Introduction
Graves’ disease (GD), the most common cause of
hyperthyroidism, usually presenting itself during early
adolescence, is an autoimmune disease in which the thyroid is
overactive, producing an excessive amount of thyroid hormones. It
has been demonstrated that hyperactivation of the thyroid is caused
by thyroid autoantibodies [thyroid stimulating hormone (TSH; also
known as thyrotropin) receptor (TSHR)-Ab)] that activate the TSHR,
thereby stimulating thyroid hormone synthesis and secretion, as
well as thyroid growth (1).
Hyperthyroidism may cause a marked combination of
neuropsychological and physical signs and symptoms. In addition to
hyperthyroidism, clinical involvement of the eyes, Graves’
ophthalmopathy (GO), develops in 25 to 50% of individuals with GD
(2). Although certain patients
with GO experience only mild ocular discomfort, approximately 5%
have severe ophthalmopathy, including excessive chemosis, proptosis
or even loss of vision (3). The
clinical symptoms and signs of GO can be explained mechanically by
the discrepancy between the increased volume of the swollen orbital
tissues and the fixed volume of the bony orbit (2). The expanded orbital tissues displace
the globe forward and impede venous outflow from the orbit. These
changes, combined with the local production of cytokines and other
mediators of inflammation, result in pain, proptosis, periorbital
edema, conjunctival injection and chemosis.
For GO treatment, the first step usually involves
the regulation of thyroid hormone levels. Topical lubrication of
the ocular surface is used to avoid corneal damage caused by
exposure. Tarsorrhaphy is an alternative option when the
complications of ocular exposure cannot be avoided solely with the
eye drops. Corticosteroids are efficient in reducing orbital
inflammation; however, the benefits cease after discontinuation.
Corticosteroid treatment is also limited due to the many
side-effects (4). Radiotherapy is
an alternative option to reduce acute orbital inflammation
(5). However, there remains
controversy surrounding its efficacy. A simple way of reducing
inflammation is smoking cessation, as pro-inflammatory substances
are found in cigarettes (6).
Surgery may also be performed to decompress the orbit, to improve
the proptosis and to address the strabismus causing diplopia.
Surgery is performed once the patient’s disease has been stable for
at least 6 months. Eyelid surgery is the most common surgery
performed on GO patients.
Several novel approaches for the treatment of GO
follow logically from the current understanding of pathogenesis.
Studies from several laboratories underline the pathogenic role of
both Th-1-type and macrophage-derived cytokines in early disease
pathogenesis (7). Therefore,
monoclonal antibodies that target pro-inflammatory cytokines and
chemokines may hold particular promise. Specifically, biological
agents that block the tumor necrosis factor (TNF) (infliximab,
adalimumab or etanercept) or interleukin (IL)-1 receptor (anakinra)
are attractive theoretical choices. These agents are effective in
rheumatoid arthritis and Crohn’s disease therapy, and they are
under investigation for the treatment of diverse conditions, such
as uveitis, sarcoidosis, interstitial lung disease,
graft-versus-host disease and Sjögren’s syndrome. However, although
these drugs have revolutionized the treatment of several
immune-mediated inflammatory diseases, there is growing evidence
that TNF inhibition is associated with serious side-effects. Of
particular concern are several case reports of serious infections,
including the reactivation of Mycobacterium
tuberculosis(8). In addition,
lymphoma, demyelinating disorders, hepatotoxicity, aplastic anemia
and lupus-like syndrome have been described in association with TNF
antagonists. In the future, the application of knowledge concerning
genetic variability and TNF/TNF receptor polymorphisms may aid in
the targeting of anti-TNF therapy for patients most likely to
benefit and least likely to have adverse effects.
A study by Peyster et al showed that there
was excellent correlation between proptosis and percentage fat
volume, supporting the contention that increased orbital fat is
responsible for proptosis (9). In
another study by Kumar et al, researchers determined whether
the expanded adipose tissue volume may be in part attributable to
de novo adipogenesis by measuring the levels of mRNA
encoding leptin, adiponectin, peroxisome proliferator-activated
receptor (PPAR), preadipocyte factor-1 and TSHR sgenes in orbital
adipose tissues from GO patients and normal individuals, and in
orbital preadipocyte cultures derived from GO patients and normal
subjects using quantitative real-time RT PCR. The results showed
increased leptin, adiponectin, PPAR and TSHR expression in GO
compared with normal orbital tissue samples, with positive
correlations in the GO tissues between TSHR and leptin, adiponectin
and PPAR (10). Th in vitro
differentiation of GO and normal preadipocytes resulted in enhanced
adiponectin, leptin and TSHR expression, with greater expression of
the latter 2 genes in the GO cultures. These results suggest that
de novo adipogenesis within orbital tissues with parallel
enhanced expression of TSHR may be important in the pathogenesis of
GO, and that potential therapies for GO may include the inhibition
of the adipogenic pathway. Preadipocytes may also be separated from
the orbital adipose tissue of GO patients and become differentiated
into mature adipocytes in vitro, indicating that
preadipocyte differentiation may occur (11).
Pingmu decoction is composed of a variety of Chinese
herbal medicines (Astragalus 30 g, Herba epimedii 15
g, root of red rooted Salvia 15 g, Semen brassicae 15
g, Plantago seed 15 g, Oldenlandia diffusa 30 g). In
our previous study (12), we
demonstrated that Pingmu decoction effectively alleviated GO
progression. In this study, we investigated the underlying
molecular mechanisms by determining the effect of Pingmu decoction
on adipocyte proliferation and apoptosis.
Materials and methods
Cell culture
GO orbital adipose tissue samples were minced and
placed directly in plastic culture dishes, allowing preadipocyte
fibroblasts to proliferate as described previously (13). Cells were propagated in medium 199
containing 20% fetal bovine serum (FBS; HyClone Laboratories, Inc.,
Logan, UT, USA), penicillin (100 U/ml) and gentamicin (20 μg/ml) in
a humidified 5% CO2 incubator at 37°C and were
maintained in 80-mm2 flasks with medium 199 containing
10% FBS and antibiotics. To initiate adipocyte differentiation,
orbital cells were grown to confluence in 6-well plates in medium
199 with 10% FBS. Differentiation was carried out as reported
previously (11); cultures were
changed to serum-free DMEM/Ham’s F-12 (1:1; Sigma-Aldrich Corp.,
St. Louis, MO, USA) supplemented with biotin (33 μm), pantothenic
acid (17 μm), apotransferrin (10 μg/ml), T3 (0.2 nm), insulin (1
μm), carbaprostacyclin (0.2 μm; Calbiochem, La Jolla, CA, USA) and,
for the first 4 days only, dexamethasone (1 μm) and
isobutylmethylxanthine (0.1 mm). The differentiation protocol was
continued for 10 days, during which time the medium was replaced
every 3–4 days. Undifferentiated cultures were derived from
fibroblasts obtained from the orbital tissues of the same patients
and were maintained for the same period of time in medium lacking
several of the components necessary for complete adipocyte
differentiation (i.e., carbaprostacyclin, dexamethasone and
isobutylmethylxanthine).
Rat treatment and serum preparation
Male Sprague-Dawley (SD) rats (8 rats for each
group) were fed Pingmu decoction or other combinations of
ingredients twice a day for 5 consecutive days: Pingmu decoction;
Astragalus; Herba epimedii; root of red-rooted Salvia
+ Semen brassicae + Plantago seed + Oldenlandia
diffusa; Astragalus + root of red-rooted Salvia +
Semen brassicae + Plantago seed + Oldenlandia
diffusa; Astragalus + Herba epimedii, Herba
epimedii + root of red-rooted Salvia + Semen
brassicae + Plantago seed + Oldenlandia diffusa.
Serum was drawn from the abdominal artery 1 h following the final
administration and heat inactivated
Cell apoptosis detection
Adipocytes from the different treatment groups were
trypsinized, collected, washed and then stained with Annexin V-FITC
and propidium iodide (PI) (BD Biosciences, Heidelberg, Germany) for
10 min at 4°C in the dark according to the manufacturer’s
instructions. Apoptotic cells were determined by flow cytometry
analysis (FACScan; BD Biosciences). The extent of apoptosis was
quantified as the percentage of Annexin V-positive cells.
Cell cycle analysis
Following the indicated treatments, adipocytes were
harvested and washed twice with 1× PBS and fixed in 70% ethanol at
−20°C for 16 h. The fixed cells were collected, washed twice with
PBS and suspended in PBS containing 10 μg/ml PI (Sigma-Aldrich) and
100 μg/ml RNase A, then incubated at 4°C for at least 30 min
avoiding light in order to eliminate the intracellular RNA. Cell
cycle distribution was determined using FACSCalibur flow cytometer
(FACScan; BD Biosciences).
Western blot analysis
Cells were lysed directly on the culture dishes
using lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.02%
NaN3, 1% Triton X-100, 1 mM PMSF, 1 μg/ml aprotinin and
1 μg/ml leupeptin). The protein concentration was determined by the
Bradford assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Equal
amounts of total protein were subjected to 10% SDS-PAGE and then
transferred onto PVDF membranes. Following overnight blocking with
5% non-fat milk at 4°C, the membranes were incubated with primary
antibodies as follows: anti-caspase-3, anti-caspase-8,
anti-caspase-9 anti-cyclin D1, anti-cyclin E1, anti-CDK4,
anti-Bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and
anti-GAPDH antibodies (BD Biosciences) overnight at 4°C. The
membranes were then incubated with secondary antibody conjugated to
horseradish peroxidase (Jackson ImmunoResearch, West Grove, PA,
USA). The protein was visualized using ECL western blotting
detection reagents and was then analyzed through scanning
densitometry using the Tanon Image system.
Indirect immunofluorescence
Isolated preadipocytes were cultured for 24 h and
fixed with 4% formaldehyde for 15 min. Cells were washed 3 times
with PBS and blocked with 5% BSA in PBS prior to Pref-1 primary
antibody (Santa Cruz Biotechnology) incubation. Cells were then
stained with FITC-conjugated secondary antibody
(JacksonImmunoResearch) and observed under a fluorescence
microscope.
Statistical analysis
All experiments were performed in duplicate or
triplicate. The data were treated using one-way ANOVA to determine
statistically significant differences. P<0.05 was considered to
indicate significance and is shown by asterisks in the figures.
Results
Preadipocyte separation and
identification
Orbital adipose tissues were removed from GO
patients and preadipocytes were isolated using a previously
published method (13). Following
5 to 10 days in culture, the preadipocytes were elongated cells
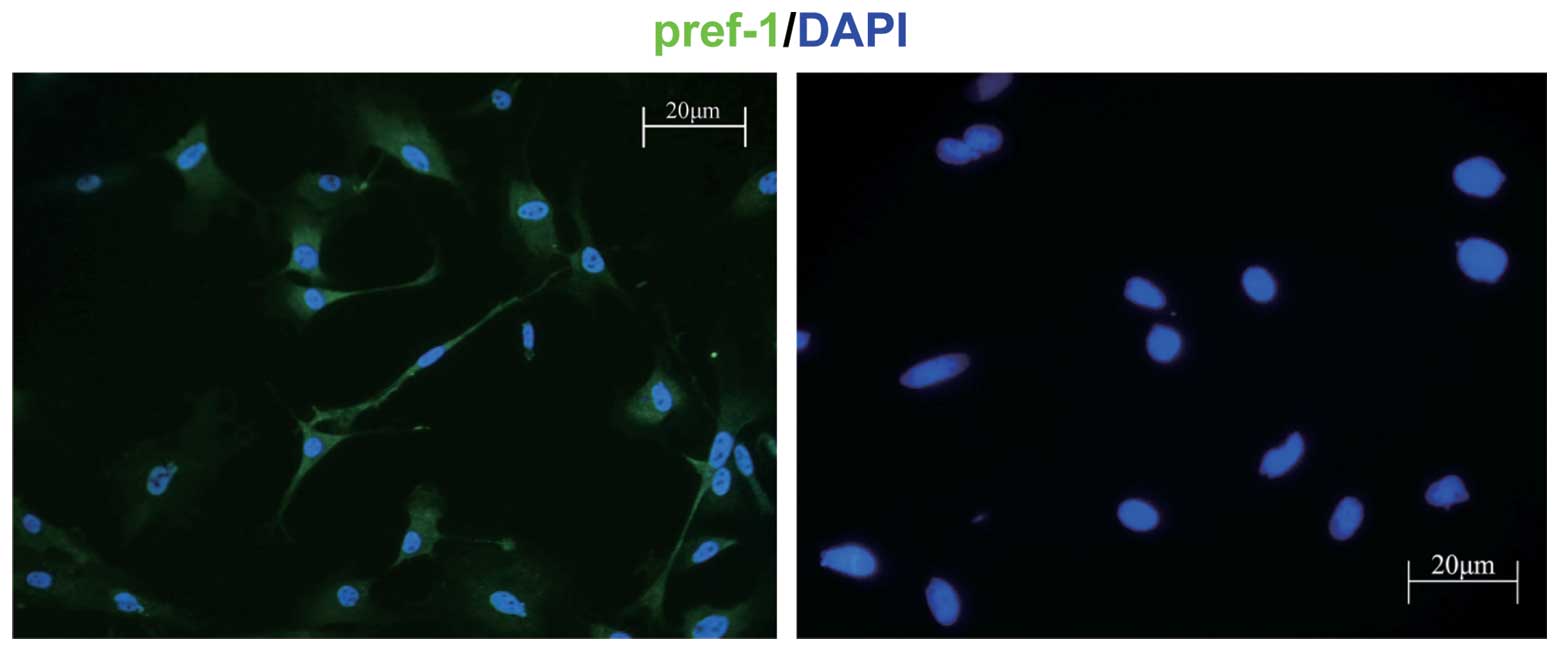
with the appearance of fibroblasts. Immunofluorescence assays with
Pref-1 antibody were carried out to identify these cells and to
determine the purity. After examination under a fluorescence
microscope, we were able to show that almost all cells stained
positive, indicating that these cells were preadipocytes (Fig. 1).
Effects of Pingmu decoction on
preadipocyte proliferation
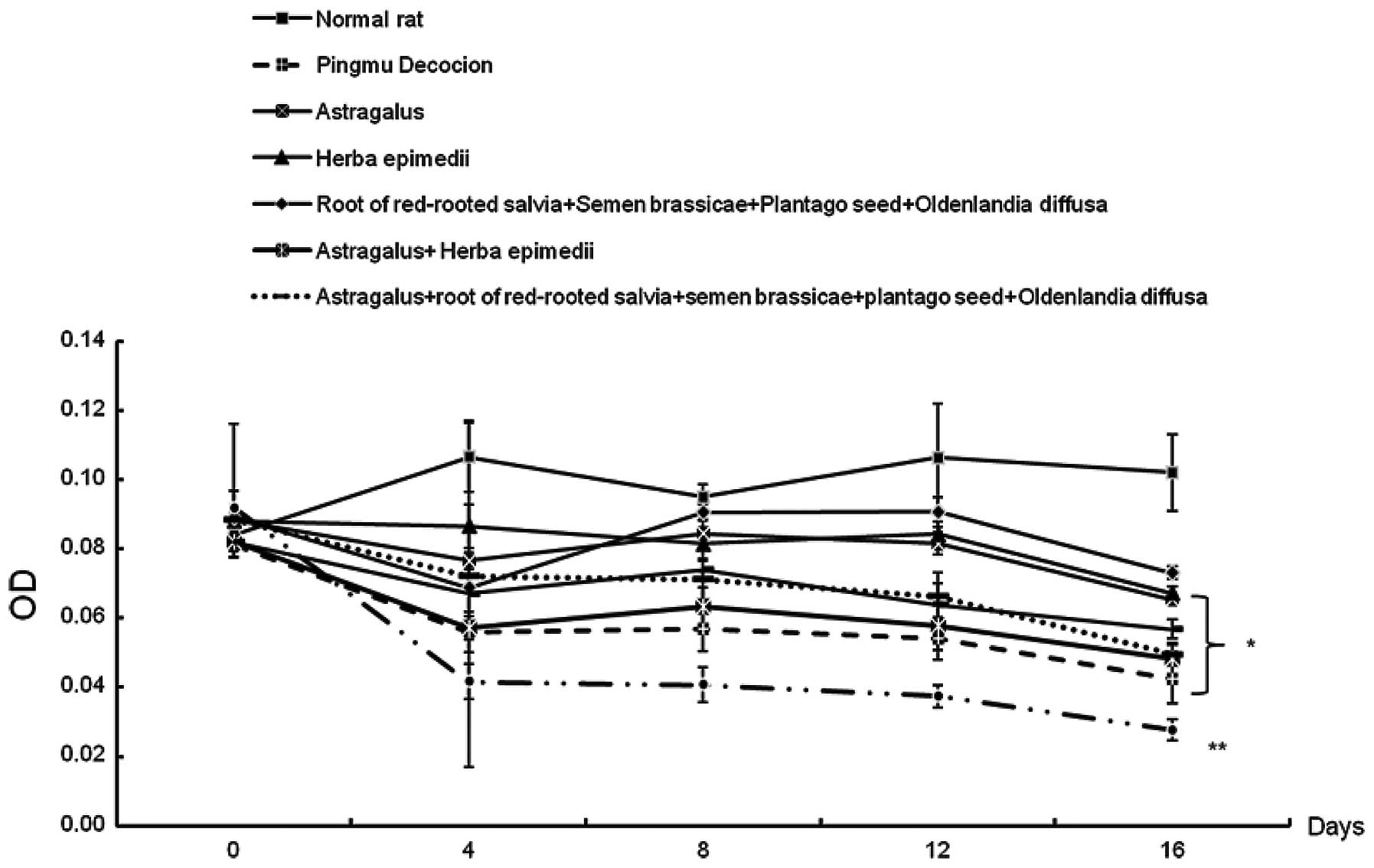
In order to determine the effect of Pingmu decoction
on preadipocyte proliferation, we incubated preadipocytes with
serum from rats fed with Pingmu decoction or other combinations of
ingredients and examined the cell cycle progression by MTT assay at
different time-points. As demonstrated in Fig. 2, Pingmu-containing serum, along
with serum from the other groups of rats fed with the other
combinations of ingredients, significantly reduced preadipocyte
proliferation.
Effects of Pingmu decoction on the cell
cycle and apoptosis of adipocyte
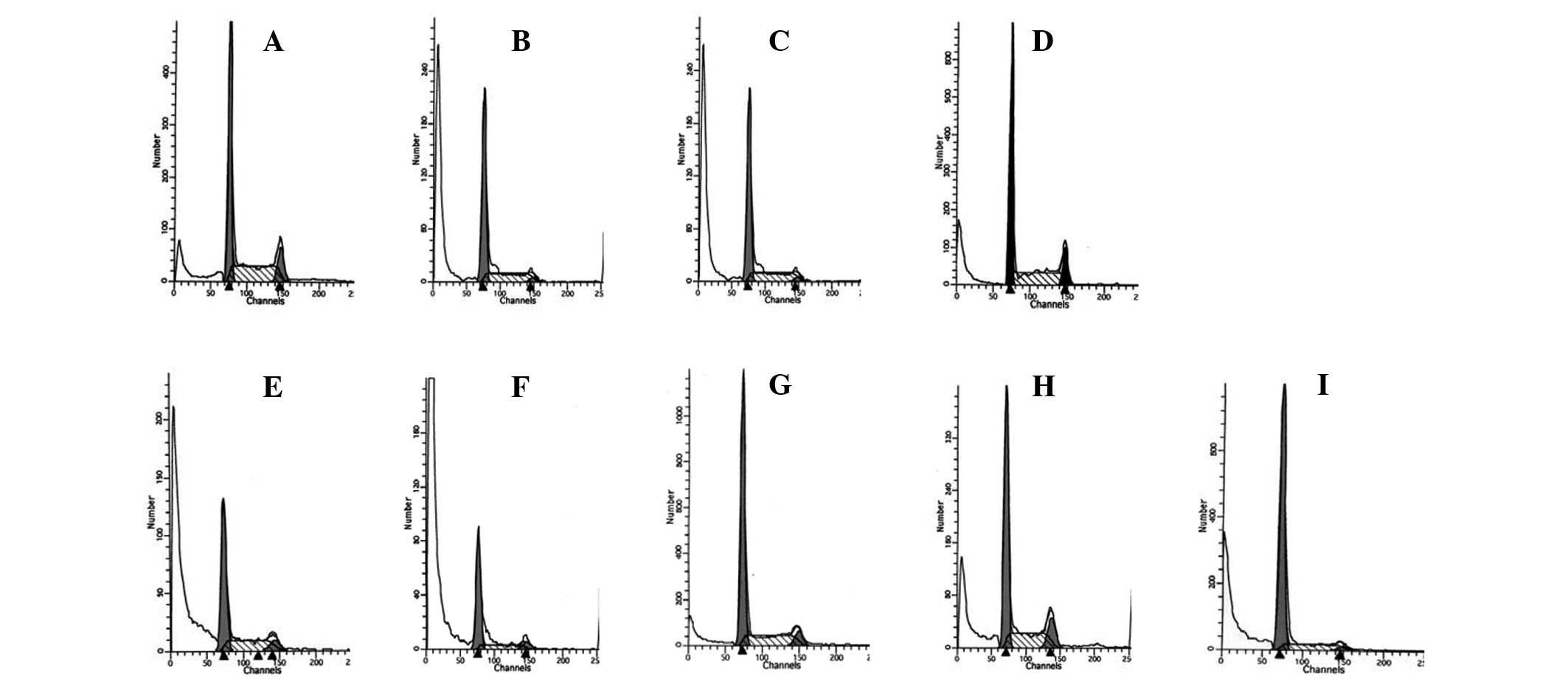
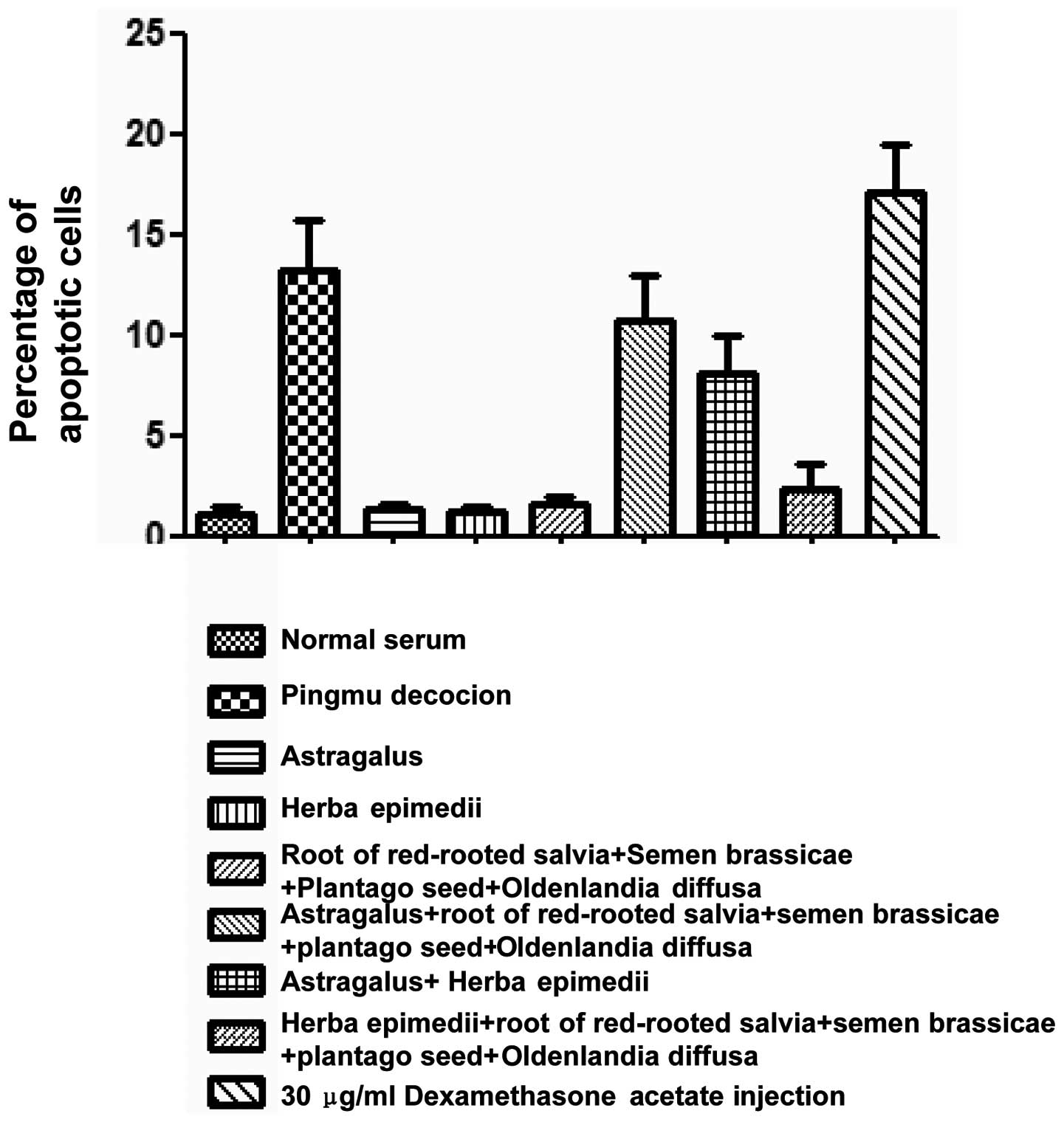
To assess the effects of Pingmu decoction on the
cell cycle and apoptosis of adipocytes, we first detected cell
cycle distribution and Annexin V/FITC staining by flow cytometry.
These experiments showed that Pingmu decoction-containing serum
caused an increase in the G1/G0 cell percentage at a level similar
to dexamethasone (Fig. 3) and
enhanced Annexin V staining levels (Fig. 4). These results were confirmed by
western blot analysis, which showed that Pingmu decoction serum had
a similar effect as dexamethasone on reducing the expression of the
cell cycle-related genes (Fig. 5).
The results indicated that Pingmu had a significant effect on the
inhibition of preadipocyte proliferation. We then examined the
cleavage of caspase-3, 8 and 9 by immunobloting, discovering that
Pingmu decoction-containing serum, with a similar efficacy as
dexamethasone, prompted the cleavage of caspase-3, 8 and 9
(Fig. 6). These data suggest that
Pingmu decoction significantly increases adipocyte apoptosis.
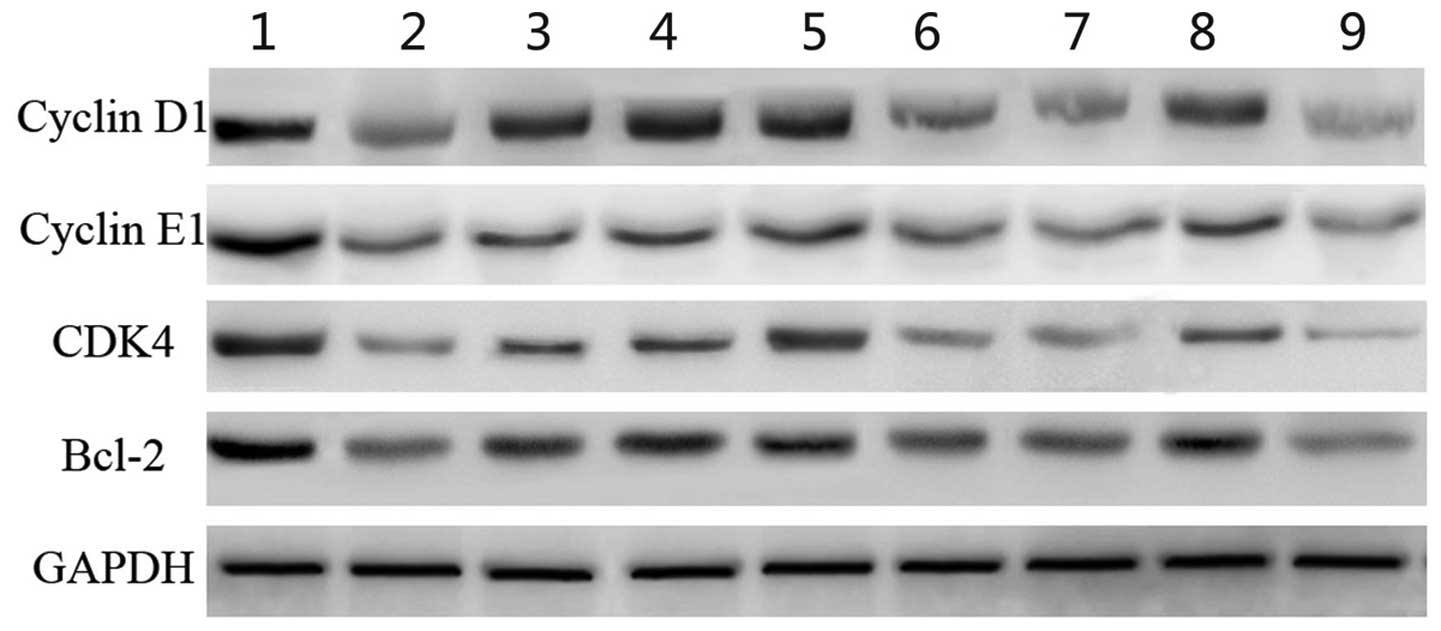 | Figure 5Pingmu decoction inhibited the
expression of cell cycle related genes. Adipocytes were treated
with serum from the different groups of rats and cultured for 2
days, and then the total proteins of the cells were extracted.
Western blot analysis was performed to determine the expression of
cell cycle-related genes, including cyclin D1, cyclin E1, CDK4 and
Bcl-2. GAPDH served as the internal control. Lane 1, normal rat;
lane 2, Pingmu decoction; lane 3, Astragalus; lane 4,
Herba epimedii; lane 5, Root of red-rooted Salvia +
Semen brassicae + Plantago seed + Oldenlandia
diffusa; lane 6, Astragalus + root of red-rooted
Salvia + Semen brassicae + Plantago seed +
Oldenlandia diffusa; lane 7, Astragalus + Herba
epimedii; lane 8, Herba epimedii + root of red-rooted
Salvia + Semen brassicae + Plantago seed +
Oldenlandia diffusa; lane 9, 30 μgml dexamethasone acetate
injection. |
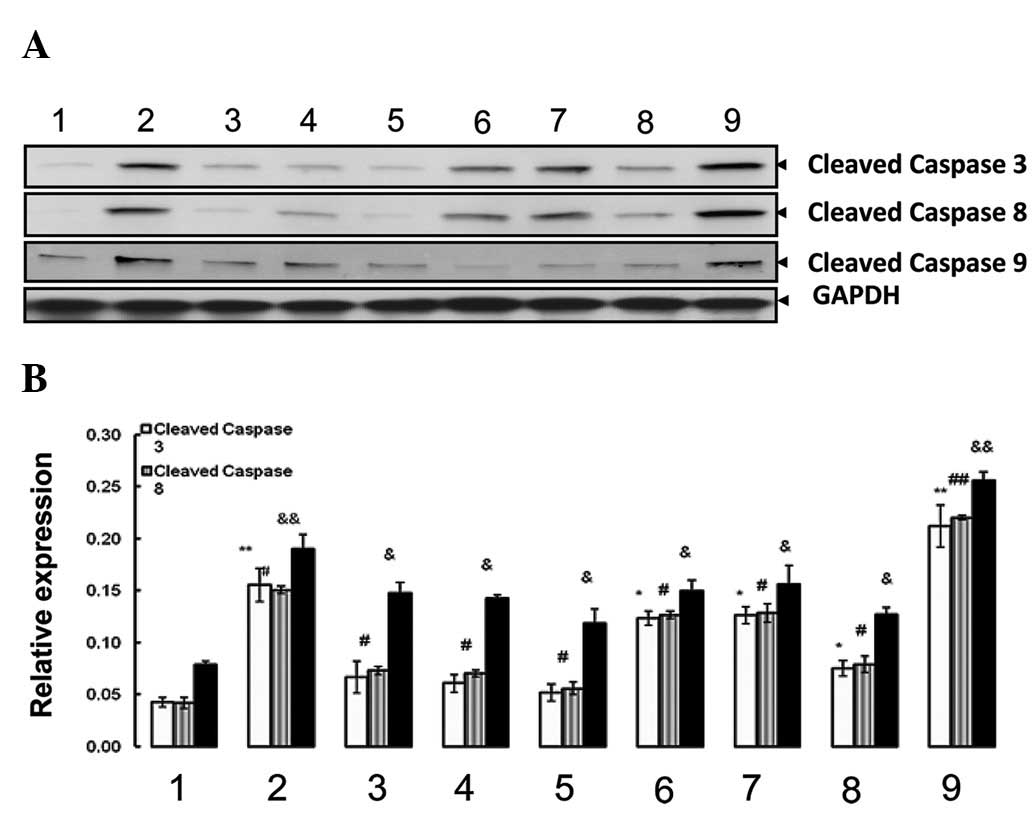 | Figure 6Pingmu decoction elevated the levels
of cleaved caspase-3, 8 and 9. Adipocytes were treated with serum
from the different groups of rats and cultured for 2 days, then
cell lysed for the detection of cleaved caspase-3, 8 and 9 by (A)
western blot analysis. (B) The optical density values of
corresponding protein bands were semi-quantified by IPP 6.0
software. Experiments were performed 3 times and representative
images are shown. Lane 1, normal rat; lane 2, Pingmu decoction;
lane 3, Astragalus; lane 4, Herba epimedii; lane 5,
root of red-rooted Salvia + Semen brassicae +
Plantago seed + Oldenlandia diffusa; lane 6,
Astragalus + root of red-rooted Salvia + Semen
brassicae + Plantago seed + Oldenlandia diffusa;
lane 7, Astragalus + Herba epimedii; lane 8, Herba
epimedii + root of red-rooted Salvia + Semen
brassicae + Plantago seed + Oldenlandia diffusa;
9, 30 μgml dexamethasone acetate injection. GAPDH was used as the
inner control to show the equal amount of loaded protein.
*P<0.05, **P <0.01, Compared to normal
group in expression of cleaved caspase 3;
&P<0.05, &&P<0.01, Compared
to normal group in expression of cleaved caspase 8;
#P<0.05, ##P<0.01, Compared to normal
group in expression of cleaved caspase 9. |
Discussion
GO is an autoimmune condition most frequently
associated with GD (12). In GD,
TSHR is the target of stimulating autoantibodies (TSAB), which
mimic the action of TSH by increasing intracellular cyclic
adenosine monophosphate (cAMP) levels (2). This leads to increased thyrocyte
function and growth, independent of the
hypothalamic-pituitary-thyroid axis. The pathogenesis of GO is less
clear. The majority of the signs and symptoms may be explained by
the increase in volume of the orbital contents. The extraocular
muscles become grossly enlarged, mostly due to edema. There are few
microscopically visible changes in the myocytes, although in
advanced disease they become heavily fibrosed. Apart from the
edema, 2 other mechanisms increase orbital volume: the production
of glycosaminoglycans (by orbital fibroblasts), which absorb water
and swell, and hyperplasia of the adipose tissue. In combination,
these cause proptosis and compression of the optic nerve, which may
result in diplopia and in extreme cases, loss of sight.
Recent studies show that adipogenesis is a major
contributor to GO progression (3).
Adipose tissue volume increases through a combination of increased
cell number (hyperplasia) and expanded cell size (hypertrophy). New
adipocyte formation plays an ongoing role in adipose tissue
enlargement throughout life. A previous study demonstrated that
cultures derived from human orbital adipose/connective tissue
contain adipocyte precursor cells (comprising 5–10% of the total),
capable of differentiating into lipid-filled adipocytes when
cultured under conditions known to stimulate adipogenesis in
fibroblasts from other sites. Adipogenesis is a complex process
associated with the activation of several adipocyte-specific genes,
including leptin, adiponectin and PPAR, and the inhibition of the
preadipocyte gene pref-1. PPAR is a nuclear hormone receptor that
is highly expressed in adipose tissue. The activation of this
receptor is critical for the complex processes of adipogenesis, and
several ligands of this receptor that have profound effects on this
process, as well as on insulin sensitivity, have been developed.
Leptin is a protein produced and secreted exclusively by mature
adipocytes.
Similarly, adiponectin is a recently identified,
adipose tissue-derived, soluble protein produced solely by mature
adipocytes. This protein has important metabolic effects related to
whole body insulin sensitivity and also possesses anti-atherogenic
properties. Serum levels of adiponectin decrease with obesity and
are higher in females than in males. Both adiponectin expression
and secretion are stimulated by activators of PPAR. It has been
demonstrated that there is a significantly increased expression of
all 3 gene markers of adipocyte differentiation (leptin,
adiponectin and PPAR) in orbital adipose tissue from patients with
GO compared with normal orbital tissue. In addition, the expression
of each of these genes correlated positively with TSHR gene
expression. A study by Kumar et al found higher levels of
leptin and adiponectin, genes produced exclusively by mature
adipocytes, in uncultured orbital adipose tissue specimens from
patients with GO compared with normal orbital tissue specimens
(10). These results suggest that
there may be a relatively greater number of mature adipocytes in GO
than in normal orbital tissues. This may result from the
stimulation of adipogenesis in orbital preadipocytes by some
unknown factor present in GD. Naturally, they suggest that the
inhibition of orbital adipogenesis by the antagonism of various
components of the adipogenic pathway may be of benefit in the
treatment of GO.
As for the treatment of GO, corticosteroids are
efficient in reducing orbital inflammation; however, the benefits
cease after discontinuation. Corticosteroid treatment is also
limited due to the many side-effects. Radiotherapy is an
alternative option to reduce acute orbital inflammation. However,
there remains controversy surrounding its efficacy. The lack of
efficient medication with minimal side-effects led us to search for
an alternative therapy. We turned to traditional Chinese
medicine.
In the present study, we examined the effect of
Pingmu decoction or other combinations of ingredients on the
proliferation and apoptosis of preadipocytes and adipocytes derived
from fat tissue of GO patients. We successfully separated
preadipocytes and induced adipocyte differentiation in conditional
medium. We then treated those cells with serum from rats feeding on
Pingmu decoction or other combinations of ingredients. We observed
a decrease in the proliferation of preadipocytes. We also showed
that Pingmu decoction-containing serum has an effect on reducing
the expression of cell cycle-related genes. These results suggest
that Pingmu decoction inhibits preadipocyte proliferation. By
immunoblotting, we observed the increase in the apoptosis of
adipocytes treated with Pingmu-containing serum, determined by
enhanced Annexin V staining, cell cycle arrest at the G0/G1 phase
and increased levels of cleaved caspase-3, 8 and 9. Moreover,
Astragalus + root of red-rooted Salvia + Semen
brassicae + Plantago seed + Oldenlandia diffusa
serum, and Astragalus + Herba epimedii serum showed
similar effects on the inhibiton of proliferation of preadipocytes
and the enhancement of adipocyte apoptosis. Collectively, these
data support the conclusion that Pingmu decoction is capable of
inhibiting preadipocyte proliferation and enhancing adipocyte
apoptosis and may potentially be applied to clinical practice.
Acknowledgements
This project is supported by Grants from the
National Natural Science Foundation of China (No. 81072793) and Key
Project of Scientific Research and Innovation of Shanghai Education
Commission (No. 11ZZ114).
References
|
1
|
Bahn RS: Graves’ ophthalmopathy. N Engl J
Med. 362:726–738. 2010.
|
|
2
|
Bahn RS and Heufelder AE: Pathogenesis of
Graves’ ophthalmopathy. N Engl J Med. 329:1468–1475. 1993.
|
|
3
|
Garrity JA and Bahn RS: Pathogenesis of
graves ophthalmopathy: implications for prediction, prevention, and
treatment. Am J Ophthalmol. 142:147–153. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartalena L, Marcocci C, Bogazzi F,
Manetti L, Tanda ML, Dell’Unto E, Bruno-Bossio G, Nardi M,
Bartolomei MP, Lepri A, Rossi G, Martino E and Pinchera A: Relation
between therapy for hyperthyroidism and the course of Graves’
ophthalmopathy. N Engl J Med. 338:73–78. 1998.
|
|
5
|
Behbehani R, Sergott RC and Savino PJ:
Orbital radiotherapy for thyroid-related orbitopathy. Curr Opin
Ophthalmol. 15:479–482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stan MN and Bahn RS: Risk factors for
development or deterioration of Graves’ ophthalmopathy. Thyroid.
20:777–783. 2010.
|
|
7
|
Kumar S and Bahn RS: Relative
overexpression of macrophage-derived cytokines in orbital adipose
tissue from patients with graves’ ophthalmopathy. J Clin Endocrinol
Metab. 88:4246–4250. 2003.PubMed/NCBI
|
|
8
|
Ellerin T, Rubin RH and Weinblatt ME:
Infections and anti-tumor necrosis factor alpha therapy. Arthritis
Rheum. 48:3013–3022. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peyster RG, Ginsberg F, Silber JH and
Adler LP: Exophthalmos caused by excessive fat: CT volumetric
analysis and differential diagnosis. AJR Am J Roentgenol.
146:459–464. 1986.PubMed/NCBI
|
|
10
|
Kumar S, Coenen MJ, Scherer PE and Bahn
RS: Evidence for enhanced adipogenesis in the orbits of patients
with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 89:930–935.
2004.
|
|
11
|
Crisp M, Starkey KJ, Lane C, Ham J and
Ludgate M: Adipogenesis in thyroid eye disease. Invest Ophthalmol
Vis Sci. 41:3249–3255. 2000.PubMed/NCBI
|
|
12
|
LI H, Xu R, Chen J and Ge F: Clinical
observation of Pingmu decocion-II in treating infiltrative
exophthalmos in non-active Graves’ disease. Shanghai J Tradit Chin
Med. 42:50–52. 2008.
|
|
13
|
Tomlinson JW, Durrani OM, Bujalska IJ, et
al: The role of 11beta-hydroxysteroid dehydrogenase 1 in
adipogenesis in thyroid-associated ophthalmopathy. J Clin
Endocrinol Metab. 95:398–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wiersinga WM and Prummel MF: Graves’
ophthalmopathy: a rational approach to treatment. Trends Endocrinol
Metab. 13:280–287. 2002.
|