS, Webb EA, Le Tissier P and
Dattani MT: Isolated growth hormone deficiency (GHD) in childhood
and adolescence: Recent advances. Endocr Rev. 35:376–432.
2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Mullis PE: Genetics of isolated growth
hormone deficiency. J Clin Res Pediatr Endocrinol. 2:52–62.
2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kautsar A, Wit JM and Pulungan A: Isolated
growth hormone deficiency type 2 due to a novel GH1 mutation: A
case report. J Clin Res Pediatr Endocrinol. 11:426–431.
2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Alatzoglou KS, Kular D and Dattani MT:
Autosomal dominant growth hormone deficiency (type II). Pediatr
Endocrinol Rev. 12:347–355. 2015.PubMed/NCBI
|
|
58
|
Feldt-Rasmussen U and Klose M: Adult
Growth Hormone Deficiency-Clinical Management. In: Endotext
[Internet]. Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A,
Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, et
al (eds). MDText.com, Inc., South Dartmouth, MA, 2000.
|
|
59
|
Murray RD: Adult growth hormone
replacement: Current understanding. Curr Opin Pharmacol. 3:642–649.
2003.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Papadogias DS, Makras P, Kaltsas GA and
Monson JP: GH deficiency in adults. Hormones (Athens). 2:217–218.
2003.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bello MO and Garla VV: Gigantism and
Acromegaly. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2025.
|
|
62
|
Rhee N, Jeong K, Yang EM and Kim CJ:
Gigantism caused by growth hormone secreting pituitary adenoma. Ann
Pediatr Endocrinol Metab. 19:96–99. 2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Hannah-Shmouni F, Trivellin G and
Stratakis CA: Genetics of gigantism and acromegaly. Growth Horm IGF
Res. 30-31:37–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Dieguez C, Lopez M and Casanueva F:
Hypothalamic GHRH. Rev Endocr Metab Disord. 26:297–303.
2025.PubMed/NCBI View Article : Google Scholar
|
|
65
|
George MM, Eugster EA and Chernausek SD:
Pituitary Gigantism. In: Endotext [Internet]. Feingold KR, Ahmed
SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de
Herder WW, Dhatariya K, Dungan K, et al (eds). MDText.com,
Inc., South Dartmouth, MA, 2020.
|
|
66
|
Quock TP, Chang E, Das AK, Speller A,
Tarbox MH, Rattana SK, Paulson IE and Broder MS: Clinical and
economic burden among older adults with acromegaly in the United
States. J Comp Eff Res. 14(e250076)2025.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Fernandez CJ, Lakshmi V, Kamrul-Hasan ABM
and Pappachan JM: Factors affecting disease control after pituitary
tumor resection in acromegaly: What is the current evidence? World
J Radiol. 17(106438)2025.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Grimberg A: Mechanisms by which IGF-I may
promote cancer. Cancer Biol Ther. 2:630–635. 2003.PubMed/NCBI
|
|
69
|
Guevara-Aguirre J, Peña G, Acosta W,
Pazmiño G, Saavedra J, Soto L, Lescano D, Guevara A and Gavilanes
AWD: Cancer in growth hormone excess and growth hormone deficit.
Endocr Relat Cancer. 30(e220402)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Simpson A, Petnga W, Macaulay VM,
Weyer-Czernilofsky U and Bogenrieder T: Insulin-Like growth factor
(IGF) pathway targeting in cancer: Role of the IGF axis and
opportunities for future combination studies. Target Oncol.
12:571–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Khan MZ, Zugaza JL and Torres Aleman I:
The signaling landscape of insulin-like growth factor 1. J Biol
Chem. 301(108047)2025.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Villanova T, Gesmundo I, Audrito V, Vitale
N, Silvagno F, Musuraca C, Righi L, Libener R, Riganti C, Bironzo
P, et al: Antagonists of growth hormone-releasing hormone (GHRH)
inhibit the growth of human malignant pleural mesothelioma. Proc
Natl Acad Sci USA. 116:2226–2231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Schally AV, Cai R, Zhang X, Sha W and
Wangpaichitr M: The development of growth hormone-releasing hormone
analogs: Therapeutic advances in cancer, regenerative medicine, and
metabolic disorders. Rev Endocr Metab Disord. 26:385–396.
2025.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Cui T, Jimenez JJ, Block NL, Badiavas EV,
Rodriguez-Menocal L, Vila Granda A, Cai R, Sha W, Zarandi M, Perez
R and Schally AV: Agonistic analogs of growth hormone releasing
hormone (GHRH) promote wound healing by stimulating the
proliferation and survival of human dermal fibroblasts through ERK
and AKT pathways. Oncotarget. 7:52661–52672. 2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Fakir S and Barabutis N: Protective
activities of growth hormone-releasing hormone antagonists against
toxin-induced endothelial injury. Endocrines. 5:116–123.
2024.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhang C, Cui T, Cai R, Wangpaichitr M,
Mirsaeidi M, Schally AV and Jackson RM: Growth hormone-releasing
hormone in lung physiology and pulmonary disease. Cells.
9(2331)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Annunziata M, Grande C, Scarlatti F,
Deltetto F, Delpiano E, Camanni M, Ghigo E and Granata R: The
growth hormone-releasing hormone (GHRH) antagonist JV-1-36 inhibits
proliferation and survival of human ectopic endometriotic stromal
cells (ESCs) and the T HESC cell line. Fertil Steril. 94:841–849.
2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Zarandi M, Cai R, Kovacs M, Popovics P,
Szalontay L, Cui T, Sha W, Jaszberenyi M, Varga J, Zhang X, et al:
Synthesis and structure-activity studies on novel analogs of human
growth hormone releasing hormone (GHRH) with enhanced inhibitory
activities on tumor growth. Peptides. 89:60–70. 2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Granata R: Peripheral activities of growth
hormone-releasing hormone. J Endocrinol Invest. 39:721–727.
2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Siejka A, Lawnicka H, Fakir S and
Barabutis N: Growth hormone-releasing hormone in the immune system.
Rev Endocr Metab Disord. 26:457–466. 2025.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Barabutis N, Siejka A, Schally AV, Block
NL, Cai R and Varga JL: Activation of mitogen-activated protein
kinases by a splice variant of GHRH receptor. J Mol Endocrinol.
44:127–134. 2010.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Barabutis N, Akhter MS, Kubra KT and
Jackson K: Growth hormone-releasing hormone in endothelial
inflammation. Endocrinology. 164(bqac209)2022.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Siejka A, Schally AV and Barabutis N:
Activation of Janus kinase/signal transducer and activator of
transcription 3 pathway by growth hormone-releasing hormone. Cell
Mol Life Sci. 67:959–964. 2010.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Fakir S, Kubra KT, Akhter MS, Uddin MA,
Sarker MMR, Siejka A and Barabutis N: Unfolded protein response
modulates the effects of GHRH antagonists in experimental models of
in vivo and in vitro lung injury. Tissue Barriers.
13(2438974)2025.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Kubra KT, Akhter MS, Apperley K and
Barabutis N: Growth hormone-releasing hormone antagonist JV-1-36
suppresses reactive oxygen species generation in A549 lung cancer
cells. Endocrines. 3:813–820. 2022.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Granata R, Leone S, Zhang X, Gesmundo I,
Steenblock C, Cai R, Sha W, Ghigo E, Hare JM, Bornstein SR and
Schally AV: Growth hormone-releasing hormone and its analogues in
health and disease. Nat Rev Endocrinol. 21:180–195. 2025.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Akhter MS and Barabutis N: Suppression of
reactive oxygen species in endothelial cells by an antagonist of
growth hormone-releasing hormone. J Biochem Mol Toxicol.
35(e22879)2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Populo H, Nunes B, Sampaio C, Batista R,
Pinto MT, Gaspar TB, Miranda-Alves L, Cai RZ, Zhang XY, Schally AV,
et al: Inhibitory effects of antagonists of growth
hormone-releasing hormone (GHRH) in thyroid cancer. Horm Cancer.
8:314–324. 2017.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Rick FG, Schally AV, Szalontay L, Block
NL, Szepeshazi K, Nadji M, Zarandi M, Hohla F, Buchholz S and Seitz
S: Antagonists of growth hormone-releasing hormone inhibit growth
of androgen-independent prostate cancer through inactivation of ERK
and Akt kinases. Proc Natl Acad Sci USA. 109:1655–1660.
2012.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Perez R, Schally AV, Popovics P, Cai R,
Sha W, Rincon R and Rick FG: Antagonistic analogs of growth
hormone-releasing hormone increase the efficacy of treatment of
triple negative breast cancer in nude mice with doxorubicin; A
preclinical study. Oncoscience. 1:665–673. 2014.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Recinella L, Chiavaroli A, Veschi S, Di
Valerio V, Lattanzio R, Orlando G, Ferrante C, Gesmundo I, Granata
R, Cai R, et al: Antagonist of growth hormone-releasing hormone
MIA-690 attenuates the progression and inhibits growth of
colorectal cancer in mice. Biomed Pharmacother.
146(112554)2022.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Kineman RD: Antitumorigenic actions of
growth hormone-releasing hormone antagonists. Proc Natl Acad Sci
USA. 97:532–534. 2000.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Letsch M, Schally AV, Busto R, Bajo AM and
Varga JL: Growth hormone-releasing hormone (GHRH) antagonists
inhibit the proliferation of androgen-dependent and -independent
prostate cancers. Proc Natl Acad Sci USA. 100:1250–1255.
2003.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Gao FY, Li XT, Xu K, Wang RT and Guan XX:
c-MYC mediates the crosstalk between breast cancer cells and tumor
microenvironment. Cell Commun Signal. 21(28)2023.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Wu HM, Schally AV, Cheng JC, Zarandi M,
Varga J and Leung PC: Growth hormone-releasing hormone antagonist
induces apoptosis of human endometrial cancer cells through
PKCδ-mediated activation of p53/p21. Cancer Lett. 298:16–25.
2010.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Chu WK, Law KS, Chan SO, Yam JC, Chen LJ,
Zhang H, Cheung HS, Block NL, Schally AV and Pang CP: Antagonists
of growth hormone-releasing hormone receptor induce apoptosis
specifically in retinoblastoma cells. Proc Natl Acad Sci USA.
113:14396–14401. 2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Buchholz S, Schally AV, Engel JB, Hohla F,
Heinrich E, Koester F, Varga JL and Halmos G: Potentiation of
mammary cancer inhibition by combination of antagonists of growth
hormone-releasing hormone with docetaxel. Proc Natl Acad Sci USA.
104:1943–1946. 2007.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Siejka A, Barabutis N and Schally AV: GHRH
antagonist inhibits focal adhesion kinase (FAK) and decreases
expression of vascular endothelial growth factor (VEGF) in human
lung cancer cells in vitro. Peptides. 37:63–68. 2012.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Rick FG, Seitz S, Schally AV, Szalontay L,
Krishan A, Datz C, Stadlmayr A, Buchholz S, Block NL and Hohla F:
GHRH antagonist when combined with cytotoxic agents induces S-phase
arrest and additive growth inhibition of human colon cancer. Cell
Cycle. 11:4203–4210. 2012.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Yeste J, Illa X, Alvarez M and Villa R:
Engineering and monitoring cellular barrier models. J Biol Eng.
12(18)2018.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Su Y, Lucas R, Fulton DJR and Verin AD:
Mechanisms of pulmonary endothelial barrier dysfunction in acute
lung injury and acute respiratory distress syndrome. Chin Med J
Pulm Crit Care Med. 2:80–87. 2024.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Sapru A, Flori H, Quasney MW and Dahmer
MK: Pediatric Acute Lung Injury Consensus Conference Group.
Pathobiology of acute respiratory distress syndrome. Pediatr Crit
Care Med. 16 (5 Suppl 1):S6–S22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Uddin MA, Akhter MS, Singh SS, Kubra KT,
Schally AV, Jois S and Barabutis N: GHRH antagonists support lung
endothelial barrier function. Tissue Barriers.
7(1669989)2019.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Granato G, Gesmundo I, Pedrolli F, Kasarla
R, Begani L, Banfi D, Bruno S, Lopatina T, Brizzi MF, Cai R, et al:
Growth hormone-releasing hormone antagonist MIA-602 inhibits
inflammation induced by SARS-CoV-2 spike protein and bacterial
lipopolysaccharide synergism in macrophages and human peripheral
blood mononuclear cells. Front Immunol. 14(1231363)2023.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Akhter MS, Kubra KT and Barabutis N:
Protective effects of GHRH antagonists against hydrogen
peroxide-induced lung endothelial barrier disruption. Endocrine.
79:587–592. 2023.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Wilson MS and Wynn TA: Pulmonary fibrosis:
Pathogenesis, etiology and regulation. Mucosal Immunol. 2:103–121.
2009.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Midgley AC, Rogers M, Hallett MB, Clayton
A, Bowen T, Phillips AO and Steadman R: Transforming growth
factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast
differentiation is mediated by hyaluronan (HA)-facilitated
epidermal growth factor receptor (EGFR) and CD44 co-localization in
lipid rafts. J Biol Chem. 288:14824–14838. 2013.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Zhang C, Cai R, Lazerson A, Delcroix G,
Wangpaichitr M, Mirsaeidi M, Griswold AJ, Schally AV and Jackson
RM: Growth hormone-releasing hormone receptor antagonist modulates
lung inflammation and fibrosis due to bleomycin. Lung. 197:541–549.
2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Gesmundo I, Pedrolli F, Vitale N, Bertoldo
A, Orlando G, Banfi D, Granato G, Kasarla R, Balzola F, Deaglio S,
et al: Antagonist of growth hormone-releasing hormone potentiates
the antitumor effect of pemetrexed and cisplatin in pleural
mesothelioma. Int J Mol Sci. 23(11248)2022.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Condor Capcha JM, Robleto E, Saad AG, Cui
T, Wong A, Villano J, Zhong W, Pekosz A, Medina E, Cai R, et al:
Growth hormone-releasing hormone receptor antagonist MIA-602
attenuates cardiopulmonary injury induced by BSL-2 rVSV-SARS-CoV-2
in hACE2 mice. Proc Natl Acad Sci USA.
120(e2308342120)2023.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Dotiwala AK, McCausland C and Samra NS:
Anatomy, Head and Neck: Blood Brain Barrier. In: StatPearls
[Internet]. StatPearls Publishing, Treasure Island, FL, 2025.
|
|
112
|
Takata F, Nakagawa S, Matsumoto J and
Dohgu S: Blood-brain barrier dysfunction amplifies the development
of neuroinflammation: Understanding of cellular events in brain
microvascular endothelial cells for prevention and treatment of BBB
dysfunction. Front Cell Neurosci. 15(661838)2021.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Galea I: The blood-brain barrier in
systemic infection and inflammation. Cell Mol Immunol.
18:2489–2501. 2021.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Jaeger LB, Banks WA, Varga JL and Schally
AV: Antagonists of growth hormone-releasing hormone cross the
blood-brain barrier: A potential applicability to treatment of
brain tumors. Proc Natl Acad Sci USA. 102:12495–12500.
2005.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Barabutis N: Insights on supporting the
aging brain microvascular endothelium. Aging Brain.
1(100009)2021.PubMed/NCBI View Article : Google Scholar
|
|
116
|
O'Toole TJ and Sharma S: Physiology,
Somatostatin. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2025.
|
|
117
|
Reubi JC and Schonbrunn A: Illuminating
somatostatin analog action at neuroendocrine tumor receptors.
Trends Pharmacol Sci. 34:676–688. 2013.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Shahid Z, Asuka E and Singh G: Physiology,
Hypothalamus. In: StatPearls [Internet]. StatPearls Publishing,
Treasure Island, FL, 2025.
|
|
119
|
Olarescu NC, Gunawardane K, Hanson TK,
Møller N and Jørgensen JOL: Normal Physiology of Growth Hormone in
Normal Adults. In: Endotext [Internet]. Feingold KR, Ahmed SF,
Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder
WW, Dhatariya K, Dungan K, et al (eds). MDText.com, Inc.,
South Dartmouth, MA, 2000.
|
|
120
|
Adigun OO, Nguyen M, Fox TJ and
Anastasopoulou C: Acromegaly. In: StatPearls [Internet]. StatPearls
Publishing, Treasure Island, FL, 2023.
|
|
121
|
Liu W, Xie L, He M, Shen M, Zhu J, Yang Y,
Wang M, Hu J, Ye H, Li Y, et al: Expression of somatostatin
receptor 2 in somatotropinoma correlated with the short-term
efficacy of somatostatin analogues. Int J Endocrinol.
2017(9606985)2017.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Debnath D and Cheriyath P: Octreotide. In:
StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL,
2023.
|
|
123
|
Roelfsema F, Biermasz NR, Pereira AM and
Romijn JA: Therapeutic options in the management of acromegaly:
Focus on lanreotide Autogel. Biologics. 2:463–479. 2008.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Velija-Asimi Z: The efficacy of octreotide
LAR in acromegalic patients as primary or secondary therapy. Ther
Adv Endocrinol Metab. 3:3–9. 2012.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Giustina A, Mazziotti G, Torri V, Spinello
M, Floriani I and Melmed S: Meta-analysis on the effects of
octreotide on tumor mass in acromegaly. PLoS One.
7(e36411)2012.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Ferrante E, Pellegrini C, Bondioni S,
Peverelli E, Locatelli M, Gelmini P, Luciani P, Peri A, Mantovani
G, Bosari S, et al: Octreotide promotes apoptosis in human
somatotroph tumor cells by activating somatostatin receptor type 2.
Endocr Relat Cancer. 13:955–962. 2006.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Fakir S and Barabutis N: Involvement of
ATF6 in octreotide-induced endothelial barrier enhancement.
Pharmaceuticals (Basel). 17(1604)2024.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Fakir S, Sigdel M, Sarker MMR, Folahan JT
and Barabutis N: Ceapin-A7 suppresses the protective effects of
Octreotide in human and bovine lung endothelial cells. Cell Stress
Chaperones. 30:1–8. 2025.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Zeng Z, Liao Q, Gan S, Li X, Xiong T, Xu
L, Li D, Jiang Y, Chen J, Ye R, et al: Structural insights into the
binding modes of lanreotide and pasireotide with somatostatin
receptor 1. Acta Pharm Sin B. 15:2468–2479. 2025.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Li ZQ, Quan Z, Tian HL and Cheng M:
Preoperative lanreotide treatment improves outcome in patients with
acromegaly resulting from invasive pituitary macroadenoma. J Int
Med Res. 40:517–524. 2012.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Caron PJ, Bevan JS, Petersenn S, Houchard
A, Sert C and Webb SM: PRIMARYS Investigators Group. Effects of
lanreotide Autogel primary therapy on symptoms and quality-of-life
in acromegaly: data from the PRIMARYS study. Pituitary. 19:149–157.
2016.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Carmichael JD: Lanreotide depot deep
subcutaneous injection: A new method of delivery and its associated
benefits. Patient Prefer Adherence. 6:73–82. 2012.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Gross CM, Kellner M, Wang T, Lu Q, Sun X,
Zemskov EA, Noonepalle S, Kangath A, Kumar S, Gonzalez-Garay M, et
al: LPS-induced Acute Lung Injury Involves NF-ĸB-mediated
Downregulation of SOX18. Am J Respir Cell Mol Biol. 58:614–624.
2018.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Sarker MMR, Fakir S, Kubra KT, Sigdel M,
Siejka A, Stepien H and Barabutis N: Lanreotide protects against
LPS-induced inflammation in endothelial cells and mouse lungs.
Tissue Barriers: Apr 17, 2025 (Epub ahead of print).
|
|
135
|
Bolanowski M, Kałużny M, Witek P and
Jawiarczyk-Przybyłowska A: Pasireotide-a novel somatostatin
receptor ligand after 20 years of use. Rev Endocr Metab Disord.
23:601–620. 2022.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Zatelli MC, Piccin D, Vignali C, Tagliati
F, Ambrosio MR, Bondanelli M, Cimino V, Bianchi A, Schmid HA,
Scanarini M, et al: Pasireotide, a multiple somatostatin receptor
subtypes ligand, reduces cell viability in non-functioning
pituitary adenomas by inhibiting vascular endothelial growth factor
secretion. Endocr Relat Cancer. 14:91–102. 2007.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Olarescu NC, Jørgensen AP, Atai S,
Wiedmann MKH, Dahlberg D, Bollerslev J and Heck A: Pasireotide as
first line medical therapy for selected patients with acromegaly.
Pituitary. 28(48)2025.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Cuevas-Ramos D and Fleseriu M:
Pasireotide: A novel treatment for patients with acromegaly. Drug
Des Devel Ther. 10:227–239. 2016.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Witek P, Bolanowski M, Krętowski A and
Głowińska A: Pasireotide-induced hyperglycemia in Cushing's disease
and Acromegaly: A clinical perspective and algorithms proposal.
Front Endocrinol (Lausanne). 15(1455465)2024.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Chinezu L, Gliga MC, Borz MB, Gliga C and
Pascanu IM: Clinical implications of molecular and genetic
biomarkers in Cushing's disease: A literature review. J Clin Med.
14(3000)2025.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Fakir S, Sarker MMR, Sigdel M and
Barabutis N: Protective effects of pasireotide in LPS-induced acute
lung injury. Pharmaceuticals (Basel). 18(942)2025.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Johnsson M, Pedroncelli AM, Hansson A and
Tiberg F: Pharmacokinetics and pharmacodynamics of a pasireotide
subcutaneous depot (CAM4071) and comparison with immediate and
long-acting release pasireotide. Endocrine. 84:1125–1134.
2024.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Gomes-Porras M, Cárdenas-Salas J and
Álvarez-Escolá C: Somatostatin analogs in clinical practice: A
review. Int J Mol Sci. 21(1682)2020.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Aversa LS, Cuboni D, Grottoli S, Ghigo E
and Gasco V: A 2024 update on growth hormone deficiency syndrome in
adults: From guidelines to real life. J Clin Med.
13(6079)2024.PubMed/NCBI View Article : Google Scholar
|