Introduction
Non-alcoholic fatty liver disease (NAFLD) is the
most common type of liver disease and is closely associated with
obesity and metabolic syndrome. There is a wide spectrum of
pathologies covered by NAFLD, ranging from simple accumulation of
triglycerides (TGs) in hepatocytes to non-alcoholic steatohepatitis
(NASH) and in certain patients, this is followed by the progression
to fibrosis and cirrhosis (1–3). The
‘two-hit’ hypothesis has become an important theoretical framework
in understanding the pathogenesis of liver damage in these patients
(3–6). Although initial and subsequent
mechanisms are not entirely distinct, the first hit mainly consists
of TG and fatty acid accumulation in the liver. The second hit
involves oxidative stress and inflammation of the liver. Both
conditions can be induced by high energy diets, and the central
role of lipid accumulation in the liver in the pathogenesis of
NAFLD has been confirmed in clinical correlation studies and animal
models (6). Thus, decreasing serum
and hepatic lipid levels are crucial for the prevention of
NAFLD.
Polydatin is a stilbenoid compound derived from the
rhizome of Polygonum cuspidatum. This plant has been used
clinically in the treatment of digestive disorders and
ischemia/reperfusion injury in traditional Chinese medicine
(7). One of the main properties of
polydatin is the hepatoprotective activity, reportedly inducing
gallbladder contraction, preventing biliary cholesterol-stone
formation and protecting against tetrachloromethane and aflatoxin
B1 hepatotoxicity (8–11). It has also been demonstrated that
polydatin suppresses the oxidative and inflammatory damage in
ischemic stroke (12). In
addition, it has been revealed that treatment with polydatin
significantly reduced the serum levels of total cholesterol (TC)
and TGs in hyperlipidemic hamsters and rabbits induced by a
high-fat diet (HFD) (13,14). Despite these hypolipidemic and
antioxidant activities, the extent to which polydatin improves
hepatic steatosis has not been well studied.
The aim of this study was to assess the effects of
polydatin on preventing hepatic lipid accumulation in high-fat
diet-induced NAFLD rats. To investigate the possible mechanism,
liver proinflammatory cytokine tumor necrosis factor-α (TNF-α) and
lipid peroxidation levels were determined. The principal regulator
of hepatic fatty acid biosynthesis, sterol-regulatory element
binding protein (SREBP-1c) and its response genes, fatty acid
synthase (FAS) and stearoly-CoA desaturase 1 (SCD1) were also
determined.
Materials and methods
Animals and treatment
Male Sprague-Dawley (SD) rats weighing 160–180 g
were purchased from the Experimental Animal Center of Xi'an
Jiaotong University. Animals were housed at 24°C with a 12 h
light-dark cycle with free access to water and food. Animal
experiments were approved by the Institute of Animal Use and Care
Committee of the Shaanxi University of Chinese Medicine.
Rats received either a regular rodent chow (normal
diet: 62.3% carbohydrate, 12.5% fat, 24.3% protein calories) or a
HFD (42.0% fat, 36.0% carbohydrate, 22.0% protein) for 16 weeks.
Lard was the major constituent of the HFD. Following
acclimatization for 1 week, the rats were randomly divided into 2
experimental groups. One group included 8 rats that received the
normal diet for 16 weeks (control group). The other group,
including 24 animals, had ad libitum access to the HFD for 8
weeks, and after this period the rats were randomly divided into 3
groups. During the remaining 8 weeks, animals were provided the
same ad libitum access to the HFD. In addition, one group
was administered polydatin 30 mg/kg/day by gavage (8 rats, HFD+P 30
mg/kg group), and another group was administered polydatin 90
mg/kg/day by gavage (8 rats, HFD+P 90 mg/kg group). The third group
received the same amount of vehicle by gavage (8 rats, HFD group).
Polydatin (3,4′,5-trihydroxystilbene-3-β-mono-D-glucoside; purity,
>99%) was obtained from Suzhou Baozetang Biotechnology Co.,
Ltd., Suzhou, China.
All rats were sacrificed after weighing at 16 weeks,
and blood was drawn via the femoral artery and stored as plasma at
−80°C. The intact liver was isolated and weighed. The liver index
was calculated as the liver/body weight ratio. Sections from the
right lobe were washed in cold saline and placed in 10% formalin
solution for histopathological analysis. The other samples were
immediately frozen in liquid nitrogen and stored at −80°C until
use.
Histological analysis
The liver sections were paraffin-embedded, sliced
into 5-μm sections and stained with hematoxylin-eosin (HE) as
previously described (15). The
pathological changes were assessed and photographed under an
Olympus BX-51 microscope. The liver biopsy was scored according to
Brunt et al(16), as
follows: 0, no steatosis; 1, fatty hepatocytes occupying <10% of
the parenchyma; 2, between 10% and 30%; 3, between 30% and 60%; and
4, fatty hepatocytes occupying >60% of the parenchyma. Pathology
was scored in a blinded manner by two independent pathologists with
expertise in rodent liver.
Biochemical analysis
The activities of alanine aminotransferase (ALT) and
aspartate aminotransferase (AST) in the plasma were measured using
commercial enzyme assay kits (Wako Pure Chemical Industries, Osaka,
Japan). Plasma TG, TC and free fatty acid (FFA) concentrations were
determined using enzymatic reagent kits from Biosino (Beijing,
China) according to the manufacturer's instructions. Hepatic lipids
were extracted with chloroform-methanol (2:1) as described by Folch
et al(17), and then
dissolved using Triton X-100. TG, TC and FFA concentrations in the
liver were determined using enzymatic reagent kits (Biosino) as
previously described (15,18).
ELISA assays for liver TNF-α
For quantification of the liver TNF-α level, liver
tissues were homogenized in extraction buffer (50 mmol/l Tris, 150
mmol/l NaCl, 1% Triton X-100 and a protease inhibitor cocktail).
The homogenate was agitated on ice for 90 min, centrifuged at 4°C
for 15 min, and then the precipitate was discarded. The TNF-α level
was detected using a commercial enzyme-linked immunosorbent assay
(ELISA) kit (BD Biosciences, Franklin Lakes, NJ, USA) according to
the manufacturer's instructions.
Measurement of lipid peroxidation
Liver tissue (100 mg) was homogenized in 10 volumes
of ice-cold Tris-HCl buffer containing 0.25 M sucrose. The
resulting homogenate was centrifuged at 3000 × g for 10 min at 4°C.
The supernatant was used for assays of malondialdehyde
(MDA)+4-hexanonenal (4-HNE). MDA+4-HNE was measured using a
commercial enzyme assay kit (LPO-586) purchased from OXIS Health
Products, Inc. (Portland, OR, USA).
Real-time RT-PCR analysis
Real-time PCR was used to detect changes in the
expression of SREBP-1c, FAS, SCD1 and TNF-α mRNA in the liver
tissues as previously described (19,20).
The isolation of total RNA from the liver tissues was performed
using RNeasy Mini kits (Qiagen, Valencia, CA, USA) according to the
manufacturer's instructions. RNA purity and concentration were
determined spectrophotometrically. For each RT-PCR reaction, 2 μg
of total RNA was converted into cDNA using reverse transcriptase
(Qiagen). Genomic DNA was eliminated using DNase I. For real-time
PCR, the reactions were conducted by placing 10 μl of material into
96-well plates with TaqMan PCR Master mix (Applied Biosystems Inc.,
Foster City, CA, USA). The specific primers and probes for
SREBP-1c, FAS, SCD1 and TNF-α mRNA were obtained from Applied
Biosystems, and RT-PCR was performed in an Applied Biosystems PRISM
7000 sequence detection system according to the manufacturer's
instructions. A comparative cycle of threshold fluorescence (CT)
method was used with β-actin as the internal control. The final
results of real-time PCR were expressed as the ratio of the mRNA of
interest to β-actin.
Statistical analysis
Results are expressed as the mean ± SD. Statistical
significance was evaluated with one-way ANOVA followed by
Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of polydatin on liver index and
plasma levels of ALT and AST in HFD rats
Following 16-week feeding, there was no difference
in body weight gain among any group, but the HFD rats demonstrated
a significantly higher liver index compared to the control rats.
Polydatin (30 and 90 mg/kg) treatment to HFD rats significantly
reduced the liver index. The effects of polydatin on body weight,
liver index and biochemical parameters of the experimental rats are
shown in Table I. In addition, the
biochemical analyses revealed increased plasma activities of ALT
and AST in the HFD-fed rats compared to those of the control rats.
HFD-induced elevations in plasma activities of ALT and AST were
significantly reversed by treating the HFD rats with polydatin
(P<0.05) (Table I).
 | Table IEffects of polydatin on body weight,
liver index and biochemical parameters of the experimental
rats. |
Table I
Effects of polydatin on body weight,
liver index and biochemical parameters of the experimental
rats.
| Control | HFD | HFD+P 30 mg/kg | HFD+P 90mg/kg |
|---|
| Body weight (g) | 482.31±31.52 | 534.07±62.47 | 507.64±52.13 | 499.83±48.21 |
| Liver index | 3.11±0.37 | 5.13±0.45b | 4.07±0.31b,d | 3.62±0.29a,d |
| Plasma |
| ALT (U/l) | 51.02±4.13 | 79.82±8.79b | 62.17±5.32b,d | 58.49±7.32d |
| AST (U/l) | 63.72±5.41 | 127.38±9.41b | 83.65±5.08b,d | 75.42±4.93b,d |
| TG (mmol/l) | 0.68±0.13 | 1.21±0.19b | 0.87±0.31d | 0.96±0.35d |
| TC (mmol/l) | 1.73±0.27 | 2.72±0.31b | 2.07±0.18a,d | 2.16±0.19b,d |
| HDL (mmol/l) | 1.42±0.13 | 1.19±0.07b | 1.35±0.09d | 1.47±0.07d |
| LDL (mmol/l) | 0.22±0.02 | 0.42±0.06b | 0.36±0.05b,d | 0.33±0.07b,d |
| FFA (mmol/l) | 0.86±0.08 | 1.24±0.25b | 1.03±0.17d | 0.96±0.11d |
| Insulin
(mU/l) | 24.17±4.21 | 30.25±5.83 | 25.24±6.17 | 22.35±6.73 |
| Glucose
(mmol/l) | 6.23±0.65 | 6.98±0.67 | 6.42±0.66 | 6.05±0.64 |
| Liver |
| TG (μmol/g) | 4.37±0.23 | 6.12±0.31b | 5.42±0.30b,d | 4.76±0.35a,d |
| TC (μmol/g) | 6.08±0.41 | 7.69±0.37b | 7.13±032d | 6.46±0.36d |
| FFA (μmol/g) | 85.63±14.17 | 157.9±24.70b | 139.11±16.9b,c |
103.24±22.05d |
Effects of polydatin on plasma and
hepatic-lipid levels in HFD rats
To analyze the possible role of polydatin in lipid
metabolism, a key factor associated with fatty liver formation,
plasma and hepatic-lipid levels in the HFD rats were investigated.
The HFD-induced elevation in plasma concentrations of TG, TC, FFA
and low-density lipoprotein (LDL) was significantly attenuated by
polydatin, while decreased plasma high-density lipoprotein (HDL)
levels were evidently reversed by treating the rats with polydatin
(Table I). The hepatic
accumulation of TC, TG, and FFA induced by the HFD was also
significantly alleviated by treating the rats with polydatin. This
suggests that polydatin is able to prevent hepatosteatosis via
downregulation of the accumulation of lipids. These results
indicate that polydatin had marked effects on the improvement of
blood and hepatic lipid levels in the experimental rats.
Effects of polydatin on liver histology
in HFD rats
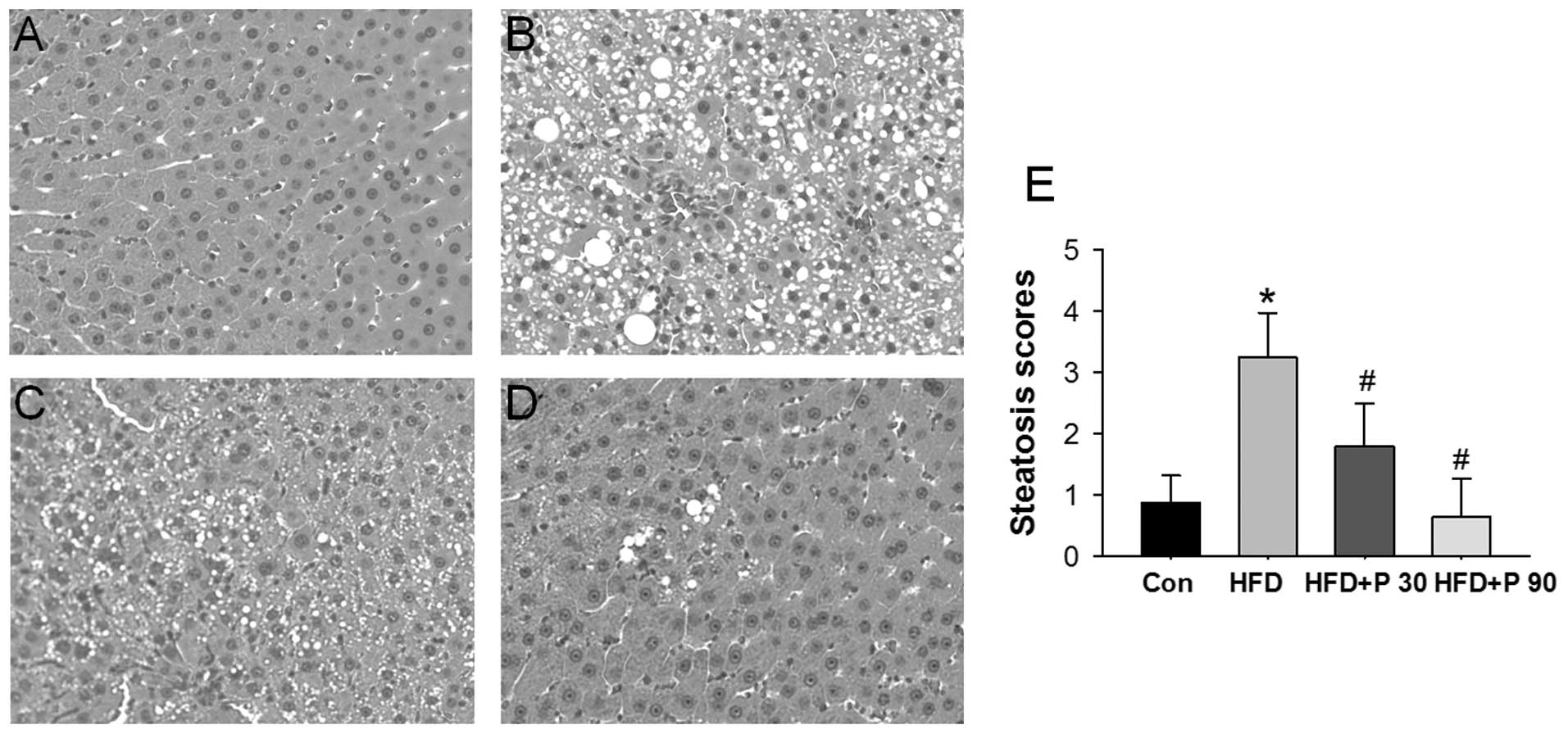
Representative histological sections of the liver
from each experimental group are shown in Fig. 1A-D. After 16 weeks, HFD-rats
demonstrated severe hepatic microvesicular and macrovesicular fat.
Rats treated with either 30 or 90 mg/kg polydatin significantly
eliminated hepatic steatosis. The quantitative evaluation of the
liver histology of all rats in each group is shown in Fig. 1E.
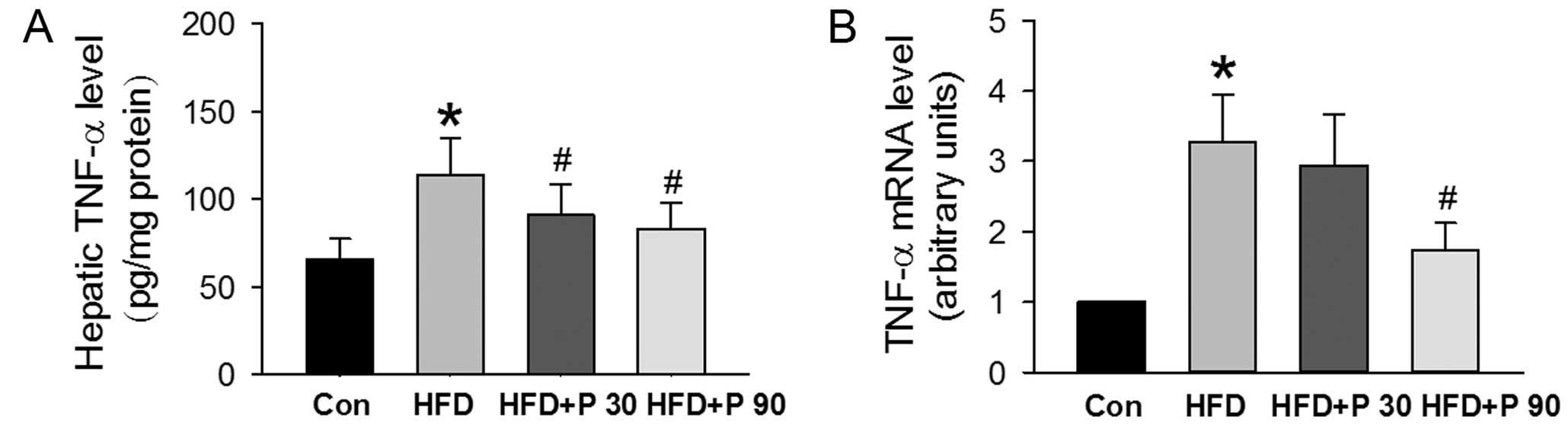
Effects of polydatin on hepatic TNF-α
level
Hepatic steatosis is associated with increased
hepatic TNF-α, which stimulates lipogenesis as well as induces
hepatic dysfunction. ELISA analysis demonstrated that hepatic TNF-α
protein was greater in HFD rats and lower in HFD rats treated with
polydatin at 30 or 90 mg/kg (Fig.
2A). In addition, hepatic TNF-α mRNA expression was 3-fold
greater in HFD rats than in normal control rats. Polydatin (90
mg/kg) administration, however, caused a significant decrease in
TNF-α mRNA expression (Fig.
2B).
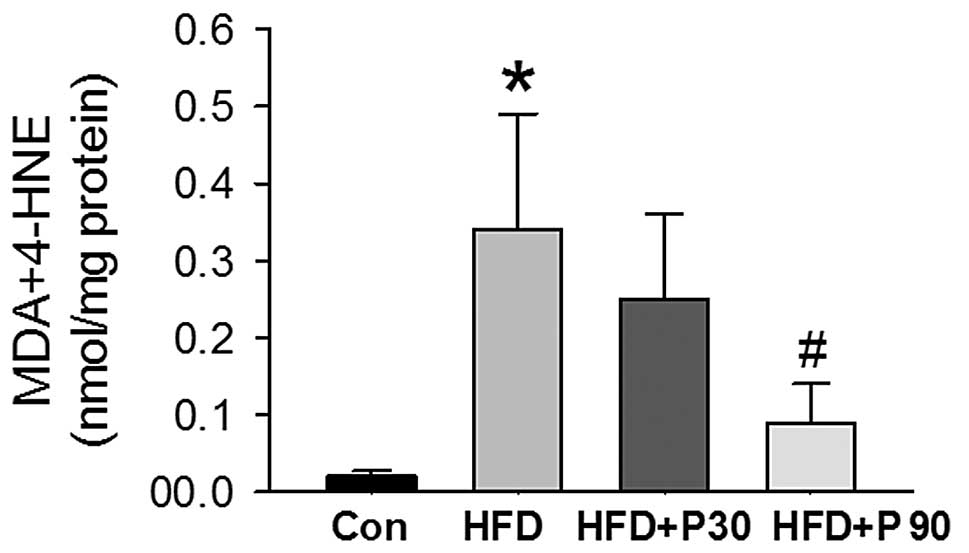
Effects of polydatin on lipid
peroxidation in liver homogenates
To investigate whether polydatin is capable of
preventing lipid peroxidation, we measured the formation of MDA and
4-HNE in liver tissues. The levels of MDA and 4-HNE were
significantly higher in the HFD rats compared to the control rats.
Supplementation of polydatin 90 mg/kg resulted in a reduction of
MDA and 4-HNE levels (P<0.01 vs. HFD rats (Fig. 3).
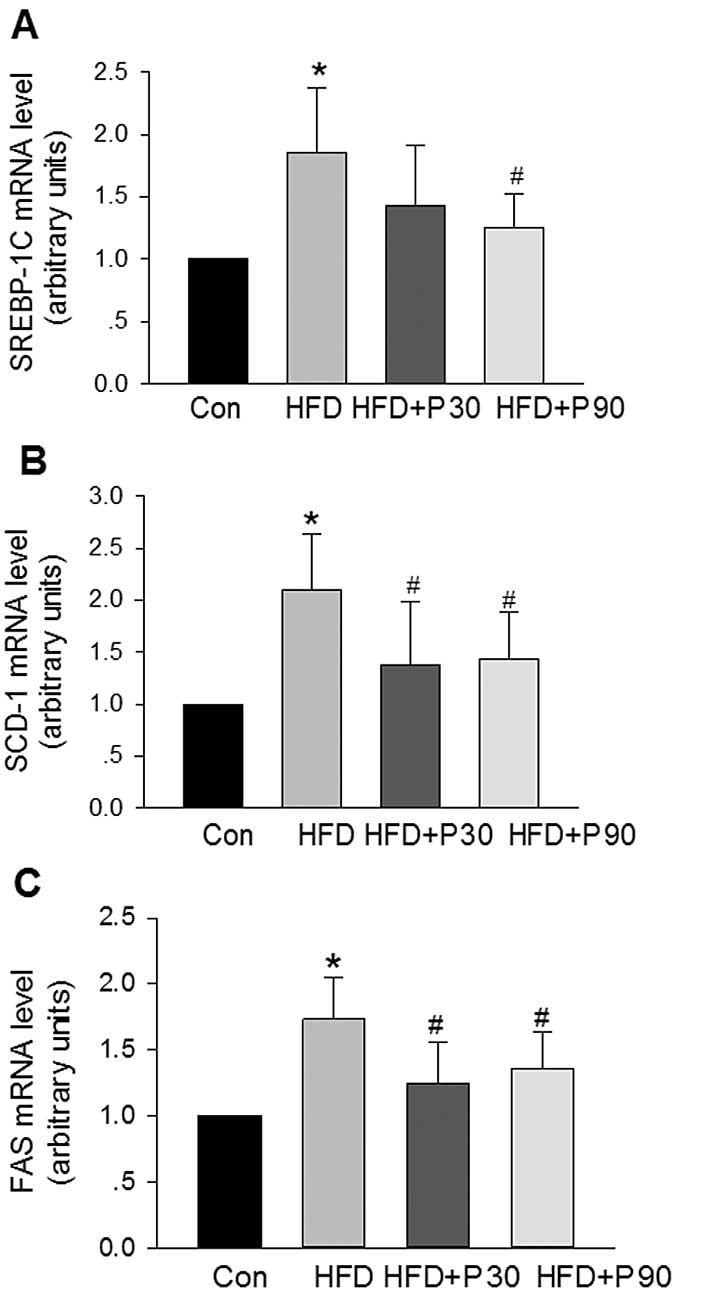
Effects of polydatin on relative mRNA
concentrations of SREBP-1c and its target genes in the liver of HFD
rats
We examined the expression of genes regulating
lipogenesis to define how polydatin inhibits hepatic steatosis. In
the HFD rats, the expression of SREBP-1c, FAS and SCD-1 was
significantly upregulated in the liver (Fig. 4). Polydatin at 90 mg/kg decreased
the expression of liver SREBP-1c mRNA; while the two doses of
polydatin decreased liver FAS and SCD-1 mRNA levels significantly
(Fig. 4).
Discussion
In the present study, the results demonstrated that
polydatin was able to decrease the TC, TG and FFA levels in serum
and liver tissue, as well as the hepatic weight index in high-fat
diet-induced fatty liver. The histopathological evaluation of liver
specimens demonstrated that polydatin may decrease lipid
accumulation, particularly in 90 mg/kg polydatin groups, and the
hepatic lipid accumulation in a few rats was reversed. These
results suggested that polydatin may exert a therapeutic effect on
high-fat diet-induced fatty liver in rats.
TNF-α is a cytokine which plays a central role in
the chronic inflammatory response in fatty liver (21,22).
Kupffer cells are the major source of increased TNF-α secretion
within the liver (21). TNF-α mRNA
is increased in liver and adipose tissues in NASH patients
(23–24). TNF receptor knockout mice have less
severe steatosis than wild-type littermates, and a significant
reduction in liver injury in steatohepatitis following anti-TNF-α
therapy supports the pathogenic role of TNF-α (25–27).
In this study, TNF-α mRNA and protein was higher in the HFD group,
but was attenuated by polydatin treatment. One of the mechanisms
for lowering TNF-α levels may have been the anti-inflammatory
properties of polydatin. Previous studies have indicated that
polydatin from Polygonum cuspidatum may regulate the
inflammatory response and immune function (10,12).
For example, polydatin was identified to regulate IL-17 production
in human peripheral blood mononuclear cells and nuclear factor κB
activation in the rat model of cerebral artery occlusion (12,28–29).
Another mechanism for regulating the inflammatory response may be
the decrease in liver fat and oxidative stress, which indirectly
decreases the damage of hepatocytes and lowers the TNF-α level. In
this study, the increase in MDA and 4-HNE, a marker of oxidative
stress, was significantly attenuated by polydatin. These results
suggest that polydatin may inhibit the inflammatory response in
NASH and further decrease damage to the liver.
In this study, we also examined the effects of
polydatin on hepatic SREBP-1c mRNA expression in rats. The results
indicated that the expression of SREBP-1c mRNA in the HFD group was
higher than that in the control group. Following administration of
polydatin 30–90 mg/kg for 8 weeks, SREBP-1c mRNA expression was
decreased. It was suggested that inhibition of SREBP-1c by
polydatin may counteract the high-fat diet-induced fatty liver via
regulation of SREBP-1c-mediated target gene expression, and
subsequently correct the imbalance of lipid metabolism toward lipid
accumulation in the level of genetic transcription.
SREBP-1c is a member of the family of SREBP
membrane-bound transcription factors. It mainly activates the
transcription of lipogenic genes that contain sterol regulatory
elements in their promoter regions, including FAS and SCD-1
(30,31). FAS is a key enzyme which controls
the rate of fatty acid synthesis. Increase in the expression of FAS
may increase fatty acid synthesis and lead to the ectopic
overaccumulation of fatty acids and TGs in the liver (32). Several lines of evidence have
suggested that the induction of SREBP-1c gene expression in the
liver contributes to the elevation of hepatic TG concentrations,
leading to the development of NAFLD (31–37).
As supporting evidence, SREBP-1c overexpression in transgenic mice
leads to fatty liver, while inactivation of the SREBP-1c gene in
the livers of ob/ob mice reduces hepatic TG content by 50%
(33,34). Shrestha et al demonstrated
that dietary catechins extracted from green tea extract could
reduce plasma and hepatic TG concentration in fructose-fed rats,
mainly through downregulation of SREBP-1c and its target gene
(37). Notably, we identified that
SREBP-1c mRNA expression was stimulated by HFD, and consumption of
polydatin suppressed its expression. As a result of low SREBP-1c
expression in rats fed polydatin, there was a concomitant
significant reduction in the expression of FAS and SCD-1 mRNA. The
results suggested that the stimulated FAS and SCD-1 transcription
may occur following SREBP-1c activation caused by HFD. Polydatin is
likely to have an inhibitory effect on SREBP-1c expression, which
in turn influences transcription in these lipogenic genes, thereby
reducing enzyme activity and resulting in a low rate of lipid
synthesis.
Recent evidence indicates that key liver genes
involved in TG homeostasis are regulated by insulin (38–42).
However, significant differences in the plasma concentrations of
insulin and glucose were not observed in our study. The failure of
HFD to affect plasma insulin concentration in this study could be
due to the fact that we focused on a single measurement of hormone
concentration at one point in time. The measurement of plasma
insulin concentration in defined time intervals following the
serving of food according to a fixed time schedule, combined with
the determination of the area under the curve of insulin, should be
elucidated in further studies.
In conclusion, this study demonstrates that
polydatin may improve the liver function of rats with non-alcoholic
steatohepatitis by lowering lipid levels in the blood and liver,
reducing oxidative damage and inflammation, and regulating the gene
expression of hepatic fatty acid biosynthesis. These polydatin
effects appear to be dose-dependent and are likely to be mediated
via alterations in adipose lipid metabolism and improvements in
hepatic anti-inflammatory responses and antioxidant defenses.
Further studies are necessary to clarify the mechanisms behind the
beneficial effects of polydatin on NAFLD.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (No. 31171101 and No. 81100175) and the
Shaanxi Provincial Department of Education (No. 08JK274).
References
|
1
|
Duvnjak M, Lerotić I, Barsić N, Tomasić V,
Virović Jukić L and Velagić V: Pathogenesis and management issues
for non-alcoholic fatty liver disease. World J Gastroenterol.
13:4539–4550. 2007.PubMed/NCBI
|
|
2
|
Angulo P: Non-alcoholic fatty liver
disease. N Engl J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yeh MM and Brunt EM: Pathology of
non-alcoholic fatty liver disease. Am J Clin Pathol. 128:837–847.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farrell GC and Larter CZ: Non-alcoholic
fatty liver disease: from steatosis to cirrhosis. Hepatology.
43:S99–S112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Postic C and Girard J: The role of the
lipogenic pathway in the development of hepatic steatosis. Diabetes
Metab. 34:643–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ayyad C and Andersen T: Long-term efficacy
of dietary treatment of obesity: a systematic review of studies
published between 1931 and 1999. Obes Rev. 1:113–119. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xue L: Progress in the pharmacological
study of Chinese herbal drug: Polygonum cuspidatum. Zhongguo
Zhong Yao Za Zhi. 25:651–653. 2000.PubMed/NCBI
|
|
8
|
Luper S: A review of plants used in the
treatment of liver disease: part two. Altern Med Rev. 4:178–188.
1999.PubMed/NCBI
|
|
9
|
Kimura Y: Pharmacological studies on
resveratrol. Methods Find Exp Clin Pharmacol. 25:297–310. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Dou C and Gu F: Advances in the
study on pharmacological actions of Polygonum cuspidatum
Sieb: clearing heat and detoxication. Zhong Yao Cai. 26:606–610.
2003.PubMed/NCBI
|
|
11
|
Huang ZS, Wang ZW, Liu MP, Zhong SQ, Li QM
and Rong XL: Protective effects of polydatin against CCl(4)-induced
injury to primarily cultured rat hepatocytes. World J
Gastroenterol. 5:41–44. 1999.PubMed/NCBI
|
|
12
|
Ji H, Zhang X, Du Y, Liu H, Li S and Li L:
Polydatin modulates inflammation by decreasing NF-κB activation and
oxidative stress by increasing Gli1, Ptch1, SOD1 expression and
ameliorates blood-brain barrier permeability for its
neuroprotective effect in pMCAO rat brain. Brain Res Bull.
87:50–59. 2012.PubMed/NCBI
|
|
13
|
Du J, Sun LN, Xing WW, et al:
Lipid-lowering effects of polydatin from Polygonum
cuspidatum in hyperlipidemic hamsters. Phytomedicine.
16:652–658. 2009. View Article : Google Scholar
|
|
14
|
Xing WW, Wu JZ, Jia M, Du J, Zhang H and
Qin LP: Effects of polydatin from Polygonum cuspidatum on
lipid profile in hyperlipidemic rabbits. Biomed Pharmacother.
63:457–462. 2009.
|
|
15
|
Zhang Q, Zhao Y, Zhang DB and Sun LJ:
Effect of Sinai san decoction on the development of non-alcoholic
steatohepatitis in rats. World J Gastroenterol. 11:1392–1395. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brunt EM, Janney CG, Di Bisceglie AM,
Neuschwander-Tetri BA and Bacon BR: Nonalcoholic steatohepatitis: a
proposal for grading and staging the histological lesions. Am J
Gastroenterol. 94:2467–2474. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Folch J, Lees M, Sloane and Starley GH: A
simple method for the isolation and purification of total lipids
from animal tissues. J Biol Chem. 226:497–509. 1957.
|
|
18
|
Bartlett GR: Phosphorous assay in column
chromatography. J Biol Chem. 234:466–468. 1959.PubMed/NCBI
|
|
19
|
Zhang Q and Tan Y: Nerve growth factor
augments neuronal responsiveness to noradrenaline in cultured
dorsal root ganglion neurons of rats. Neuroscience. 193:72–79.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Q, Yao F, Raizada MK, O'Rourke ST
and Sun C: Apelin gene transfer into the rostral ventrolateral
medulla induces chronic blood pressure elevation in normotensive
rats. Circ Res. 104:1421–1428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Diehl AM, Li ZP, Lin HZ and Yang SQ:
Cytokines and the pathogenesis of non-alcoholic steatohepatitis.
Gut. 54:303–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tilg H and Diehl AM: Cytokines in
alcoholic and nonalcoholic steatohepatitis. N Engl J Med.
343:1467–1476. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crespo J, Cayon A, Fernandez-Gil P, et al:
Gene expression of tumor necrosis factor alpha and TNF-receptors,
p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology.
34:1158–1163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abiru S, Migita K, Maeda Y, et al: Serum
cytokine and soluble cytokine receptor levels in patients with
non-alcoholic steatohepatitis. Liver Int. 26:39–45. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poniachik J, Csendes A, Diaz JC, et al:
Increased production of IL-1alpha and TNFalpha in
lipopolysaccharide-stimulated blood from obese patients with
non-alcoholic fatty liver disease. Cytokine. 33:252–257. 2006.
View Article : Google Scholar
|
|
26
|
Tomita K, Tamiya G, Ando S, et al: Tumour
necrosis factor alpha signaling through activation of Kupffer cells
plays an essential role in liver fibrosis of non-alcoholic
steatohepatitis in mice. Gut. 55:415–424. 2006. View Article : Google Scholar
|
|
27
|
Hui JM, Hodge A, Farrell GC, Kench JG,
Kriketos A and George J: Beyond insulin resistance in NASH:
TNF-alpha or adiponectin. Hepatology. 40:46–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lanzilli G, Cottarelli A, Nicotera G,
Guida S, Ravagnan G and Fuggetta MP: Anti-inflammatory effect of
resveratrol and polydatin by in vitro IL-17 modulation.
Inflammation. 35:240–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Song R, Bian HN, Brunk UT, Zhao M
and Zhao KS: Polydatin protects arterial smooth muscle cells
against mitochondrial dysfunction and lysosomal destabilization
following hemorrhagic shock. Am J Physiol Regul Integr Comp
Physiol. Jan 25–2002.(Epub ahead of print).
|
|
30
|
Shimano H: Sterol regulatory
element-binding proteins (SREBPs): transcriptional regulators of
lipid synthetic genes. Prog Lipid Res. 40:439–452. 2001. View Article : Google Scholar
|
|
31
|
Raghow R, Yellaturu C, Deng X, Park EA and
Elam MB: SREBPs: the crossroads of physiological and pathological
lipid homeostasis. Trends Endocrinol Metab. 19:65–73. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan D, Lehto M, Rasilainen L, et al:
Oxysterol binding protein induces upregulation of SREBP-1c and
enhances hepatic lipogenesis. Arterioscler Thromb Vasc Biol.
27:1108–1114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yahagi N, Shimano H, Hasty AH, Matsuzaka
T, Ide T and Yoshikawa T: Absence of sterol regulatory
element-binding protein-1 (SREBP-1) ameliorates fatty livers but
not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol
Chem. 277:19353–19357. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shimomura I, Bashmakov Y and Horton JD:
Increased levels of nuclear SREBP-1c associated with fatty livers
in two mouse models of diabetes mellitus. J Biol Chem.
274:30028–30032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahmed MH and Byrne CD: Modulation of
sterol regulatory element binding proteins (SREBPs) as potential
treatments for non-alcoholic fatty liver disease (NAFLD). Drug
Discov Today. 12:740–747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ntambi JM, Miyazaki M, Stoehr JP, et al:
Loss of stearoyl-CoA desaturase-1 function protects mice against
adiposity. Proc Natl Acad Sci USA. 99:11482–11486. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shrestha S, Ehlers SJ, Lee JY, Fernandez
ML and Koo SI: Dietary green tea extract lowers plasma and hepatic
triglycerides and decreases the expression of sterol regulatory
element-binding protein-1c mRNA and its responsive genes in
fructose-fed, ovariectomized rats. J Nutr. 139:640–645. 2009.
View Article : Google Scholar
|
|
38
|
Saltiel A and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Browning J and Horton J: Molecular
mediators of hepatic steatosis and liver injury. J Clin Invest.
114:147–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Utzschneider KM and Kahn SE: Review: the
role of insulin resistance in nonalcoholic fatty liver disease. J
Clin Endocrinol Metab. 91:4753–4761. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Previs SF, Withers DJ, Ren JM, White MF
and Shulman GI: Contrasting effects of IRS-1 versus IRS-2 gene
disruption on carbohydrate and lipid metabolism in vivo. J Biol
Chem. 275:38990–38994. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Samuel VT, Liu ZX, Qu X, et al: Mechanism
of hepatic insulin resistance in non-alcoholic fatty liver disease.
J Biol Chem. 279:32345–32353. 2004. View Article : Google Scholar : PubMed/NCBI
|