1. Introduction
Non-invasive fluid biopsies in in vitro
fertilization (IVF) aim to address several critical clinical gaps
that limit the efficacy and safety of current reproductive
diagnostic methods (1,2). Traditional invasive techniques, such as
embryo biopsy for genetic testing, carry risks of damaging embryos
and causing patient discomfort, and are typically limited to
single-time sampling, which restricts the ability to monitor
dynamic changes throughout assisted reproductive technology (ART)
cycles (3,4). These methods also rely heavily on
subjective morphological assessments for embryo selection, which
may not accurately predict embryo viability or genetic competence
(5,6). Additionally, invasive genetic testing
can be affected by maternal DNA contamination and require
specialized laboratory resources, rendering it less accessible and
potentially less reliable (7,8). There
is also a lack of standardized protocols, leading to variability in
results (9,10), and valuable biological materials such
as granulosa and cumulus cells are often discarded, despite
containing crucial molecular information (11). By enabling the repeated, low-risk
sampling of accessible body fluids and providing objective
molecular biomarkers, non-invasive fluid biopsies provide a
promising solution to improve embryo selection, enhance genetic
testing accuracy, and support more personalized and sustainable
reproductive therapies in IVF (10).
Current invasive methods in IVF, such as embryo
biopsy for preimplantation genetic testing (PGT), present a number
of limitations and risks (12).
These procedures can potentially harm the embryo, reduce its
viability and lead to lower implantation and pregnancy rates
(13). Additionally, invasive
sampling is uncomfortable for patients and is typically restricted
to a single time point, which limits the ability to monitor dynamic
changes throughout the ART cycle. The reliance on subjective
morphological assessments for embryo selection further compounds
the issue, as these visual evaluations may not accurately reflect
the embryo's genetic competence or developmental potential
(14). Invasive genetic testing also
faces challenges, such as maternal DNA contamination and the need
for specialized laboratory resources, rendering it less accessible
and at times, less reliable (7).
Non-invasive fluid biopsy alternatives, by contrast, enable the
repeated, low-risk sampling of accessible body fluids, such as
follicular fluid, embryo culture media and uterine secretions
(9,15). These approaches provide objective
molecular biomarkers, such as cell-free nucleic acids,
extracellular vesicles and microRNAs (miRNAs/miRs), that can more
accurately predict embryo quality and viability (10,16). By
minimizing harm to embryos and patients, and providing more
reliable, accessible and dynamic diagnostic information,
non-invasive fluid biopsies have the potential to overcome the
major limitations of current invasive methods and revolutionize
fertility diagnostics and therapeutic decision-making in IVF
(17,18).
The present review summarizes recent findings on key
biomolecular components found in accessible body fluids, such as
follicular fluid, embryo culture medium, uterine secretions and
saliva, namely cell-free nucleic acids, extracellular vesicles and
miRNAs.
2. About non-invasive fluid biopsy samples
and related molecules
Non-invasive fluid biopsies play a crucial role in
medical diagnosis and monitoring, providing advantages, such as the
ease of collection, reduced patient discomfort and the potential
for repeated sampling. Various body fluids, including urine,
saliva, interstitial fluid and nasal secretions, have been explored
for their biomarker content to aid in the non-invasive detection of
various diseases (19-21).
These biomarkers include circulating tumor cells and trophoblastic
cells, as well as more numerous, cell-free nucleic acids (cfNAs)
such cell-free DNA (cfDNA), cell-free RNA (cfRNA) and circulating
miRNAs. Furthermore, cfNAs not only circulate in isolation, but can
also associate with protective protein complexes or be encapsulated
in extracellular vesicles (EVs) (22).
cfNAs, including cfDNA and cfRNA originate from
cultured cells, non-malignant somatic tissues, tumors, embryos, or
fetuses and are released when cells undergo necrosis or apoptosis
(23). cfNAs can be characterized by
their length, physical size, surface molecules, electrical charge
and density. cfDNA can also be detected in the blastocellular fluid
of human embryos and the IVF culture medium used, allowing minimal
and non-invasive genetic testing, respectively (24). In fact, the presence of mitochondrial
DNA (mtDNA) in the culture medium of the embryo has been associated
with embryo lysis caused by apoptosis or necrosis. Stigliani et
al (25) found a strong
association between mtDNA levels in the culture medium and human
embryo fragmentation, which suggests that higher mtDNA
concentrations indicate cellular distress and apoptosis in embryos.
Furthermore, another study highlighted that the amount of DNA
present in the culture medium is negatively associated with the
competence of human embryos and clinical pregnancy outcomes,
implying that the release of mtDNA may reflect the underlying
embryonic competence (26).
3. Extracellular vesicles and their usage as
non-invasive fluid samples
EVs are crucial mediators of intercellular
communication, facilitating the exchange of biological signals
among both prokaryotic and eukaryotic cells. These vesicles, which
include exosomes, microvesicles and apoptotic bodies, vary in
composition and size, ranging from 30 to 1,000 nm, and contain a
variety of proteins, lipids and nucleic acids (27). These vesicles function as carriers of
biomarkers and play essential roles in tissue regeneration,
rendering them potential targets for therapeutic interventions in
various diseases (28).
The implantation of the embryo is a critical step
for a successful pregnancy and requires a complex interaction
between the embryo and the endometrium. In a previous study
investigating the involvement of EVs in embryo implantation and
endometrial diseases, EVs were isolated from uterine fluid,
cultured endometrial, epithelial/stromal and trophectodermal cells
(2s). Endometrial, epithelial and stromal/decidual cell-derived EVs
are covered by trophoblast cells, which regulate several gene
sequences involved in adhesion, invasion and migration. Conversely,
embryo-derived EVs are internalized by epithelial and immune cells
of the endometrium for the biosensing and immunomodulation required
for successful implantation. EVs have also been shown to play a
role in infertility, recurrent implantation failure, endometriosis,
endometritis and endometrial cancer (29). Further research will pave the way for
the use of EVs as non-invasive ‘liquid biopsy’ tools for assessing
endometrial health.
Ng et al (30)
analyzed a panel of 227 endometrial exosomal miRNAs and
demonstrated that a number of their target genes regulate key
pathways involved in implantation. Exosomal miRNAs not only
regulate key pathways, such as the VEGF pathway, Toll-like receptor
pathway and Jak-STAT pathway, but also adhere to extracellular
matrix-receptor interactions and junctions. Vilella et al
(31) demonstrated that hsa-miR-30d
derived from the endometrial exosome, when taken up by
trophoblasts, increased the gene expression of integrin subunit
alpha 7, integrin subunit beta-3 and cadherin 5 required for
blastocyst implantation. Greening et al (32) further demonstrated that endometrial
EVs increased their adhesiveness through the focal adhesion kinase
signaling pathway when internalized by the trophectoderm.
4. Non-invasive fluid samples and
preimplantation genetic testing
The use of PGT has expanded significantly in recent
years, driven by advances made in genetic testing technologies and
an increased understanding of genetic disorders. Major reproductive
societies endorse PGT for various indications, including aneuploidy
screening, HLA matching and the prevention of genetic diseases
(33). The most common indication
for PGT remains aneuploidy screening, particularly for couples with
an advanced maternal age or those experiencing recurrent
implantation failure (34). Despite
its benefits, traditional PGT methods continue to face challenges,
including the potential for damage to embryos and the need for
specialized laboratory resources (1). While non-invasive PGT provides the
potential to reduce embryo harm and patient risk, its current
limitations, particularly maternal contamination, a low cfDNA
yield, diagnostic variability, the lack of standardization and
biological uncertainties, need to be critically addressed through
further research and protocol optimization before it can reliably
replace invasive methods in clinical IVF practice (2-4,35).
Kuznyetsov et al (7) demonstrated that combining spent embryo
culture media and blastocoel fluid enhanced the quantity and
quality of cfDNA available for aneuploidy testing, potentially
improving the accuracy of genetic assessments. This approach
minimizes the risks associated with invasive biopsies, while still
providing valuable genetic information.
Moreover, Brouillet et al (8) conducted a systematic review, indicating
that cfDNA in spent embryo culture medium could serve as a viable
alternative to embryo biopsy for PGT, highlighting its potential to
streamline the testing process and reduce costs. The analysis of
EVs and miRNAs in non-invasive samples has also shown that they
hold promise as additional biomarkers for embryo health and
viability (36).
The integration of non-invasive testing could lead
to higher implantation rates, reduced miscarriage rates and
improved overall outcomes for couples undergoing IVF. Furthermore,
advancements in next-generation sequencing and bioinformatics are
expected to enhance the accuracy and reliability of non-invasive
genetic testing methods, rendering them more accessible to a
broader range of patients (33).
Follicular fluid
Embryo selection procedures in IVF aim to identify
high-quality embryos with the highest implantation potential. cfDNA
of apoptotic granulosa cells is present in follicular fluid, which
influences follicle maturation and oocyte growth in vivo.
This biomarker can be used to sample the developmental competence
of the contained oocyte during the oocyte retrieval phase of IVF
treatment. Low levels of cfDNA in follicular fluid are
significantly associated with a low embryo fragmentation rate and
are indicative of high-quality embryos (37). Furthermore, extracellular mtDNA is
actively released by granulosa cells into the follicular fluid in
response to mitotic failure, and a low mtDNA content is associated
with high oocyte developmental ability, and this allows for the
determination of the viability of the embryo (38).
Cumulus-oocyte complex (COC) and
granulosa cells
The COC is involved in several key processes,
including oocyte growth, metabolic regulation and cellular adhesion
to the oocyte membrane. It also facilitates intercellular
communication between the cumulus cells and the oocyte, which is
essential for oocyte maturation and successful fertilization.
Additionally, the COC is necessary for the expansion of the cumulus
oophorous and plays a role in the spatial distribution of granulosa
cells within the follicle (39). The
complex also regulates gap junctions and cytoskeletal changes and
influences polar body displacement after treatments to remove
cumulus-corona cells in preparation for assisted reproductive
methods (40). The COC is a dynamic
structure that undergoes changes during in vitro culture,
influenced by various factors, such as biological (e.g.,
age-related) and external (e.g., co-enzyme Q10 supplementation)
conditions (41).
In cases of mitochondrial malfunction, it has been
shown that cumulus cells surrounding the oocyte during development
can increase the levels of cf-mtDNA in IVF culture media (42). Researchers are investigating the
effects of mitochondrial dysfunction to predict the developmental
competence and implantation potential of embryos, as well as to
better understand embryo quality (43-45).
Since the expression of specific genes in cumulus cells is linked
to embryo potential and pregnancy outcomes, cumulus cell gene
expression is a reliable indicator of oocyte quality (24).
Granulosa cells are involved in various
ovary-related diseases, including polycystic ovary syndrome
(46). The interaction between
granulosa cells and theca cells is crucial for early progesterone
synthesis (47). Granulosa cells
express specific biomarkers and receptors, such as aromatase and
thyroid hormone receptors, highlighting their functional importance
(48). Despite their physiological
importance, granulosa cells and associated structures, such as the
corona cumulus, are often discarded as waste during IVF treatments.
However, their potential alternative applications or effects as
waste have not been extensively investigated. Culture media
enriched with various components used in IVF treatments support the
growth and maturation of these cells and the oocyte (49). Further research into the potential
use or analysis of these excreted components could enhance the
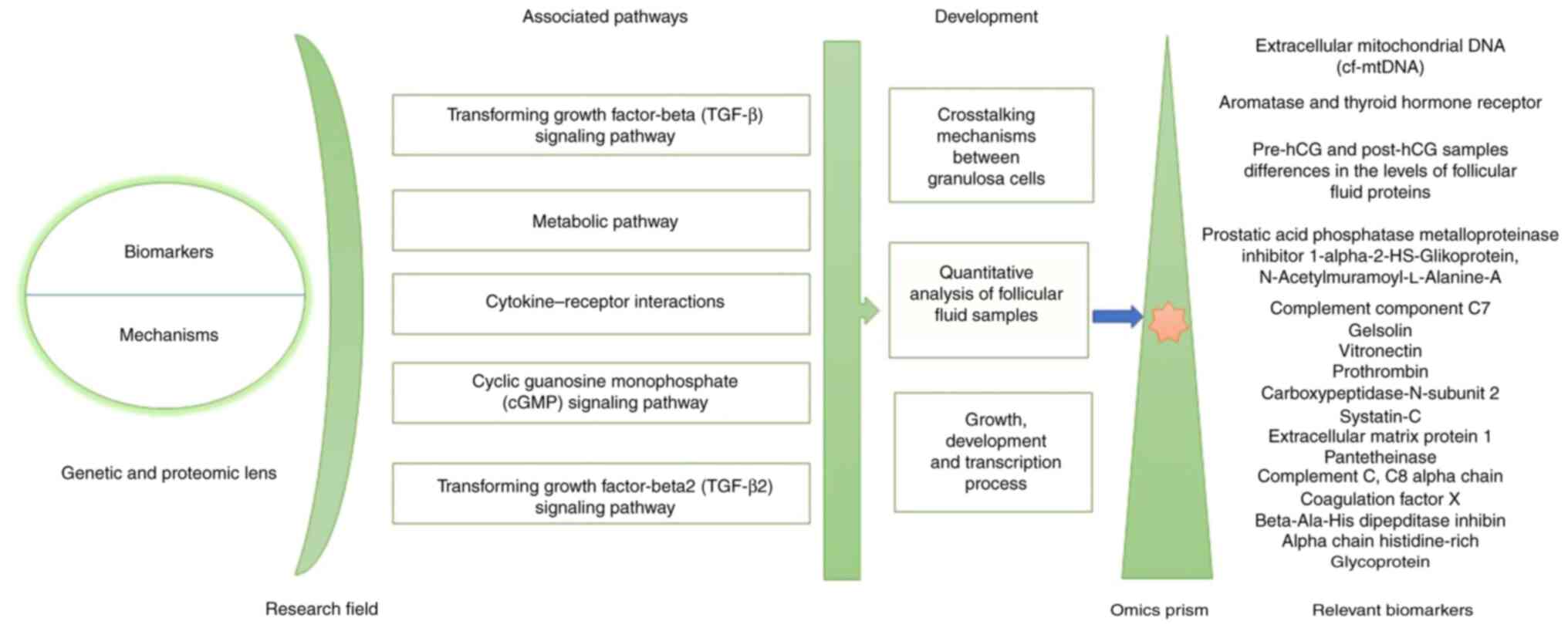
understanding of reproductive biology. A summary of genetic,
epigenetic and proteomic influences on granulosa cells in
follicular fluid and their associated pathways according to
developmental mechanisms and transcription process with relevant
biomarkers is provided below and is illustrated in Fig. 1.
Genetic research on granulosa cells in
follicular fluid
The transcription information from granulosa cells,
which are the somatic cells most adjacent to the oocyte, may mirror
the developmental competence of the associated oocyte. Therefore,
comparing the follicular fluid microenvironment and granulosa cell
gene expression, particularly in patients with a poor ovarian
response treated with various ovarian stimulation methods is
critical for understanding the effects of different treatments on
follicular physiology (5).
At present, exogenous gonadotropin (Gn) is used as a
required drug for ovarian stimulation in ART by affecting the way
granulosa cells, follicular fluid and oocytes interact, which in
turn promotes follicular growth. In the study by Liu et al
(50), the transcriptome of the
granulosa cell was analyzed to determine whether Gn stimulation may
potentially cause meiotic mistakes in human oocytes following
natural and Gn stimulation cycles.
Proteomic research on granulosa cells
in follicular fluid
To gain a better understanding of the
intrafollicular environment during oocyte maturation, Zamah et
al (51) performed a proteomic
analysis of follicular fluid from anonymous oocyte donors
undergoing IVF oocyte retrieval. As a result, a total of 742
follicular fluid proteins were identified in healthy ovum donors,
which included 413 previously unnotified ones. These mentioned
proteins belong to various functional groups, such as insulin
growth factors and its binding protein families, immunity, growth
factors, anti-apoptotic proteins, receptor signaling and matrix
metalloprotease-associated proteins. Moreover, a quantitative
analysis of follicular fluid samples among the women with matched
ages and between the pre-hCG and post-hCG samples indicated the
vital differences in the levels of 17 follicular fluid proteins,
which play a role in inflammation, cell adhesion and protease
inhibition processes (Fig. 1)
(51).
The study by Al-Saleh et al (52) examined the differences in hormone and
cytokine levels between the follicular fluids of the mild ovarian
stimulation and conventional groups. As a result, the follicle
stimulating hormone, prolactin and progesterone levels in the
follicular fluid were significantly lower in the mild ovarian
stimulation group compared to the conventional one (52). Furthermore, the cytokine
concentrations in the mild group were significantly higher in
TGF-β2 and lower in growth differentiation factor-9 (GDF-9), while
the bone morphogenic protein-15 (BMP-15) levels were comparable
(Fig. 1) (33).
Oxidative stress research on granulosa
cells in follicular fluid
Reactive oxygen species (ROS), such as superoxide
anion, hydroxyl radicals and hydrogen peroxide, are produced during
mitochondrial electron transport for energy production, and are
essential for controlling follicular growth, oocyte maturation,
ovulation, fertilization, embryo implantation and fetal development
(53). A surge in luteinizing
hormone and neovascularization within the follicle during ovulation
provides essential stimulation for follicular rupture and oocyte
maturation, which in response stimulates the production of ROS
(54).
When there is an imbalance between oxidation and the
antioxidant system, ROS can oxidatively damage DNA, proteins and
lipids either directly or indirectly and it can lead to gene
mutations, protein denaturation and lipid peroxidation (55). Al-Saleh et al (52) indicated that oxidative stress
biomarkers, including 8-oxo-2'-deoxyguanosine, total antioxidant
capacity and malondialdehyde may be useful tools for assessing
clinical features in patients undergoing IVF due to their
association with reproductive hormones and pregnancy outcomes in
ART. As aforementioned, follicular development and oocyte
maturation are strongly influenced by the interaction between the
oxidative, antioxidative system and metabolic products within a
follicular fluid. Furthermore, this fluid is appropriate for
determining the levels of oxidative stress in the follicular milieu
and, as a result, the developmental potential of oocytes as it is
simple and non-invasive to acquire (56). The complex role of oxidative stress,
which may have undetermined advantageous functions in some
circumstances, may help to elucidate the mechanisms through which
oxidative stress can improve oocyte quality and result in normal
fertility if the level of ROS in the cell is at an appropriate
level (57).
6. Research on the cumulus-oocyte complex in
follicular fluid
Genetic research on the COC in
follicular fluid
Cumulus cells (CCs) from follicles with varying
diameters exhibit variations in gene expression related to
metabolic processes, cell differentiation, and adhesion, according
to one study's genetic ontology analysis. Additionally, research
has discovered genetic biomarkers expressed in CCs that can predict
the developmental competence of oocytes and provide a non-invasive
manner to evaluate the quality of oocytes (58). Gene expression in CCs has been linked
to embryonic development and pregnancy, suggesting a fundamental
role of cumulus cell pathways (PAP1, RAS and ErbB pathways) in
these processes (59).
In the study conducted by Su et al (60), oocytes were shown to regulate
metabolic activities in CCs by upregulating the expression of
certain genes that encode amino acid transporters and enzymes
essential for oocyte-deficient metabolic processes. CC-associated
genes, such as hyaluronan synthase homolog,
prostaglandin-endoperoxide synthase 2 (Ptgs2) and TNF-stimulated
gene 6 (Tnfsg6) have been shown to be abundant in CCs, which has
implications for cumulus growth and subsequent embryonic
development (61). Ligand-encoding
genes with specific expression in oocytes or CC have been linked to
biological functions, possibly linked to the coordinated formation
of transzonal projections from CC that reach the oocyte membrane
(62). The expression of
expansion-associated genes, such as Ptgs2 or cyclooxygenase-2,
TNF-alpha-induced protein 6 (Tnfaip6) and hyaluronan synthase 2
(Has2) in CCs is essential for the synthesis and stabilization of
the extracellular matrix by CCs, and is crucial for cumulus
expansion and ovulation (Tables I
and II) (61). Furthermore, the downregulation of the
Wnt signaling pathway in dysmature CCs was identified as a marker
for assessing oocyte quality, demonstrating the importance of gene
expression in CC as an indicator of embryonic development (Tables I and II) (63).
 | Table IOverview of key non-invasive
biomarkers relevant to IVF, listing their biological sources and
summarizing their clinical implications. |
Table I
Overview of key non-invasive
biomarkers relevant to IVF, listing their biological sources and
summarizing their clinical implications.
| Biomarker | Source | Clinical
implications |
|---|
| Cell-free DNA
(cfDNA) | Follicular fluid,
embryo culture media | Indicates oocyte
and embryo quality, apoptosis, and developmental competence;
non-invasive genetic testing; lower levels linked to higher
pregnancy rates. |
| Cell-free
mitochondrial DNA (cf-mtDNA) | Follicular fluid,
embryo culture media | Associated with
cellular distress, apoptosis, and embryo fragmentation; lower
levels linked to higher embryo viability. |
| Extracellular
vesicles (EVs) | Uterine fluid,
embryo culture media, endometrial secretions | Mediate
intercellular communication; contain proteins, lipids, and nucleic
acids; biomarkers for implantation, endometrial health, and embryo
viability. |
| MicroRNAs (miRNAs,
sncRNAs) | Follicular fluid,
embryo culture media, endometrial exosomes | Regulate gene
expression related to implantation and embryo development;
non-invasive markers for embryo health and implantation
potential. |
| Proteins (PTX3,
TNFAIP6, BMP15, GDF9, MMPs, VEGF, FSH, LH, hCG) | Follicular fluid,
cumulus cells, embryo culture media | Involved in cumulus
expansion, oocyte maturation, embryo development, and ovarian
response; markers for oocyte and embryo quality. |
| Oxidative stress
markers (MDA, 8-OHdG, AOPP, TAC, SOD, GPx, GST, thiol groups) | Follicular fluid,
cumulus cells | Reflect oxidative
stress status; associated with reproductive hormones, embryo
quality, and IVF outcomes. |
| Metabolites
(glucose, lactate, glycerophosphocholine, androsterone sulfate,
elaidic carnitine) | Follicular fluid,
embryo culture media | Indicate metabolic
activity, ovarian response, and predict clinical outcomes
(pregnancy, miscarriage, live birth rates). |
| Gene expression
profiles | Granulosa cells,
cumulus cells, uterine fluid | Predict oocyte and
embryo quality, endometrial receptivity, and pregnancy outcomes;
used for personalized embryo selection. |
| Immunologic
markers | Follicular fluid,
blood, endometrial tissue | Indicate immune
status, inflammation, and endometrial receptivity; potential
predictors of implantation success. |
| Antioxidant enzymes
(glutathione peroxidase, catalase, SOD) | Follicular fluid,
cumulus cells | High levels are
linked to better oocyte and embryo quality; protect against
oxidative damage. |
| Time-lapse
Imaging/morphokinetic parameters | Embryo culture
media (image analysis) | Non-invasive
assessment of embryo developmental kinetics and morphology;
associated with live birth rates. |
| Transcriptomic
biomarkers | Uterine fluid,
endometrial secretions | Predict endometrial
receptivity and window of implantation; guide timing of embryo
transfer. |
| Seminal plasma
biomarkers (cfDNA, proteins, metabolites) | Semen | Non-invasive
assessment of male fertility, sperm retrieval potential, and ART
outcomes. |
 | Table IICumulus-oocyte complex in genetics,
proteomics and oxidative stress research. |
Table II
Cumulus-oocyte complex in genetics,
proteomics and oxidative stress research.
| A, Genetic research
on the COC in follicular fluid |
|---|
| Key genes | Functional
roles | (Refs.) |
|---|
| Hyaluronan synthase
homolog prostaglandin-endoperoxide synthase 2, TNF-stimulated gene
6 (Hsh, Ptgs2 and Tnfsg6) | Genes affecting
cumulus growth and subsequent embryonic development | (61,63) |
| Prostaglandin
synthase-2 or cyclooxygenase-2, tumor necrosis factor alpha-induced
protein and hyaluronan synthase 2 (Ptgs2, Tnfaip6 and Has2) | Cumulus expansion
and ovulation | (61) |
| Hyaluronan synthase
2, prostaglandin synthase-2 or cyclooxygenase-2, and gremlin1
(Has2, Ptgs2 and Grem1) | Oocyte and embryo
quality | (6) |
| B, Proteomic
research on the COC in follicular fluid |
| Structural
proteins | Roles in the
cumulus matrix | (Refs.) |
| Pentraxin 3, tumor
necrosis factor-induced protein 6, bone morphogenetic protein 15
and growth differentiation factor 9 (Ptx3, Tnfip6, Bmp15 and
Gdf9) | Organization and
function of the cumulus matrix | (65) |
| PTX3 and
TNFIP6 | Key components of
the cumulus matrix required for cumulus expansion and in
vivo fertilization | (65) |
| Inter-Α-trypsin
inhibitor (Iαi or ITI), tumor necrosis factor-induced protein-6
(TSG6 Or TNFIP6) and pentraxin 3 (PTX3 Or TSG14) | Proteins required
for the formation and stability of the COC matrix | (66) |
| Protein levels of
phospho-H2AX, breast cancer susceptibility gene 1 (BRCA1),
ataxia-telangiectasia mutated (ATM), meiotic recombination 11
homolog A (MRE11) and radiation repair gene (RAD51) | Significantly
increased in aging CCs, suggesting that these proteins are involved
in the aging process | (67) |
| C, Oxidative stress
research on the COC in follicular fluid |
| Oxidative stress
biomarkers | Impact for oocyte
development | (Refs.) |
| Malondialdehyde
(MDA), 8-Oxo-2'-Deoxyguanosine (8-OHdG), Advanced Oxidation Protein
Products (AOPP), Total Antioxidant Capacity (TAC), Superoxide
Dismutase (SOD), Glutathione Peroxidase (GPx), Glutathione
S-transferase (GST), Thiol Groups | Fragmented DNA,
poorer embryological development, and increased miscarriage
rates | (69) |
The expression of certain genes in CCs has been
identified as potential markers for oocyte and subsequent embryo
quality. The study by Uyar et al (6) demonstrated that the expression levels
of genes in CCs are related to oocyte maturation, fertilization and
embryo quality. In particular, the expression of Has2, Ptgs2 or
cyclooxygenase-2 and gremlin1 in CCs was shown to be associated
with oocyte and embryo quality (Tables
I and II) (6). Akino et al (63) used next-generation sequencing to
analyze gene expression in immature CCs, revealing the
downregulation of the Wnt signaling pathway as a marker for
determining oocyte quality. Furthermore, it has been demonstrated
that the expression of certain genes in CCs is associated with
embryonic developmental competence, suggesting their potential as
markers for oocyte quality (Tables I
and II) (64).
Proteomic research on the COC in
follicular fluid
Several proteins essential for the organization and
function of the cumulus matrix have been identified, including
pentraxin 3 (PTX3), TNF-induced protein 6 (TNFAIP6), BMP15 and
GDF9. Following the pre-ovulatory luteinizing hormone, these
proteins play a role in cumulus expansion, oocyte-cumulus cell
contact and cumulus cell function control (65). Furthermore, proteins secreted by CCs
have been shown to form ligand-receptor pairs that transmit
paracrine signaling between the oocyte and CCs, demonstrating the
complex communication and regulatory networks between these two
components (62). PTX3 and TNFIP6
were identified as key components of the cumulus matrix required
for cumulus expansion and IVF (Tables
I and II) (65). Inter-α-trypsin inhibitor, TNFAIP6 and
PTX3 were identified as proteins required for the formation and
stability of the COC matrix (Tables
I and II) (66). Protein levels of phospho-H2AX, breast
cancer susceptibility gene 1, ataxia-telangiectasia mutated,
meiotic recombination 11 homolog A and radiation repair gene were
previously significantly increased in aging CCs, suggesting that
these proteins are involved in the aging process (Tables I and II) (67).
Oxidative stress research on the COC
in follicular fluid
The presence of CCs during IVF has been shown to
protect the oocyte against oxidative stress, thus improving initial
division and subsequent development. The exposure of oocytes to
hydrogen peroxide has been shown to result in oocyte death and the
blockade of the first division, highlighting the protective role of
CCs against oxidative stress during fertilization (68). Moreover, oxidative stress has been
associated with fragmented DNA, poorer embryological development
and increased miscarriage rates (Table
I and II) (69).
7. Conclusion and future perspectives
The advent of non-invasive fluid biopsies has
transformed the landscape of medical diagnostics and reproductive
health, providing a more patient-friendly alternative to
traditional biopsy methods. By leveraging biomarkers, such as EVs,
circulating tumor cells and cfNAs, researchers have expanded the
potential for early disease detection, prognostic evaluation, and
personalized therapeutic strategies. These innovations have been
particularly impactful in oncology, where liquid biopsies offer a
means to detect tumor-derived genetic material and monitor
treatment responses in real time. Additionally, in reproductive
medicine, the ability to analyze cfDNA and cfRNA from embryo
culture media has opened new avenues for non-invasive PGT, reducing
the need for embryo biopsies and mitigating potential risks to
embryo viability.
Despite these advancements, several challenges
remain to be addressed before non-invasive fluid biopsies can
become routine in clinical practice. The sensitivity and
specificity of these methods vary across different conditions,
necessitating the further refinement of isolation and detection
techniques. Standardization remains a critical issue, as
differences in sample collection, processing and analytical
methodologies can lead to inconsistent results. Moreover, the low
concentration of biomarkers in some fluids, such as ctDNA in
early-stage cancer, poses a significant limitation, requiring
improvements in enrichment and amplification technologies to
enhance detection accuracy.
In reproductive health, while non-invasive methods
such as the analysis of cfDNA in embryo culture media shows
promise, their clinical utility remains under debate. The presence
of maternal contamination, the accuracy of chromosomal assessments
and the reproducibility of findings across different patient
populations warrant further investigation. Additionally, the
clinical application of EVs as biomarkers in fertility and
pregnancy-related complications is an emerging field that requires
extensive validation through large-scale studies.
Looking ahead, future research is required to focus
on integrating multi-omics approaches, combining genomics,
transcriptomics, proteomics and metabolomics, to develop more
comprehensive and reliable diagnostic tools.
In conclusion, non-invasive fluid biopsies hold
immense potential to revolutionize disease diagnosis, treatment
monitoring and reproductive medicine. While significant progress
has been made, continued advancements in technology,
standardization and clinical validation are essential for these
approaches to become fully integrated into mainstream medical
practice. With ongoing innovation, non-invasive sampling techniques
could ultimately lead to a paradigm shift in personalized medicine,
offering safer, more efficient and widely applicable diagnostic
solutions for a range of medical conditions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ÇÖ conceived the study. SAÖ, NÖK, DK and ÇÖ
performed the literature review and the analysis of data from the
literature. The manuscript was drafted by SAÖ, NÖK, DK and ÇÖ and
was critically reviewed by all authors. All authors have read and
agreed to the published version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang S, Xu B, Zhuang Y, Zhang Q, Li J and
Fu X: Current research status and clinical applications of
noninvasive preimplantation genetic testing: A review. Medicine.
10(e39964)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Volovsky M, Scott RT Jr and Seli E:
Non-invasive preimplantation genetic testing for aneuploidy: Is the
promise real? Hum Reprod. 39:1899–1908. 2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tsai NC, Chang YC, Su YR, Lin YC, Weng PL,
Cheng YH, Li YL and Lan KC: Validation of non-invasive
preimplantation genetic screening using a routine IVF laboratory
workflow. Biomedicines. 10(1386)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chow JFC, Lam KKW, Cheng HHY, Lai SF,
Yeung WSB and Ng EHY: Optimizing non-invasive preimplantation
genetic testing: Investigating culture conditions, sample
collection, and IVF treatment for improved non-invasive PGT-A
results. J Assist Reprod Genet. 41:465–472. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Adriaenssens T, Wathlet S, Segers I,
Verheyen G, De Vos A, Van Der Elst J, Coucke W, Devroey P and Smitz
J: Cumulus cell gene expression is associated with oocyte
developmental quality and influenced by patient and treatment
characteristics. Hum Reprod. 25:1259–1270. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Uyar A, Torrealday S and Seli E: Cumulus
and granulosa cell markers of oocyte and embryo quality. Fertile
Steril. 99:979–997. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kuznyetsov V, Madjunkova S, Abramov R,
Antes R, Ibarrientos Z, Motamedi G, Zaman A, Kuznyetsova I and
Librach CL: Minimally invasive cell-free human embryo aneuploidy
testing (miPGT-A) utilizing combined spent embryo culture medium
and blastocoel fluid-towards development of a clinical essay. Sci
Rep. 10(7244)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brouillet S, Martinez G, Coutton C and
Hamamah S: Is cell-free DNA in spent embryo culture medium an
alternative to embryo biopsy for preimplantation genetic testing? A
systematic review. Reprod Biomed Online. 40:779–796.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Alizadegan A, Dianat-Moghadam H, Shadman
N, Nouri M, Hamdi K, Ghasemzadeh A, Akbarzadeh M, Sarvarian P,
Mehdizadeh A, Dolati S and Yousefi M: Application of cell free DNA
in ART. Placenta. 24:18–24. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Abreu C, Thomas V, Knaggs P, Bunkheila A,
Cruz A, Teixeira SR, Alpuim P, Francis LW, Gebril A, Ibrahim A, et
al: Non-invasive molecular assessment of human embryo development
and implantation potential. Biosens Bioelectron.
157(112144)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Massoud G, Spann M, Vaught KC, Das S, Dow
M, Cochran R, Baker V, Segars J and Singh B: Biomarkers assessing
the role of cumulus cells on IVF outcomes: A systematic review. J
Assist Reprod Genet. 41:253–275. 2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Aoyama N and Kato M: Trophectoderm biopsy
for preimplantation genetic test and technical tips: A review.
Reprod Med Biol. 19:222–231. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scott Jr RS, Upham K, Forman E, Zhao T and
Treff NR: Cleavage-stage biopsy significantly impairs human
embryonic implantation potential while blastocyst biopsy does not:
A randomized and paired clinical trial. Fertil Steril. 100:624–630.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang H, DeWan AT, Desai MM and Vermund SH:
Preimplantation genetic testing for aneuploidy: Challenges in
clinical practice. Hum Genomics. 16(69)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bellassai N, Biricik A, Surdo M, Bianchi
V, D'Agata R, Breveglieri G, Gambari R, Spinella F and Spoto G:
Non-invasive preimplantation genetic testing: Cell-free DNA
detection in embryo culture media using a plasmonic biosensor. Anal
Chem. 97:19241–19248. 2025.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Battaglia R, Caponnetto A, Ferrara C,
Fazzio A, Barbagallo C, Stella M, Barbagallo D, Ragusa M, Vento ME,
Borzì P, et al: Up-regulated microRNAs in blastocoel fluid of human
implanted embryos could control circuits of pluripotency and be
related to embryo competence. J Assist Reprod Genet. 42:1635–1649.
2025.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xing X, Wu S, Xu H, Ma Y, Bao N, Gao M,
Han X, Gao S, Zhang S, Zhao X, et al: Non-invasive prediction of
human embryonic ploidy using artificial intelligence: A systematic
review and meta-analysis. EClinicalMedicine.
24(102897)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ersoy Z, Ozdemirkan ZA, Zeyneloglu P,
Cekmen N, Torgay A, Kayhan Z and Haberal M: Pulse contour cardiac
output system monitoring in pediatric patients undergoing
orthotopic liver transplantation. Int J Innov Res Med Sci.
7(11)2022.
|
|
19
|
Njoku K, Chiasserini D, Jones ER, Barr CE,
O'Flynn H, Whetton AD and Crosbie EJ: Urinary biomarkers and their
potential for the non-invasive detection of endometrial cancer.
Front Oncol. 10(559016)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tran BQ, Miller PR, Taylor RM, Boyd G,
Mach PM, Rosenzweig CN, Baca JT, Polsky R and Glaros T: Proteomic
characterization of dermal interstitial fluid extracted using a
novel microneedle-assisted technique. J Proteome Res. 17:479–485.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Woźniak M, Paluszkiewicz C and Kwiatek WM:
Saliva as a non-invasive material for early diagnosis. Acta Biochim
Pol. 66:383–388. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zeng H, He B, Yi C and Peng J: Liquid
biopsies: DNA methylation analyses in circulating cell-free DNA. J
Genet Genomics. 45:185–192. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schwarzenbach H, Hoon DSB and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Assou S, Aït-Ahmed O, El Messaoudi S,
Thierry AR and Hamamah S: Non-invasive pre-implantation genetic
diagnosis of X-linked disorders. Med Hypotheses. 83:506–508.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stigliani S, Anserini P, Venturini PL and
Scaruffi P: Mitochondrial DNA content in embryo culture medium is
significantly associated with human embryo fragmentation. Hum
Reprod. 28:2652–2660. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Caamaño D, Cabezas J, Aguilera C, Martinez
I, Wong YS, Sagredo DS, Ibañez B, Rodriguez S, Castro FO and
Rodriguez-Alvarez L: DNA content in embryonic extracellular
vesicles is independent of the apoptotic rate in bovine embryos
produced in vitro. Animals (Basel). 14(1041)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang WL, Liu Y, Jiang J, Tang YJ, Tang YL
and Liang XH: Extracellular vesicle long non-coding RNA-mediated
crosstalk in the tumor microenvironment: Tiny molecules, huge
roles. Cancer Sci. 111:2726–2735. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Birjandi AA and Sharpe P: Potential of
extracellular space for tissue regeneration in dentistry. Front
Physiol. 13(1034603)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mishra A, Ashary N, Sharma R and Modi D:
Extracellular vesicles in embryo implantation and disorders of the
endometrium. Am J Reprod Immunol. 85(e13360)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ng YH, Rome S, Jalabert A, Forterre A,
Singh H, Hincks CL and Salamonsen LA: Endometrial
exosomes/microvesicles in the uterine microenvironment: A new
paradigm for embryo-endometrial cross talk at implantation. PLoS
One. 8(e0058502)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Vilella F, Moreno-Moya JM, Balaguer N,
Grasso A, Herrero M, Martínez S, Marcilla A and Simón C:
Hsa-miR-30d, secreted by the human endometrium, is taken up by the
pre-implantation embryo and might modify its transcriptome.
Development. 142:3210–3221. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Greening DW, Nguyen HPT, Elgass K, Simpson
RJ and Salamonsen LA: Human endometrial exosomes contain
hormone-specific cargo modulating trophoblast adhesive capacity:
Insights into endometrial-embryo interactions. Biol Reprod.
94(38)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bedrick BS, Tipping AD, Nickel KB, Riley
JK, Jain T and Jungheim ES: State-mandated insurance coverage and
preimplantation genetic testing in the United States. Obstet
Gynecol. 139:500–508. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Snoek R, Stokman MF, Lichtenbelt KD, van
Tilborg TC, Simcox CE, Paulussen ADC, Dreesen JCMF, van Reekum F,
Lely AT, Knoers NVAM, et al: Preimplantation genetic testing for
monogenic kidney disease. Clin J Am Soc Nephrol. 15:1279–1286.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
de Albornoz EC, Arroyo JAD, Iriarte YF,
Vendrell X, Vidal VM and Roig MC: Non invasive preimplantation
testing for aneuploidies in assisted reproduction: A SWOT Analysis.
Reprod Sci. 32:1–14. 2025.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hawke DC, Watson AJ and Betts DH:
Extracellular vesicles, microRNA and the preimplantation embryo:
Non-invasive clues of embryo well-being. Reprod Biomed Online.
42:39–54. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Scalici E, Traver S, Molinari N, Mullet T,
Monforte M, Vintejoux E and Hamamah S: Cell-free DNA in human
follicular fluid as a biomarker of embryo quality. Hum Reprod.
29:2661–2669. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Stigliani S, Persico L, Lagazio C,
Anserini P, Venturini PL and Scaruffi P: Mitochondrial DNA in day 3
embryo culture medium is a novel, non-invasive biomarker of
blastocyst potential and implantation outcome. Mol Hum Reprod.
20:1238–1246. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dompe C, Kulus M, Stefańska K, Kranc W,
Chermuła B, Bryl R, Pieńkowski W, Nawrocki MJ, Petitte JN, Stelmach
B, et al: Human granulosa cells-stemness properties, molecular
cross-talk and follicular angiogenesis. Cells.
10(1396)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nakano R, Radaelli MRM, Fujihara LS,
Yoshinaga F, Nakano E and Almodin CG: Efficacy of a modified
transvaginal ultrasound-guided fresh embryo transfer procedure.
JBRA Assist Reprod. 26:78–83. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ben-Meir A, Kim K, McQuaid R, Esfandiari
N, Bentov Y, Casper RF and Jurisicova A: Co-enzyme Q10
supplementation rescues cumulus cells dysfunction in a maternal
aging model. Antioxidants (Basel). 8(58)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kansaku K, Munakata Y, Itami N, Shirasuna
K, Kuwayama T and Iwata H: Mitochondrial dysfunction in
cumulus-oocyte complexes increases cell-free mitochondrial DNA. J
Reprod Dev. 64:261–266. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lukaszuk K and Podolak A: Does
trophectoderm mitochondrial DNA content affect embryo developmental
and implantation potential? Int J Mol Sci. 23(5976)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hashimoto S and Morimoto Y: Mitochondrial
function of human embryo: Decline in their quality with maternal
aging. Reprod Med Biol. 21(e12491)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Van Blerkom J: Mitochondrial function in
the human oocyte and embryo and their role in developmental
competence. Mitochondrion. 11:797–813. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Guo H, Li T and Sun X: LncRNA HOTAIRM1,
miR-433-5p and PIK3CD function as a ceRNA network to exacerbate the
development of PCOS. J Ovarian Res. 14(19)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tajima K, Orisaka M, Yata H, Goto K,
Hosokawa K and Kotsuji F: Role of granulosa and theca cell
interactions in ovarian follicular maturation. Microsc Res Tech.
69:450–458. 2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kato N, Uchigasaki S, Fukase M and Kurose
A: Expression of P450 aromatase in granulosa cell tumors and
sertoli-stromal cell tumors of the ovary: Which cells are
responsible for estrogenesis? Int J Gynecol Pathol. 35:41–47.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sciorio R and Rinaudo R: Culture
conditions in the IVF laboratory: State of the ART and possible new
directions. J Assist Reprod Genet. 40:2591–2607. 2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liu X, Mai H, Chen P, Zhang Z, Wu T, Chen
J, Sun P, Zhou C, Liang X and Huang R: Comparative analyses in
transcriptome of human granulosa cells and follicular fluid
micro-environment between poor ovarian responders with conventional
controlled. Reprod Biol Endocrinol. 20(54)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zamah AM, Hassis ME, Albertolle ME and
Williams KE: Proteomic analysis of human follicular fluid from
fertile women. Clin Proteomics. 12(5)2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Al-Saleh I, Coskun S, Al-Rouqi R,
Al-Rajudi T, Eltabache C, Abduljabbar M and Al-Hassan S: Oxidative
stress and DNA damage status in couples undergoing in vitro
fertilization treatment. Reprod Fertil. 2:117–139. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Reiter RJ, Sharma R, Romero A, Manucha W,
Tan DX, de Campos Zuccari DAP and Chuffa LGA: Aging-related ovarian
failure and infertility: Melatonin to the rescue. Antioxidants
(Basel). 12(695)2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Tamura H, Jozaki M, Tanabe M, Shirafuta Y,
Mihara Y, Shinagawa M, Tamura I, Maekawa R, Sato S, Taketani T, et
al: Importance of melatonin in assisted reproductive technology and
ovarian aging. Int J Mol Sci. 21(1135)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Drejza MA, Rylewicz K, Majcherek E,
Gross-Tyrkin K, Mizgier M, Plagens-Rotman K, Wójcik M,
Panecka-Mysza K, Pisarska-Krawczyk M, Kędzia W and
Jarząbek-Bielecka G: Markers of oxidative stress in obstetrics and
Gynaecology-a systematic literature review. Antioxidants (Basel).
11(1477)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Huang J, Feng Q, Zou L, Liu Y, Bao M, Xia
W and Zhu C: Humanin exerts a protective effect against
D-galactose-induced primary ovarian insufficiency in mice.
Reproductive BioMedicine Online. 48(103330)2025.
|
|
57
|
Dong L, Teh DBL, Kennedy BK and Huang Z:
Unraveling female reproductive senescence to enhance healthy
longevity. Cell Res. 33:11–29. 2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wigglesworth K, Lee KB, Emori C, Sugiura K
and Eppig JJ: Transcriptomic diversification of developing cumulus
and mural granulosa cells in mouse ovarian follicles. Biol Reprod.
92(23)2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Demiray SB, Goker ENT, Tavmergen E, Yilmaz
O, Calimlioglu N, Soykam HO, Oktem G and Sezerman U: Differential
gene expression analysis of human cumulus cells. Clin Exp Reprod
Med. 46:76–86. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Su YQ, Sugiura K and Eppig JJ: Mouse
oocyte control of granulosa cell development and function:
Paracrine regulation of cumulus cell metabolism. Semin Reprod Med.
27:32–42. 2009.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hsieh M, Lee D, Panigone S, Horner K, Chen
R, Theologis A, Lee DC, Threadgill DW and Conti M: Luteinizing
hormone-dependent activation of the epidermal growth factor network
is essential for ovulation. Mol Cell Biol. 27:1914–1924.
2007.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Biase FH and Kimble KM: Functional
signaling and gene regulatory networks between the oocyte and the
surrounding cumulus cells. BMC Genomics. 19(351)2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Akino R, Matsui D, Kawahara-Miki R, Amita
M, Tatsumi K, Ishida E, Kang W, Takada S, Miyado K and Sekizawea A:
Next-generation sequencing reveals downregulation of the Wnt
signaling pathway in human dysmature cumulus cells as a hallmark
for evaluating oocyte. Reprod Med. 1:205–215. 2020.
|
|
64
|
Vigone G, Merico V, Prigione A, Mulas F,
Sacchi L, Gabetta M, Bellazzi R, Redi CA, Mazzini G, Adjaye J, et
al: Transcriptome based identification of mouse cumulus cell
markers that predict the developmental competence of their enclosed
antral oocytes. BMC Genomics. 14(380)2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Salustri A, Garlanda C, Hirsch E, De
Acetis M, Maccagno A, Bottazi B, Doni A, Bastone A, Mantovani G,
Peccoz PB, et al: PTX3 plays a key role in the organization of the
cumulus oophorus extracellular matrix and in in vivo fertilization.
Development. 131:1577–1586. 2004.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Scarchilli L, Camaioni A, Bottazzi B,
Negri V, Doni A, Deban L, Bastone A, Salvatori G, Mantovani A,
Siracusa G and Salustri A: PTX3 Interacts with Inter-α-trypsin
Inhibitor: IMPLICATIONS FOR HYALURONAN ORGANIZATION AND CUMULUS
OOPHORUS EXPANSION. J Biol Chem. 12(41)2007.
|
|
67
|
Sun XL, Jiang H, Han DX, Fu Y, Liu JB, Gao
Y, Hu SM, Yuan B and Zhang JB: The activated DNA double-strand
break repair pathway in cumulus cells from aging patients may be
used as a convincing predictor of poor outcomes after in vitro
fertilization-embryo transfer treatment. PLoS One.
13(e0204524)2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Fatehi AN, Roelen BAJ, Colenbrander B,
Schoevers EJ, Gadella BM, Bevers MM and van den Hurk R: Presence of
cumulus cells during in vitro fertilization protects the bovine
oocyte against oxidative stress and improves first cleavage but
does not affect further. Zygote. 13:177–185. 2005.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Chua SC, Yovich SJ, Hinchliffe PM and
Yovich JL: How well do semen analysis parameters correlate with
sperm DNA fragmentation? A retrospective study from 2567 semen
samples analyzed by the. J Pers Med. 13(518)2023.PubMed/NCBI View Article : Google Scholar
|