Introduction
It has been reported that the incidence of diabetes
has increased to up to 9.7% of the population over the age of 20 in
China (almost 92.4 million in total) (1). Globally, China has become the country
with the fastest growth in diabetes incidence, overtaking India.
Type 2 diabetes mellitus (T2DM) patients account for 90% of all
patients with diabetes, and the number of diabetes patients overall
has increased primarily due to the increase in T2DM patients. Over
the years, the clinical treatment of T2DM has involved using
traditional conservative therapy, including drugs to stimulate
insulin secretion and insulin replacement therapy. However,
according to a recent study, drug therapy and insulin replacement
therapy cannot delay the complications in high-risk T2DM patients
and cannot improve the prognosis (2). A weight-loss surgeon discovered
coincidentally that the blood glucose in obese Roux-en-Y gastric
bypass (RYGB) patients with T2DM decreased to normal levels
postoperatively and was maintained in the long-term (3). Currently, as the medical treatment of
T2DM and control of complications is generally ineffective, this
discovery will undoubtedly bring a glimmer of hope for the majority
of T2DM patients.
The main clinical manifestations of T2DM include
damaged islet cell function and progressive increases of insulin
resistance (4). In the
pathogenesis of T2DM, insulin resistance is an important link,
which is mainly caused by decreasing the glucose uptake and use of
the peripheral tissues. In the course of peripheral tissue glucose
utilization, the transmembrane transport of glucose, which is
mediated by glucose transporter 4 (GLUT4), is the main
rate-limiting step.
Certain studies have shown that peroxisome
proliferator-activated receptor γ2 (PPARγ2), tumor necrosis factor
α (TNFα) and GLUT4 are closely related to insulin resistance
(5–8). The expression of serum TNFα in
patients with T2DM was upregulated significantly (9,10).
Upregulation of GLUT4 may improve insulin resistance, while
downregulation of GLUT4 expression may induce or exacerbate insulin
resistance (11,12).
Currently, the mechanism of RYGB in the treatment of
T2DM has become an international hotspot for research. However, its
exact mechanism remains unclear. In this study, we supposed that
RYGB may improve blood glucose through regulating certain fat
factors and glucose transporter proteins. Therefore, investigating
the changes in PPARγ2, TNFα, phosphatidylinositol-3-kinase subunit
p85α (PI3Kp85α) and GLUT4 expression following RYGB surgery is
essential in order to reveal the mechanism involved.
Materials and methods
Animal grouping
The experimental animals [8-week-old Goto-Kakizaki
(GK) rats] were all purchased from SLAC, Ltd. (Shanghai, China).
When the 60 male GK rats were adapted to the environment after two
weeks, they were randomly divided into 6 groups (n=10). The surgery
group 1 (GB1), sham operation group 1 (SO1) and control group 1
(CO1) were monitored 20 days after RYGB surgery, while GB2, SO2 and
CO2 were monitored 30 days after RYGB surgery. The animals were
housed in a SPF animal experiment center in Shengjing Hospital of
China Medical University, and the feeding conditions were as
follows: a normal day-and-night cycle, temperature 25±1°C, relative
humidity 45±2%, with free access to water. The animal studies were
agreed with the China Medical University Animal Research
Committee.
Modeling
The GK rats underwent a 12-h fast and 4-h water
deprivation prior to surgery and then were anesthetized with 10%
chloral hydrate (3 ml/100 g intraperitoneal injection). We
performed a 2.5-cm long incision in the middle upper abdomen.
Gastric bypass surgery was performed in the surgery groups, in
which approximately 10% of the stomach was kept at the end and the
rest was removed, then the far end was closed. The jejunum was cut
off 10 cm from the Treitz ligaments, and the distal intestinal loop
lined residual gastrojejunostomy. End-to-side anastomosis was
performed 10 cm away from the anastomosis at the proximal bowel and
jejunum. In the sham groups, the cutting and in-situ
anastomosis was performed using the equivalent anesthesia method
and position, with the gastrointestinal tract anatomy unchanged. A
similar surgical procedure was performed to ensure the same length
of time, and consistency with 0/7 thread. The abdominal cavity was
flushed by a 3 ml solution with 800,000 units penicillin. Free
access to water was provided after the rats regained consciousness
from anesthesia and free food after 24 h. Free access to water was
provided in the control group.
Western blotting
Total protein from cells was extracted in lysis
buffer and quantified using the Bradford method. In total, 50 μg of
protein was separated by SDS-PAGE (12%). After transferring to
polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA,
USA), the membranes were incubated overnight at 4°C with antibodies
against PPARγ2, PI3Kp85α and GLUT4 (1:1000; Abcam, Ltd., MA, USA).
Following incubation with peroxidase-coupled anti-mouse IgG (Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA) at 37°C for 2 h,
bound proteins were visualized using ECL (Pierce Biotechnology,
Rockford, IL, USA) and detected using Bio Imaging Systems (UVP
Inc., Upland, CA, USA). The relative protein levels were calculated
based on β-actin protein as a loading control. The experiments were
repeated 3 times independently.
Real-time PCR
Expression of TNFα, PI3Kp85α and GLUT4 mRNA were
assayed using the TaqMan assay according to the manufacturer’s
instructions (Applied Biosystems, Foster City, CA, USA). Specific
RT primers and TaqMan probes were used to quantify the expression
of TNF-α, PI3Kp85α and GLUT4. For quantification of cell samples,
RT-PCR analysis was performed in three independent experiments,
each using three independent samples.
Statistical methods
The experimental data were expressed as the means ±
standard deviation and statistics were analyzed using SPSS 13.0 for
Windows software. A t-test was used to compare the weight change
prior to and following surgery. The single-factor analysis of
variance was used to compare the change between groups and within
the groups. The linear correlation analysis was used to compare the
correlation between two indicators. P<0.05 was considered to
indicate a statistically significant difference.
Results
Body weight and blood glucose decreased
following RYGB surgery
Twenty and 30 days after RYGB, the body weight of
the rats decreased to 247.54±4.44 g and 244.54±3.48 g,
respectively. The loss of weight was approximately 10%, and the
difference was statistically significant when compared with the
preoperative weight (P<0.01). However, the difference of body
weight in the GB1 and GB2 groups was relatively stable and showed
no significant difference (P>0.05). The weight in the GB group
decreased significantly compared with the SO and CO groups, and the
difference was statistically significant (P<0.01). The
concentration of fasting and postprandial 2-h blood glucose in the
GB group decreased significantly as compared to the SO and CO
groups, and the difference was statistically significant
(P<0.01). The blood glucose levels continued to decline at 20
days post-operation, but the difference was not statistically
significant when compared with that in the 30 days post-operation
group (P>0.05). The serum TNFα after 20 and 30 days showed no
significant difference, and was almost equal between the GB, SO and
CO groups (Table I).
 | Table IComparison of body weight, blood
glucose and TNFα. |
Table I
Comparison of body weight, blood
glucose and TNFα.
| Body weight (g) | Blood glucose |
|---|
|
|
|
|---|
| Groups | Pre-operation | Post-operation | Fasting | 2-hour | TNFα |
|---|
| CO1 | 273.8±3.83 | 273.27±2.89 | 11.2±1.03 | 22.1±1.05 | 129.05±0.77 |
| SO1 | 275.3±2.52 | 274.64±2.32 | 10.7±1.09 | 21.8±1.04 | 129.44±3.04 |
| GB1 | 275.0±2.91 | 247.54±4.44a,b | 6.6±0.58b | 7.6±0.6b | 129.84±5.54 |
| CO2 | 273.2±2.97 | 273.29±2.98 | 11.0±1.33 | 22.3±1.23 | 129.10±1.47 |
| SO2 | 275.8±4.06 | 275.52±4.34 | 10.8+1.13 | 21.4±1.09 | 128.84±2.27 |
| GB2 | 275.7±3.31 | 244.54±3.48a,b | 6.2±0.48b | 6.0±0.5b | 128.83±0.85 |
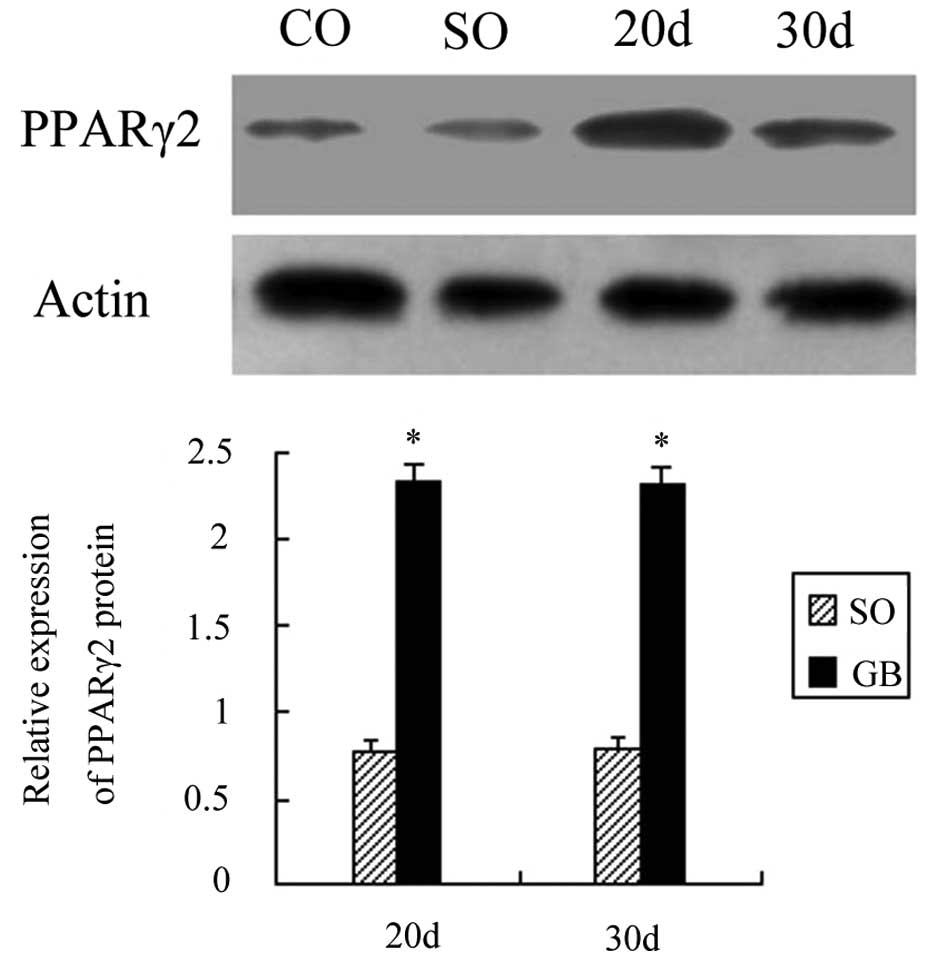
PPARγ2 protein expression increased
following RYGB surgery
A total of 20 days after RYGB surgery, the PPARγ2
protein expression was increased by 1.36 times as compared to the
CO group and the difference was statistically significant
(P<0.01). Thirty days after surgery, the PPARγ2 protein
expression was increased by 1.35 times and the difference was also
significant (P<0.01). The PPARγ2 protein expression between the
SO and CO groups showed no statistical significance (P>0.05).
The PPARγ2 protein expression also showed no significant difference
between the GB1 and GB2 groups (P>0.05; Fig. 1).
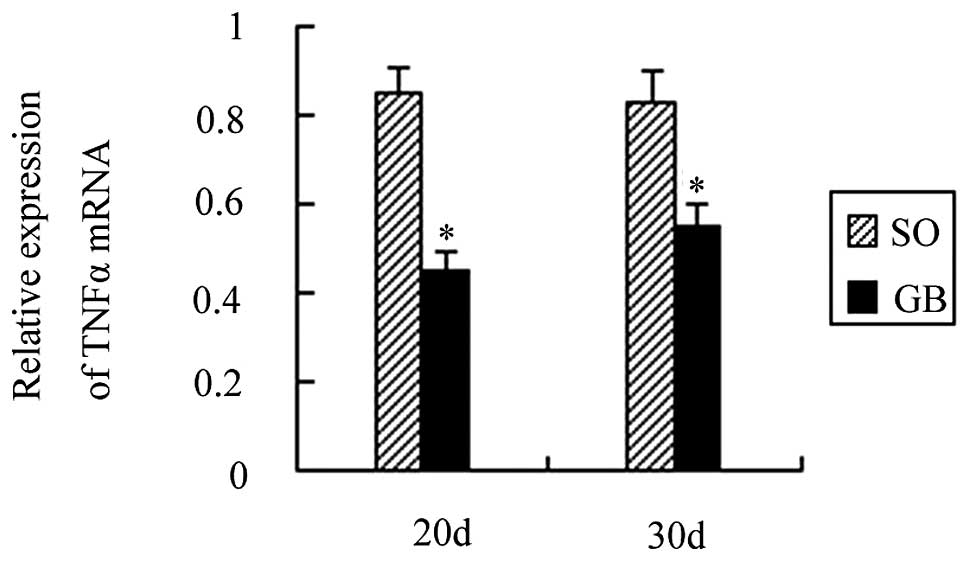
TNFα mRNA expression decreased following
RYGB surgery
A total of 20 days after surgery, the TNFα mRNA
expression was decreased by 0.51 times as compared to the CO group
and the difference was statistically significant (P<0.01). A
total of 30 days after surgery, TNFα mRNA expression was decreased
by 0.42 times as compared to the CO group and the difference was
also statistically significant (P<0.01). The TNFα mRNA
expression between the SO and CO groups was not statistically
significant (P>0.05). The TNFα mRNA expression was also not
significant between the GB1 and GB2 groups (P>0.05; Fig. 2).
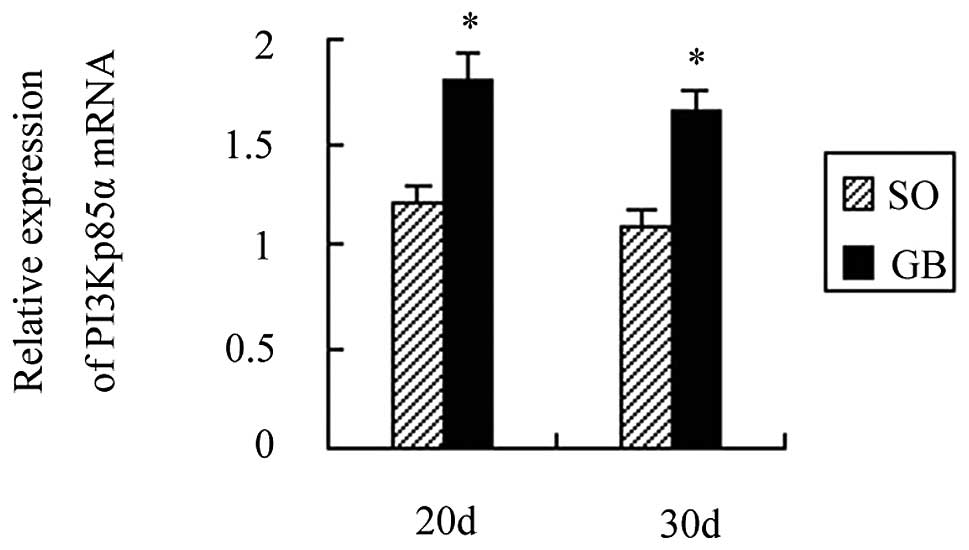
PI3Kp85α mRNA and protein expression
increased following RYGB surgery
A total of 20 days after surgery, PI3Kp85α mRNA
expression was increased by 1.89 times as compared to the CO group
and the difference was statistically significant (P<0.01). A
total of 30 days after surgery, PI3Kp85α mRNA expression was
increased by 1.85 times and the difference was also statistically
significant (P<0.01). PI3Kp85α mRNA expression between the SO
and CO groups was not statistically significant (P>0.05).
PI3Kp85α mRNA expression also showed no significant difference
between the GB1 and GB2 groups (P>0.05; Fig. 3).
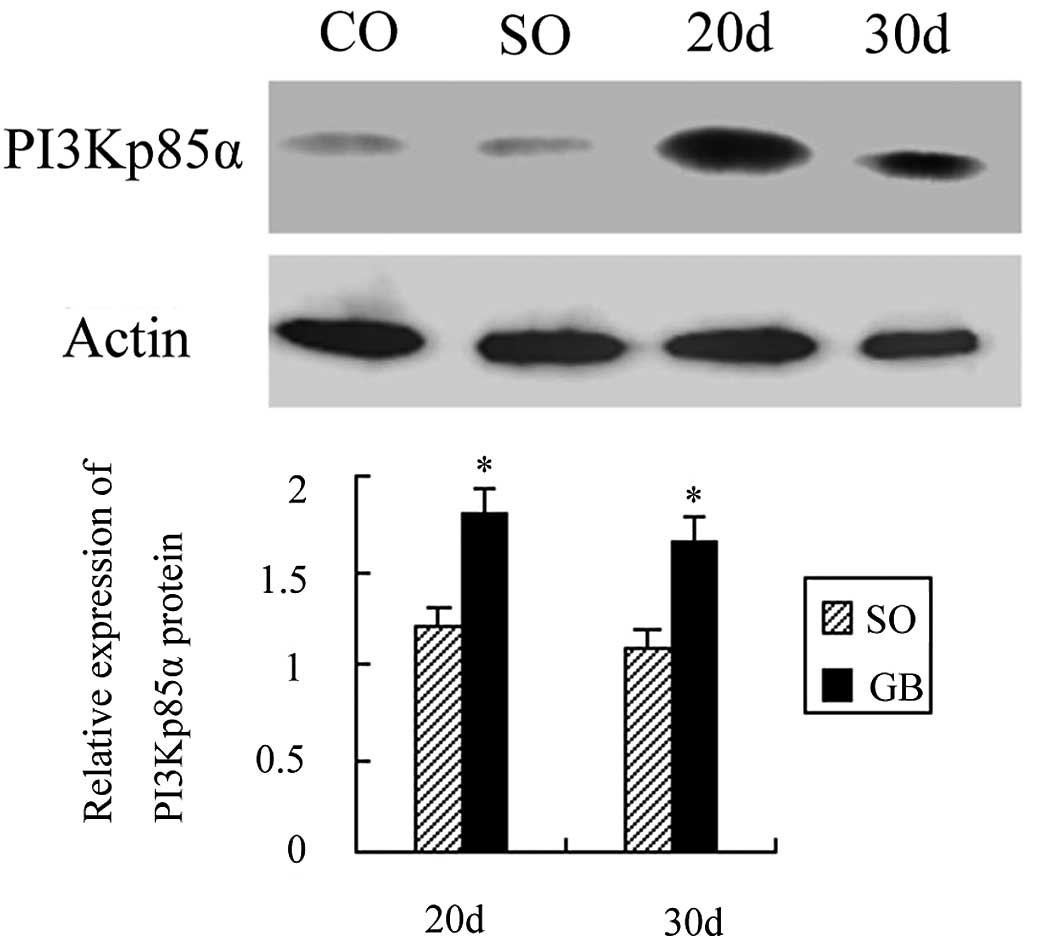
A total of 20 days after surgery, PI3Kp85α protein
expression was increased by 1.05 times as compared to the CO group
and the difference was statistically significant (P<0.01). A
total of 30 days after surgery, PI3Kp85α protein expression was
increased by 0.95 times and the difference was also statistically
significant (P<0.01). PI3Kp85α protein expression between the SO
and CO groups was not statistically significant (P>0.05).
PI3Kp85α protein expression was also not significantly different
between the GB1 and GB2 groups (P>0.05; Fig. 4).
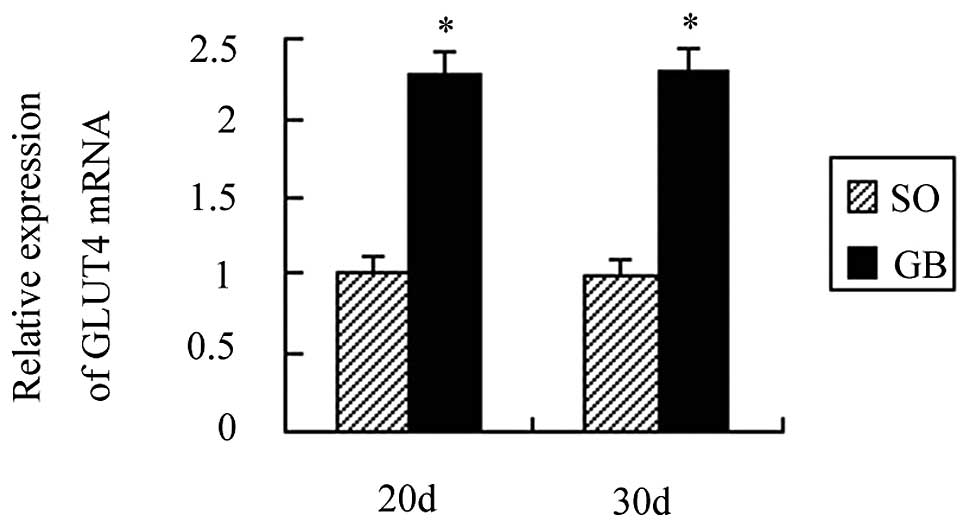
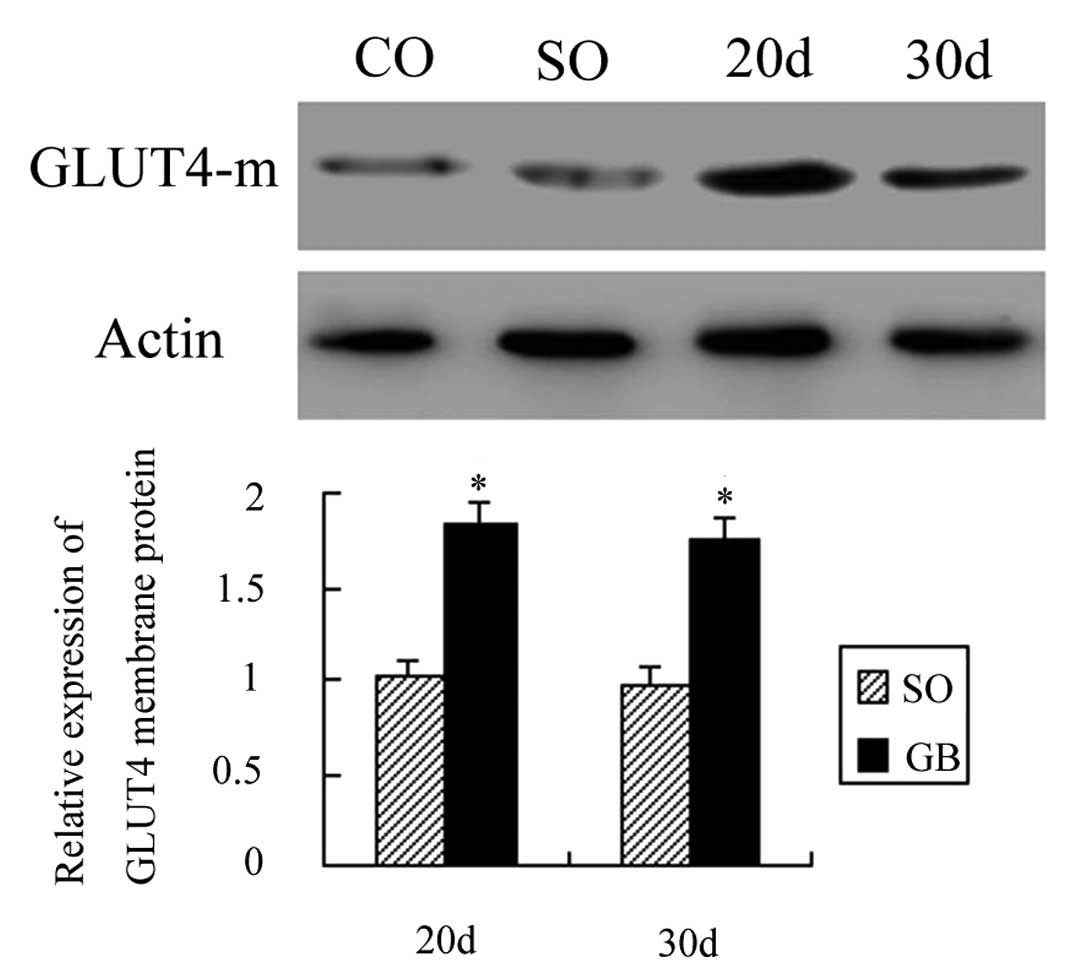
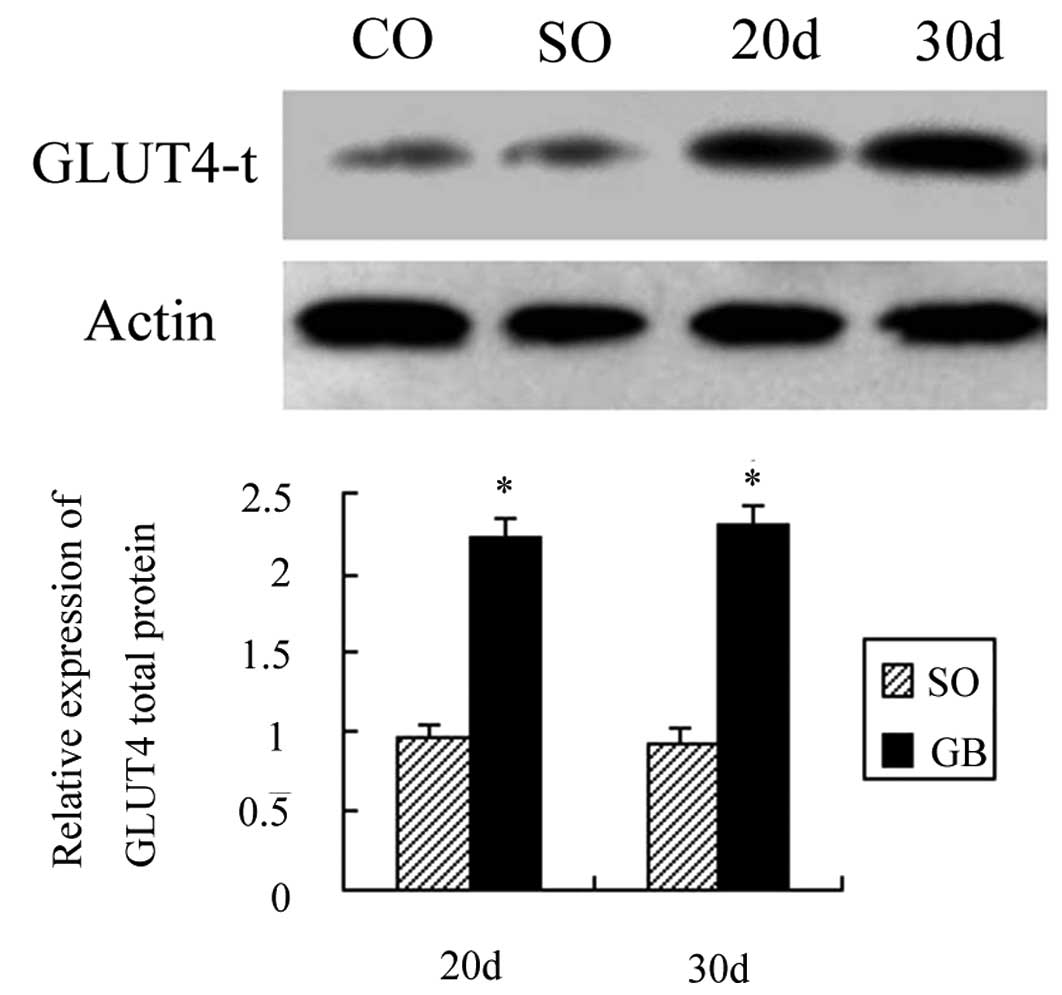
The GLUT4α mRNA, membrane protein and
total protein expression increased following RYGB surgery
A total of 20 days after surgery, GLUT4α mRNA
expression was increased by 1.24 times as compared to the CO group
and the difference was statistically significant (P<0.01). A
total of 30 days after surgery, GLUT4α mRNA expression was
increased by 1.29 times as compared to the CO group and the
difference was also statistically significant (P<0.01). GLUT4α
mRNA expression between the SO and CO groups was not statistically
significant (P>0.05). GLUT4α mRNA expression in the GB group was
also not significant after 20 and 30 days (P>0.05; Fig. 5).
A total of 20 days after surgery, the membrane GLUT4
protein expression was increased by 0.95 times as compared to the
CO group and the difference was statistically significant
(P<0.01). Thirty days after surgery, the membrane GLUT4 protein
expression was increased by 0.80 times as compared to the CO group
and the difference was also statistically significant (P<0.01).
The membrane GLUT4 protein expression between the SO and CO groups
was not statistically significant (P>0.05). The membrane GLUT4
protein expression in the GB group was also not significant after
20 and 30 days (P>0.05; Fig.
6).
A total of 20 days after surgery, the total GLUT4
protein expression was increased by 1.22 times as compared to the
CO and SO groups and the difference was statistically significant
(P<0.05). Thirty days after surgery, the total GLUT4 protein
expression was increased by 1.35 times as compared to the CO and SO
groups and the difference was also statistically significant
(P<0.05). The total GLUT4 protein expression was not significant
after 20 and 30 days (P>0.05; Fig.
7).
The correlation coefficient between fasting plasma
glucose and GLUT4 protein was equal to 0.618 and P<0.01. Fasting
plasma glucose and GLUT4 protein were negatively correlated, and
the difference was statistically significant.
Discussion
T2DM conventional treatment negatively impacts the
patient’s quality of life and requires long-term use of drugs. RYGB
provides a new method of therapy for T2DM patients, which has
changed the T2DM conventional treatment, improved patient quality
of life and reduced the diabetes-related mortality (13). At present, increasing numbers of
T2DM patients are selecting RYGB treatment (14). The possible mechanisms involved in
RYGB are as follows: i) reducing food intake; ii) weight loss; iii)
intestinal-insulin shaft mechanism. However, related studies
indicated that dietary restrictions could not improve the blood
glucose long-term. RYGB postoperative glucose was reduced earlier
than weight loss, indicating that weight loss and reducing the food
intake may not be the real cause of RYGB improving glucose levels.
However, the exact mechanism involved remains unclear (15–18).
In our study, RYGB postoperative fasting blood
glucose dropped to normal and the postprandial blood glucose
decreased markedly as compared to the control group and sham
operation group. It was confirmed that the RYGB not only reduced
fasting blood glucose, but also reduced the postprandial blood
glucose. Twenty and 30 days after surgery, TNFα mRNA expression was
decreased and GLUT4 mRNA, PI3Kp85α, PPARγ2, and membrane and total
GLUT4 protein expression were all elevated in postoperative adipose
tissue. However, serum TNFα levels did not change significantly.
Increased PPARγ2 may inhibit the expression of TNFα mRNA, reduce
TNFα expression in the fatty tissue, and not only increase PI3K
expression through an autocrine manner and promote the
translocation of GLUT4, but also increase the total GLUT4 protein
in the fat cells, thereby improving insulin resistance (19,20).
We suggest that increasing PPARγ2 protein,
inhibiting TNFα transcription, upregulating PI3Kp85α mRNA and
protein, promoting GLUT4 protein translocation and upregulating
total GLUT4 protein are the possible mechanisms by which RYGB
improves insulin resistance and reduces fasting blood glucose in
adipose tissue.
Adipose tissue is a target tissue of insulin action.
Many fat factors are closely related to insulin resistance
(21). PPARγ is specifically
expressed in adipose tissue and the possible mechanism by which
PPARγ improves insulin resistance may be as follows: i) PPARγ
promotes fat cell differentiation. By this mechanism, activated
PPARγ is capable of promoting white adipose cell differentiation
and increasing the number of small fat cells and decreasing the
number of large fat cells. Small fat cells respond more strongly to
insulin and promote glucose uptake more easily. ii) PPARγ enhances
the insulin signal transduction. PI3K is the key kinase that
mediates transport of glucose into the target cells. The pathway,
which is mediated by PI3K, is the main method of insulin signal
transduction. The physiologically active PPARγ is capable of
upregulating PI3Kp85α expression and promoting signal transduction,
thereby improving insulin resistance. iii) PPARγ inhibits the lipid
metabolism. PPARγ is involved in the expression and regulation of
related genes in lipid metabolism. Activated PPARγ may decrease
fatty acids, which are transferred to the muscles and liver tissue.
Adipose synthesis is then reduced, thereby improving insulin
resistance.
TNFα is an adipose factor that is secreted from
adipose tissue to cause insulin resistance. The mechanism by which
TNFα affects adipose tissue insulin resistance may be as follows:
i) TNFα inhibits tyrosine phosphorylation of insulin receptor
substrate 1, thereby inhibiting PI3K activity and impairing the
insulin signaling pathway (22,23);
ii) TNFα promotes the decomposition of fat cells and increases free
fatty acids; iii) TNFα affects GLUT4 mRNA and protein expression
(24–26); iv) TNFα synergizes other cytokines
(7,10,27).
In ob/ob mice and Zucker obese rats, TNFα mRNA is overexpressed and
neutralizing TNFα is capable of alleviating insulin resistance
(28).
GLUT4 protein is the main effector molecule in
insulin-mediated glucose uptake and improving insulin resistance.
Overexpression of GLUT4 protein may alleviate insulin resistance
(29,30). The animals with overexpression of
GLUT4 protein may have improved glucose uptake and utilization, and
this may significantly increase insulin sensitivity. Abel et
al(31) selectively reduced
the GLUT4 expression in fat cells by recombinant DNA technology and
found that insulin-stimulated glucose uptake capacity reduced
significantly in fat cells.
Our results showed that PPARγ expression was
elevated following RYGB in adipose tissue and then TNFα mRNA
transcription was reduced, while the serum TNFα concentration was
normal. The possible mechanism is that TNFα plays a role through a
paracrine or autocrine method (31). While increasing the PI3Kp85α
expression, the switch of GLUT4 from vesicles to the cell membrane
was increased. At the same time, reducing TNFα content in adipose
tissue may alleviate the inhibition effect on GLUT4 mRNA and
increase the total GLUT4 content, thereby increasing glucose
transport and insulin sensitivity.
In short, RYGB may improve insulin resistance and
treat type 2 diabetes through upregulation of PPARγ2 protein,
downregulation of TNFα mRNA transcription, upregulation of PI3Kp85α
expression, induction of the translocation of GLUT4, upregulation
of GLUT4 mRNA transcripts and increased total GLUT4 content in
adipose tissue. However, the mechanism by which PPARγ2 protein is
upregulated requires further study.
References
|
1
|
Yang W, Lu J, Weng J, et al: Prevalence of
diabetes among men and women in China. N Engl J Med. 362:1090–1101.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zimmet P, Alberti KG and Shaw J: Global
and societal implications of the diabetes epidemic. Nature.
414:782–787. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Couzin J: Medicine. Bypassing medicine to
treat diabetes. Science. 320:438–440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lazar MA: How obesity causes diabetes: not
a tall tale. Science. 307:373–375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kadowaki T, Hara K, Kubota N, et al: The
role of PPARgamma in high-fat diet-induced obesity and insulin
resistance. J Diabetes Complications. 16:41–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mishima Y, Kuyama A, Tada A, Takahashi K,
Ishioka T and Kibata M: Relationship between serum tumor necrosis
factor-alpha and insulin resistance in obese men with Type 2
diabetes mellitus. Diabetes Res Clin Pract. 52:119–123. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruan H and Lodish HF: Insulin resistance
in adipose tissue: direct and indirect effects of tumor necrosis
factor-alpha. Cytokine Growth Factor Rev. 14:447–455. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murakami K, Bujo H, Unoki H and Saito Y:
High fat intake induces a population of adipocytes to co-express
TLR2 and TNFalpha in mice with insulin resistance. Biochem Biophys
Res Commun. 354:727–734. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen H, Ren A, Hu S, Mo W, Xin X and Jia
W: The significance of tumor necrosis factor-alpha in newly
diagnosed type 2 diabetic patients by transient intensive insulin
treatment. Diabetes Res Clin Pract. 75:327–332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Yang G, Shi S, Yang M, Liu H and
Boden G: The adipose triglyceride lipase, adiponectin and visfatin
are downregulated by tumor necrosis factor-alpha (TNF-alpha) in
vivo. Cytokine. 45:12–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garvey WT, Maianu L, Huecksteadt TP,
Birnbaum MJ, Molina JM and Ciaraldi TP: Pretranslational
suppression of a glucose transporter protein causes insulin
resistance in adipocytes from patients with non-insulin-dependent
diabetes mellitus and obesity. J Clin Invest. 87:1072–1081. 1991.
View Article : Google Scholar
|
|
12
|
Zisman A, Peroni OD, Abel ED, et al:
Targeted disruption of the glucose transporter 4 selectively in
muscle causes insulin resistance and glucose intolerance. Nat Med.
6:924–928. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adams TD, Gress RE, Smith SC, et al:
Long-term mortality after gastric bypass surgery. N Engl J Med.
357:753–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buchwald H and Oien DM:
Metabolic/bariatric surgery Worldwide 2008. Obes Surg.
19:1605–1611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laville M and Disse E: Bariatric surgery
for diabetes treatment: why should we go rapidly to surgery.
Diabetes Metab. 35:562–563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mingrone G and Castagneto-Gissey L:
Mechanisms of early improvement/resolution of type 2 diabetes after
bariatric surgery. Diabetes Metab. 35:518–523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scheen AJ, De Flines J, De Roover A and
Paquot N: Bariatric surgery in patients with type 2 diabetes:
benefits, risks, indications and perspectives. Diabetes Metab.
35:537–543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andreelli F, Amouyal C, Magnan C and
Mithieux G: What can bariatric surgery teach us about the
pathophysiology of type 2 diabetes? Diabetes Metab. 35:499–507.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rieusset J, Chambrier C, Bouzakri K, et
al: The expression of the p85alpha subunit of phosphatidylinositol
3-kinase is induced by activation of the peroxisome
proliferator-activated receptor gamma in human adipocytes.
Diabetologia. 44:544–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iwata M, Haruta T, Usui I, et al:
Pioglitazone ameliorates tumor necrosis factor-alpha-induced
insulin resistance by a mechanism independent of adipogenic
activity of peroxisome proliferator--activated receptor-gamma.
Diabetes. 50:1083–1092. 2001. View Article : Google Scholar
|
|
21
|
Antuna-Puente B, Feve B, Fellahi S and
Bastard JP: Adipokines: the missing link between insulin resistance
and obesity. Diabetes Metab. 34:2–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hotamisligil GS, Budavari A, Murray D and
Spiegelman BM: Reduced tyrosine kinase activity of the insulin
receptor in obesity-diabetes. Central role of tumor necrosis
factor-alpha. J Clin Invest. 94:1543–1549. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hotamisligil GS, Peraldi P, Budavari A,
Ellis R, White MF and Spiegelman BM: IRS-1-mediated inhibition of
insulin receptor tyrosine kinase activity in TNF-alpha- and
obesity-induced insulin resistance. Science. 271:665–668. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stephens JM and Pekala PH: Transcriptional
repression of the C/EBP-alpha and GLUT4 genes in 3T3-L1 adipocytes
by tumor necrosis factor-alpha. Regulations is coordinate and
independent of protein synthesis. J Biol Chem. 267:13580–13584.
1992.
|
|
25
|
Fernandez-Veledo S, Hernandez R, Teruel T,
Mas JA, Ros M and Lorenzo M: Ceramide mediates TNF-alpha-induced
insulin resistance on GLUT4 gene expression in brown adipocytes.
Arch Physiol Biochem. 112:13–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mingrone G, Rosa G, Di Rocco P, et al:
Skeletal muscle triglycerides lowering is associated with net
improvement of insulin sensitivity, TNF-alpha reduction and GLUT4
expression enhancement. Int J Obes Relat Metab Disord.
26:1165–1172. 2002. View Article : Google Scholar
|
|
27
|
Chung S, Lapoint K, Martinez K, Kennedy A,
Boysen Sandberg M and McIntosh MK: Preadipocytes mediate
lipopolysaccharide-induced inflammation and insulin resistance in
primary cultures of newly differentiated human adipocytes.
Endocrinology. 147:5340–5351. 2006. View Article : Google Scholar
|
|
28
|
Hotamisligil GS, Shargill NS and
Spiegelman BM: Adipose expression of tumor necrosis factor-alpha:
direct role in obesity-linked insulin resistance. Science.
259:87–91. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carvalho E, Kotani K, Peroni OD and Kahn
BB: Adipose-specific overexpression of GLUT4 reverses insulin
resistance and diabetes in mice lacking GLUT4 selectively in
muscle. Am J Physiol Endocrinol Metab. 289:E551–561. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brozinick JT Jr, McCoid SC, Reynolds TH,
et al: GLUT4 overexpression in db/db mice dose-dependently
ameliorates diabetes but is not a lifelong cure. Diabetes.
50:593–600. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abel ED, Peroni O, Kim JK, et al:
Adipose-selective targeting of the GLUT4 gene impairs insulin
action in muscle and liver. Nature. 409:729–733. 2001. View Article : Google Scholar : PubMed/NCBI
|