Introduction
Colorectal cancer is a growing health problem. It is
a leading cause of cancer mortality in men and women and affects
more than one million people annually worldwide (1). The features of the disease usually
occur progressively over a protracted period due to increased
genomic instability, leading to the upregulation of oncogenes and
the downregulation of tumor suppressor genes (2). Studies have shown that major
intracellular signaling pathways are altered during tumorigenesis,
leading to the dysregulation of processes such as proliferation and
survival (3).
The induction of apoptosis in tumor cells is a
strategy used in antitumor therapy. The balance between survival-
and apoptosis-signaling pathways controls tumor pathogenesis. A
number of flavonoids exert potent antitumor activity through the
induction of apoptosis and cell cycle arrest in several tumor cell
lines (4,5). Flavonoids, a group of polyphenolic
compounds, are natural products present in numerous fruits and
vegetables and in all vascular plants (6). The antitumor activity of flavonoids
has been the subject of much attention.
Scutellaria baicalensis, known as Chinese
skullcap and Huang Qin, contains several flavonoids and is a widely
used herb in traditional Chinese medicine with antitumor,
antiviral, antibacterial and anti-inflammatory properties (7–11).
Baicalein and wogonin are flavonoid-like chemical compounds which
are found in Scutellaria baicalensis and have been reported
to inhibit cell growth and induce apoptosis in various tumors
(12,13). However, the precise mechanisms by
which baicalein and wogonin induce apoptosis are not yet known.
We compared the antitumor efficacies and mechanisms
of action of baicalein and wogonin in HT-29 human colorectal tumor
cells. Additionally, a mouse xenograft model was used to evaluate
the antitumor activities of baicalein and wogonin in vivo.
Understanding the underlying mechanisms of baicalein- and
wogonin-induced apoptosis may benefit the development of
chemopreventives and chemotherapeutics for colon tumors.
Materials and methods
Cell culture and reagents
The HT-29 cells were obtained from the Korean Cell
Line Bank (KCLB, Korea) and were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin. The cells were subsequently incubated for
2 h at 37°C in an atmosphere of 5% CO2. Wogonin was
purchased from Wako (Osaka, Japan). Baicalein, MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide],
dimethylsulfoxide (DMSO) and propidium iodide (PI) were purchased
from Sigma-Aldrich (St. Louis, MO, USA). The RPMI-1640 medium,
penicillin/streptomycin and trypsin-EDTA were obtained from Hyclone
Laboratories, Inc. (Logan, UT, USA). FBS was obtained from
Gibco-BRL (Grand Island, NY, USA). Anti-Akt, anti-phospho-Akt
(Ser473), anti-Bcl-2, anti-Bax, anti-p53, anti-p21,
anti-cleaved-poly(ADP-ribose) polymerase (PARP),
anti-phospho-GSK-3β, anti-cyclin-B1, anti-survivin and anti-β-actin
were purchased from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Anti-cyclin D1 (HD11) was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Cell lysis buffer and
4′,6-diamidino-2-phenylindole (DAPI) were obtained from Invitrogen
Life Technologies (Carlsbad, CA, USA). The fluorescein
isothiocyanate (FITC)-conjugated annexin V apoptosis detection kit
was purchased from BD Biosciences (San Diego, CA, USA).
Cell viability assessment
Cells were seeded in 12-well plates at
5×104 cells/well (24 h) and 4×104 cells/well
(48 h) and then incubated with either baicalein or wogonin at 0,
25, 50 and 100 μM for the indicated times. The medium was removed
and the cells were incubated with 1000 μl MTT solution (2 mg/ml MTT
in PBS) for 4 h. The optical densities of the solutions were
determined using a spectrophotometer (Ultrospec 2100 pro; Amersham
Biosciences, Piscataway, NJ, USA) at 540 nm. The cell viability was
expressed as the optical density ratio of the treated cells to the
control.
Nuclear morphology
Cells were seeded in 12-well plates at
4×104 cells/well and then incubated with either
baicalein or wogonin at 0, 25, 50 and 100 μM for 48 h. Following
treatment, the cells were fixed with 4% paraformaldehyde in PBS for
30 min in an incubator. The fixed cells were washed twice with PBS
and the cell nuclei were stained with DAPI in PBS. Specific
fluorescence was observed using a fluorescence microscope (Bx-41;
Olympus, Tokyo, Japan).
Annexin V staining for apoptosis
analysis
Adherent and floating HT-29 cells grown in
75-cm2 flasks were collected following mild
trypsinization. The trypsinized cells were washed once with PBS,
then resuspended in 100 μl annexin V binding buffer and mixed with
FITC-conjugated annexin V and phycoerythrin (PE)-conjugated PI. The
resuspended cells were incubated at room temperature in the dark
for 15 min. Apoptotic cells (annexin V + PI) were measured within 1
h using a flow cytometer (FACSCalibur; BD Biosciences).
Flow cytometric analysis of the cell
cycle
HT-29 cells (1.5–2×105) were seeded in
25-cm2 flasks. After 24 h, the medium was replaced with
fresh medium (control) or fresh medium supplemented with baicalein
or wogonin. After 2 days of incubation (at 50–60% cell confluence),
the cells were trypsinized, collected and centrifuged for 5 min at
1,700 rpm. The cell pellets were washed twice with PBS and then
fixed with 70% ethanol for 30 min. DNA fragments were then stained
with 50 μg/ml PI and 100 μg/ml of RNase (Sigma-Aldrich) in PBS for
30 min at room temperature. The viable cells were sorted and the
fluorescence intensity was measured by flow cytometry.
Western blotting
Briefly, floating cells were collected and protein
concentrations were measured using the Bradford assay (14). Equal amounts of protein were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and then electrophoretically transferred onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The transferred membranes were blocked with Tris-buffered
saline containing 5% non-fat dry milk and 0.1% Tween-20 for 2 h at
4°C.
The blocked membranes were incubated with primary
antibodies overnight at 4°C with gentle agitation. The membranes
were then incubated with horseradish peroxidase-conjugated goat
anti-mouse and anti-rabbit IgG secondary antibodies for 1 h at room
temperature with gentle agitation. After washing, the bands were
visualized using enhanced chemiluminescence (ECL) detection
reagents (Pierce Biotechnology, Inc., Rockford, IL, USA).
Nude mouse xenograft assay
Male nude mice (6 weeks old) were purchased from
Orient Ltd. (Seoul, Korea). Xenografts were established by
subcutaneous injection of in vitro-cultured HT-29 cells
(106 cells/200 μl) into the flanks of donor nude mice.
All procedures involving the use of the animals were approved by
the Institutional Animal Care and Use Committee of Kong-Ju National
University and were carried out in accordance with the ethical
guidelines.
When the tumors reached ~1000 mm3 in
size, the mice were anesthetized with diethyl ether and the tumor
masses were obtained surgically from the mice. The masses were
sliced into 2×2 mm fragments using a grid and the tumor fragments
were implanted surgically into the subcutaneous tissue of the right
flank of each mouse (15). The
mice were randomized into three groups each comprising four mice
(two tumors/mouse). In the treatment groups, animals were treated
orally with baicalein or wogonin (10 mg/kg) three times/week for 43
days starting on the day of tumor cell implantation. The control
mice received an equal volume of the vehicle. The tumors were
measured in two diameters with calipers to permit calculation of
the tumor volume using the formula: V = {(D+d)/2}3,
where D and d are the larger and smaller diameters, respectively.
The statistical significance of differences in tumor volume, wet
tumor weight and body weight between the control and treated mice
were assessed by the Student’s t-test.
Preparation and administration of
baicalein and wogonin
Groups of four mice were randomly assigned to
receive one of the treatments starting on the day of implantation
of the tumor fragment. In the control group, each mouse was
administered corn oil (0.2 ml/day) every day for 5 weeks. In the
treatment groups, each mouse was administered 10 mg/kg baicalein or
wogonin every day for 5 weeks.
Immunohistochemistry
Briefly, 4-μm-thick sections were cut from
paraffin-embedded tissue blocks. The sections were deparaffinized
and hydrated by sequential immersion in xylene and graded alcohol
solutions. The endogenous peroxidase activity was quenched by
treatment with 3% hydrogen peroxide for 5 min at room temperature.
The sections were incubated with primary antibodies for 1 h at 37°C
and then with the secondary antibody for 30 min at room
temperature. Staining was performed using diaminobenzidine (DAB)
and counterstaining was performed using methyl green. For the
negative control, the incubated antibody diluent was used as a
substitute for the primary antibody.
Apoptotic cell detection using the
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) assay
Tumor tissues were examined using the Dead End™
Colorimetric TUNEL System (Promega Corporation, Madison, WI, USA).
After dewaxing and rehydration, the sections were treated with
proteinase K (20 μg/ml) for 15 min at room temperature. Endogenous
peroxidase was blocked with 0.3% hydrogen peroxide in PBS for 5
min. Digoxigenin dUTP end-labeled DNA was detected with
anti-digoxigenin peroxidase, followed by peroxidase detection with
0.05% DAB containing 0.02% hydrogen peroxide. Tissue sections were
counterstained with methyl green. Three randomly selected fields in
each tumor section were counted for apoptotic bodies.
Statistical analysis
All data are expressed as the mean ± SE (standard
error). One-way ANOVA was used to analyze statistical differences
among multiple comparisons. P<0.05 was considered to indicate a
statistically significant result.
Results
Cell growth inhibition assessed by the
colorimetric MTT assay
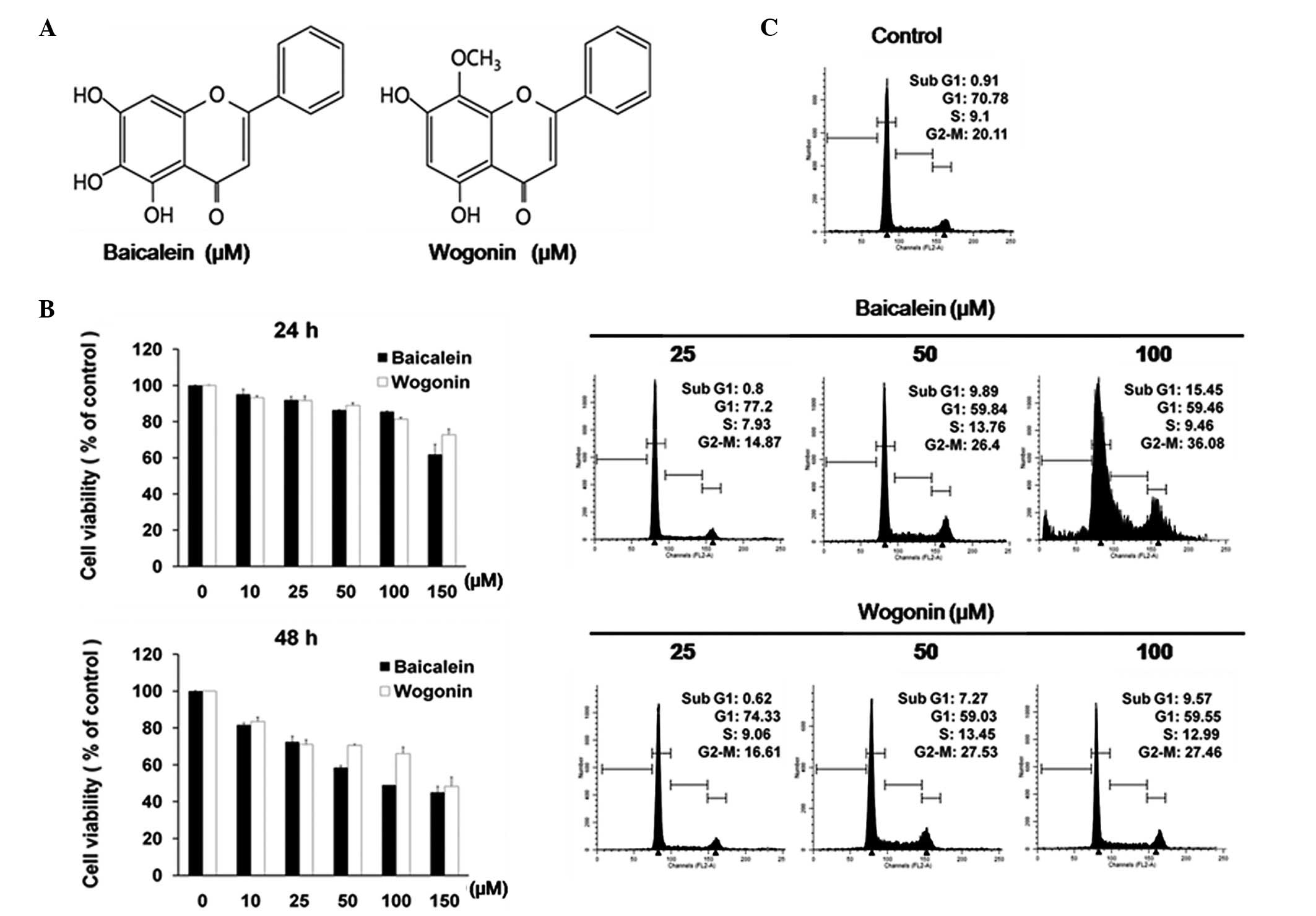
The chemical structures of baicalein and wogonin are
shown in Fig. 1A. The growth
inhibitory effects of the two agents on the HT-29 cells were
determined as the percentage of viable cells in the treated cells
compared with the untreated control. As shown in Fig. 1B, treatment with 10–150 μM
baicalein or wogonin for 24 or 48 h resulted in a slight decrease
in the cell viability of the HT-29 cells. Baicalein and wogonin
demonstrated significant cytotoxicity after 48 h. In the 24-h and
48-h treatment groups, wogonin (150 μM) reduced cell viability by
27.28 and 51.66%, respectively. Baicalein (100 μM) reduced cell
viability by more than 50% in the 48-h treatment group.
Effects of baicalein and wogonin on cell
cycle arrest and induction of apoptosis in HT-29 cells
The results shown in Fig. 1C indicate that baicalein and
wogonin induced sub-G1 and G2/M arrest. In the cells treated with
baicalein, when the drug concentration was increased from 25 to 100
μM, the percentage of cells in the sub-G1 phase increased from 0.8
to 15.45% and the percentage of cells in the G2/M phase (apoptotic
fraction) increased from 14.87 to 36.08%. However, the S phase was
not markedly altered by baicalein.
In the cells treated with wogonin, when the drug
concentration was increased from 25 to 100 μM, the percentage of
cells in the sub-G1 phase increased from 0.62 to 9.57% and the
percentage of cells in the G2/M phase increased from 16.61 to
27.46%. No significant change in the S phase was observed following
wogonin treatment in our experiments.
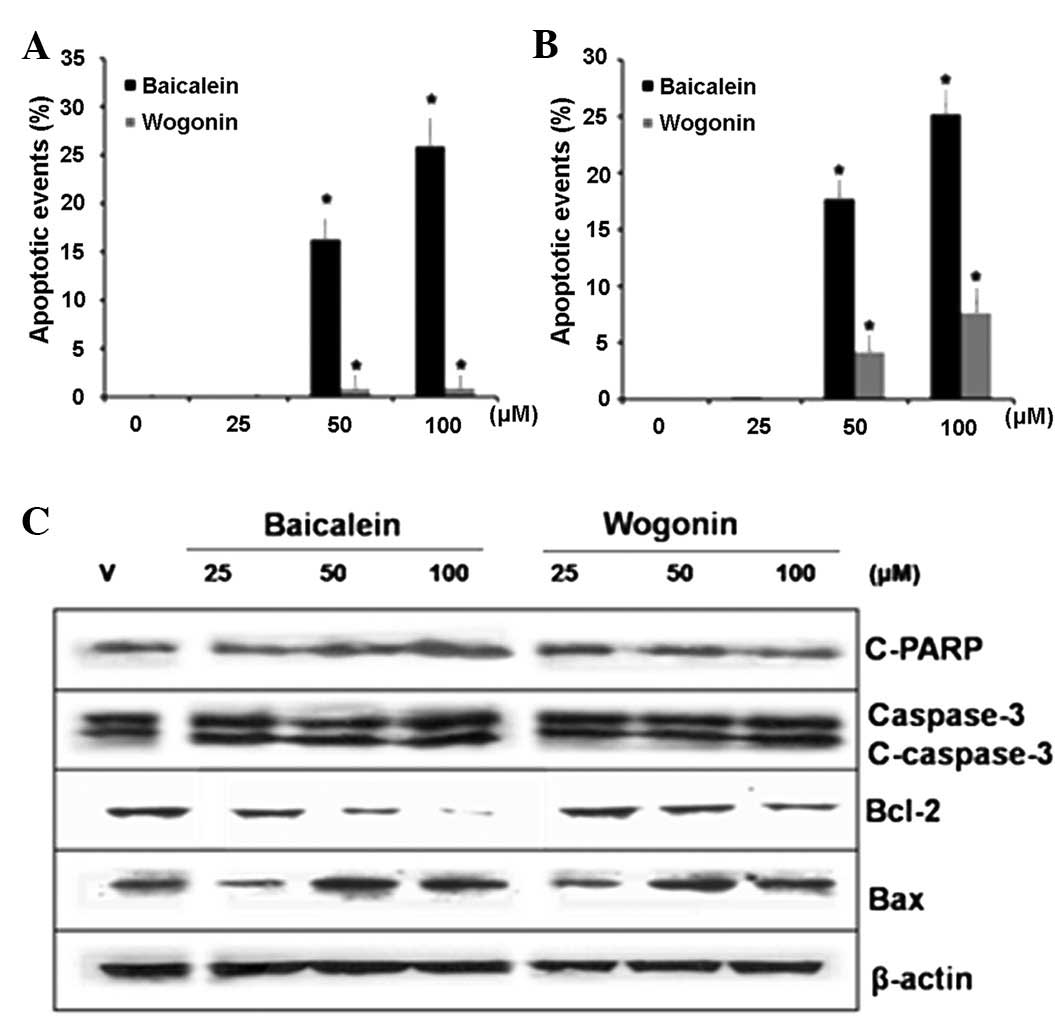
Apoptosis assessment by DAPI staining and
annexin V and PI double staining
The quantified results of the DAPI staining are
presented in Fig. 2A. A
dose-dependent increase was observed in the number of HT-29 cells
showing nuclear condensation and fragmentation following treatment
with either baicalein or wogonin (25, 50 and 100 μM) for 48 h
(Fig. 2A). Treatment of the HT-29
cells with wogonin for 48 h significantly increased the percentage
of apoptotic cells from 0.11% in the control group to 0.12% (25
μM), 0.88% (50 μM) and 0.93% (100 μM). Treatment of the HT-29 cells
with baicalein for 48 h significantly increased the percentage of
apoptotic cells from 0.11% in the control group to 16.27% (50 μM)
and 25.92% (100 μM).
Additionally, flow cytometric analysis with annexin
V/PI double staining was used to determine the levels of apoptosis
elicited by baicalein and wogonin. The percentages of apoptotic
cells induced by 25, 50 and 100 μM baicalein were 0.15, 17.74 and
25.22%, respectively, whereas only 0.01% of the control cells were
apoptotic (Fig. 2B). The results
clearly indicate that baicalein induced apoptosis in the HT-29
cells.
Intracellular signaling in baicalein- and
wogonin-induced apoptosis
To obtain further evidence of the
apoptosis-inducing effect of baicalein in HT-29 cells, the
expression levels of caspase-3, which is the key indicator of
intracellular signaling in apoptosis, were determined. Cleaved
caspase-3 levels were increased following treatment with 25, 50 and
100 μM baicalein and 100 μM wogonin for 48 h (Fig. 2C).
We also investigated whether the apoptosis was
linked to the expression of the Bcl-2 (anti-apoptotic) and Bax
(pro-apoptotic) proteins, which are central regulators of
apoptosis. Following incubation with 25–100 μM baicalein or wogonin
for 48 h, the expression level of Bcl-2 decreased markedly, whereas
that of Bax increased significantly. Additionally, baicalein
induced a significant increase in PARP cleavage compared with the
control group (Fig. 2C). To assess
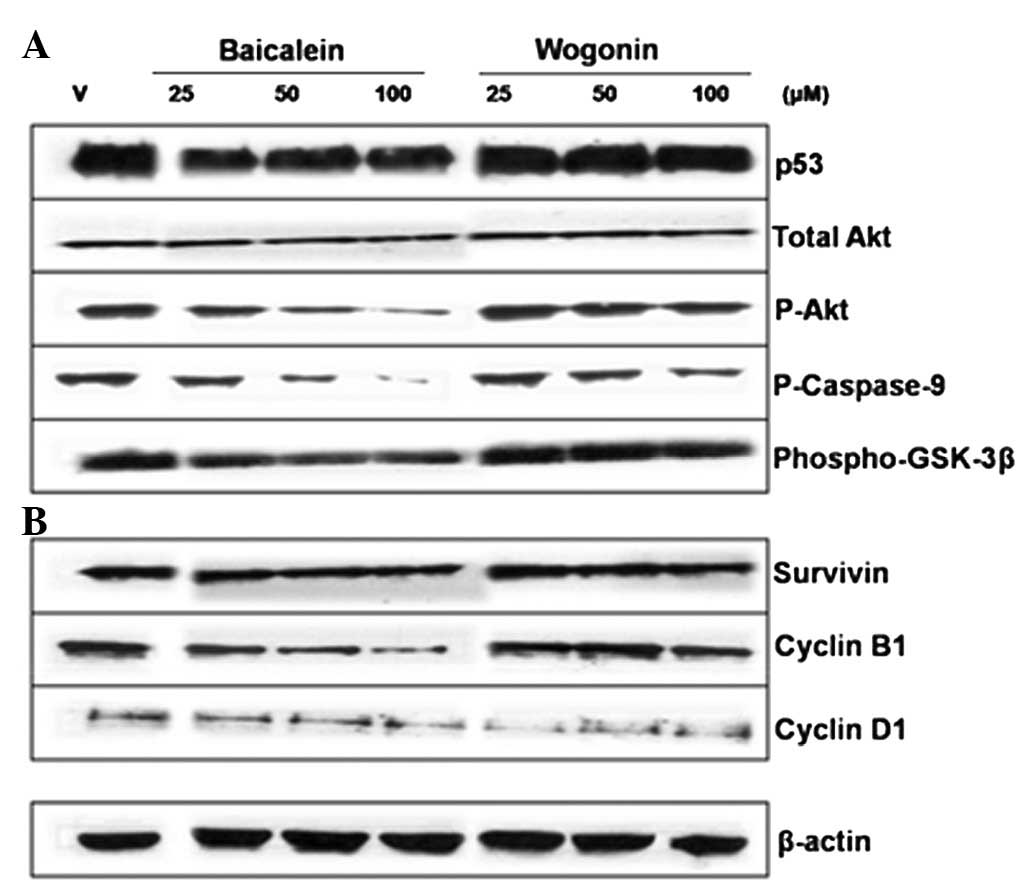
the involvement of the Akt pathway in the apoptosis, the levels of
phosphorylated Akt protein were investigated by western blotting.
The amount of active phosphorylated Akt was clearly reduced by the
baicalein treatment while the total Akt expression level was
unchanged (Fig. 3). As observed in
Fig. 3A, the p53 protein level was
reduced by the baicalein treatment. Since phospho-GSK-3β is another
downstream effector of Akt, we also investigated the phospho-GSK-3β
expression levels by western blotting. As shown in Fig. 3A, the phospho-GSK-3β expression
level was markedly downregulated by the baicalein treatment,
consistent with the inhibition of Akt phosphorylation following
treatment. Also, the baicalein and wogonin treatments clearly
reduced phospho-caspase-9 levels.
As shown in Fig.
3B, the protein levels of cyclin D1 and cyclin B1, as assessed
by immunoblotting, were reduced in a concentration-dependent manner
(Fig. 3B). The amount of survivin
was also reduced by the baicalein treatment (Fig. 3B).
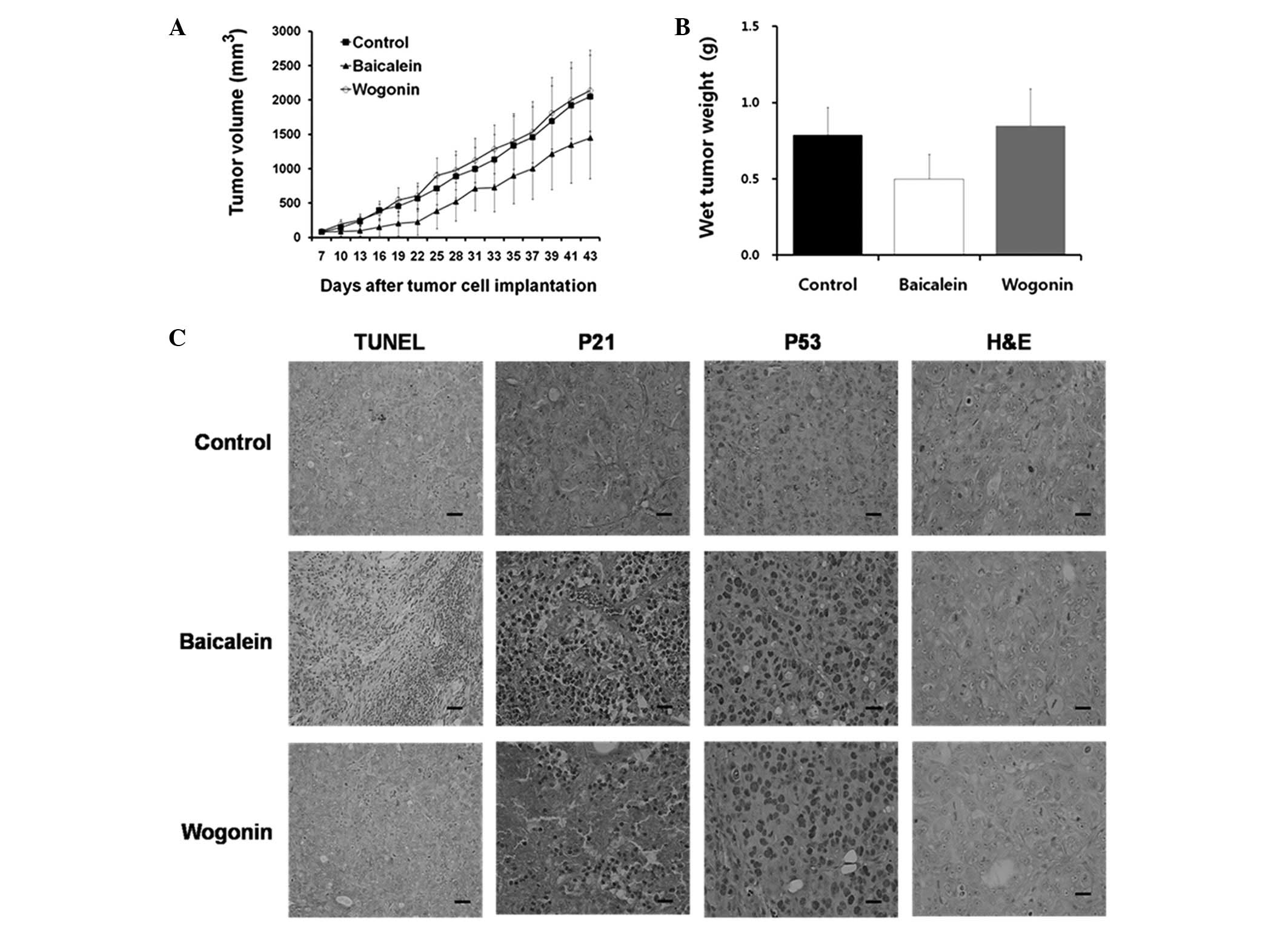
Inhibition of colon tumor growth by
baicalein and wogonin in xenograft models
Next, we investigated the effects of baicalein and
wogonin on colon tumor cells in vivo. Our results revealed
that significant tumor growth inhibition occurred in the
baicalein-treated mice compared with the untreated mice (Fig. 4A). Additionally, baicalein was
shown to significantly decrease tumor weights (Fig. 4B). No significant difference in
tumor weight was observed between the wogonin-treated and control
groups.
The average tumor volumes following 5 weeks of
treatment were 1,448.60 mm3 (baicalein; 10 mg/kg/day),
2,137.85 mm3 (wogonin; 10 mg/kg/day) and 2,049.85
mm3 (control) (Fig.
4A). The inhibition rates for baicalein and wogonin were 29.33
and -4.29%, respectively (Table
I). Baicalein inhibited the colorectal tumor growth to 87% of
control values (n=4; P<0.05), whereas the colorectal tumor
growth following wogonin treatment was not significantly different
from that of the controls. The difference in trends was
statistically significant (P<0.001). The average tumor volumes
are shown in Table I and reveal
that baicalein suppressed colon tumor growth in the xenograft
model.
 | Table ITumor inhibition rate of mice
implanted with colon cancer cells treated with wogonin and
baicalein. |
Table I
Tumor inhibition rate of mice
implanted with colon cancer cells treated with wogonin and
baicalein.
| Group | Pre-experiment | Post-experiment | Inhibition rate
(%) |
|---|
|
|
|---|
| n | Size
(mm3) | n | Size
(mm3) |
|---|
| Control | 4 | 80.3 | 4 | 2049.9 | |
| Baicalein | 4 | 81.2 | 4 | 1448.6 | 29.33 |
| Wogonin | 4 | 92.6 | 4 | 2137.9 | -4.29 |
Additionally, in the mice treated with 10 mg/kg
baicalein or wogonin, when body weight, organ histology and kidney
function were compared with those of the controls, no evidence of
drug-related toxicity was observed (data not shown).
Effects of baicalein and wogonin on p21
and p53 levels in HT-29 cells
A TUNEL assay was performed on the tumor tissues
from the experimental animals. A marked increase in the number of
apoptotic cells in the baicalein-treated HT-29 tumor xenograft
tissue was observed, as indicated by the dark brown nuclear
staining of the apoptotic cells. However, no significant induction
of apoptosis was observed in the wogonin-treated cells (Fig. 4C). These in vivo results
support the in vitro findings that baicalein had an
apoptotic effect in HT-29 cells.
The morphological aspects of the apoptosis were
studied immunohistochemically in the HT-29 cells following
treatment with baicalein and wogonin. Increases in the p53 protein
levels were detected in the baicalein- and wogonin-treated HT-29
cells. Although p21 protein is rarely observed in the
wogonin-treated and control tumor tissues, it was definitely
identified in the baicalein-treated tissues. These data indicate
that increased levels of p53 and p21 are involved in
baicalein-induced apoptosis, whereas only a slight induction of p21
was detected in the wogonin-treated cells (Fig. 4C).
Morphological analysis of hematoxylin and
eosin-stained tissue sections was also performed (Fig. 4C). However, only a few mitotic
nuclei cells were observed following the baicalein and wogonin
treatments.
Discussion
We evaluated and compared the antitumor effects of
baicalein and wogonin, two compounds derived from S.
baicalensis. The human colon tumor cell line HT-29 was cultured
in the absence and presence of varying concentrations of baicalein
and wogonin. Moreover, to understand whether the baicalein- and
wogonin-induced inhibition of cell viability was due to cell cycle
arrest, PI staining was performed.
Flavonoid treatment resulting in histone
hyperacetylation leads to manifold effects in tumor cells,
including the modulation of gene expression and cell death, and
occurs to varying extents in different cell types. As shown in
Fig. 1, wogonin induced cell death
in HT-29 cells with a higher percentage of necrosis than apoptosis.
Since PARP causes ATP depletion, it appears plausible that its
inhibition favors apoptosis over necrosis, as apoptosis is an
ATP-dependent, energy-consuming process (16). Thus, the prevention of
mitochondrial dysfunction and ATP depletion appear to be involved
in switching the mode of wogonin-induced cell death in the HT-29
cells from a necrotic to a mainly apoptotic type. The primary
trigger of wogonin-induced cell death, downstream of histone
acetylation, remains to be identified.
Evidence suggests that the proteolytic degradation
of specific substrates is responsible for many of the morphologic
and biochemical features of apoptosis. Increased levels of nuclear
fragmentation and total apoptosis were observed in the cells by
DAPI staining (Fig. 2A) and
annexin V/PI double staining (Fig.
2B), respectively. The assays demonstrated that baicalein
significantly inhibited cell growth and induced apoptosis more
potently than wogonin in the HT-29 cells (Fig. 2A and B).
Previous studies have demonstrated that Bcl-2
protein family members significantly regulate apoptosis, either as
activators (e.g. Bax) or as inhibitors (e.g. Bcl-2) (17,18).
In the present study, we identified that baicalein and wogonin
treatment significantly induced mitochondrial damage and apoptosis,
modulated Bcl-2 and Bax protein expression and led to mitochondrial
dysfunction, caspase activation and PARP cleavage in HT-29 cells
(Fig. 2C). Our data demonstrate
that baicalein- and wogonin-induced apoptosis is related to
increased levels of Bax and decreased levels of Bcl-2 and also the
induction of mitochondrial dysfunction, leading to the apoptosis of
the HT-29 cells.
Caspase-3 is one of the key executioners of
apoptosis, as it is either partially or completely responsible for
the proteolytic cleavage of a number of key proteins, including
PARP. PARP is critical for cell viability. The cleavage of PARP
facilitates cellular disassembly and serves as a marker of cells
undergoing apoptosis (16). Our
in vitro results in HT-29 cells indicate the concurrent
occurrence of apoptotic DNA fragmentation, caspase-3 activation and
PARP cleavage in the baicalein-treated HT-29 cells (Fig. 2C). These results indicate that the
induction of apoptosis by baicalein is mediated by a p53-dependent
or p53-independent pathway followed by the stimulation of the
activities of caspase-3 and endonuclease. The cross-linking between
the p53-dependent and p53-independent pathways in the baicalein-
and wogonin-induced apoptosis remains unclear.
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway
is associated with cell survival through the activation of
anti-apoptotic downstream effectors (19,20).
Therefore, we investigated the expression of phosphorylated
caspase-9, a downstream target of Akt, and found that the baicalein
treatment significantly reduced the levels of phospho-caspase-9
(Ser196). These data suggest that the baicalein-induced apoptosis
was caspase-9-dependent. Also, changes in the levels of
phospho-Akt, phospho-GSK-3β, cyclin B1 and cyclin D1 suggest that
the Akt-mediated canonical Wnt-signaling pathway was downregulated
by baicalein in the colon tumor cells (Fig. 3A and B). Inhibition of the Akt
pathway downregulates survivin expression and enhances apoptosis in
tumor cells (21). Thus, baicalein
elicits activation of Akt that may be caused by self-protection of
tumor cells, which resist cell death by defending the survivin
level. The blockade of the PI3K-Akt pathway by PI3K and Akt
inactivation enhanced the reduction of survivin levels and promoted
cytotoxicity in the baicalein-exposed cells.
We then investigated whether the growth-inhibitory
activity observed with baicalein treatment in vitro would
also occur in vivo. Our results demonstrated that tumor
growth was significantly inhibited in the baicalein-treated mice
compared with the untreated mice (Fig.
4A and B).
The in vivo experiments also served to
examine the mechanism of the baicalein-induced apoptosis in the
nude mice. Several studies have shown that the upregulation of p53
and p21 proteins results in the inhibition of molecules related to
tumor cell growth and proliferation (22,23).
We identified that the levels of the apoptosis-related proteins
induced by baicalein, particularly p53 and p21, were higher in the
tissues of the baicalein- and wogonin-treated groups than those of
the control group (Fig. 4C).
Additionally, an increase in TUNEL-positive cells was found in the
baicalein-treated group compared with the control group (Fig. 4C). These in vivo findings
support the in vitro data and suggest that baicalein
moderates apoptotic cell death in the HT-29 colon tumor xenografts
through a p53-mediated apoptotic response.
Together, these results indicate that baicalein
induces apoptosis in HT-29 cells via Akt inactivation and in a
p53-dependent manner and that baicalein is a potential
chemopreventive and therapeutic agent for colon tumors. These
results should further contribute to the understanding of the
antitumor activities of flavonoids.
Acknowledgements
This study was supported by a research grant from
the Kongju National University in 2010.
References
|
1
|
Jemal A, Murray T, Ward E, et al: Cancer
statistics, 2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar
|
|
2
|
Samowitz WS and Slattery ML: Missense
mismatch repair gene alterations, microsatellite instability, and
hereditary nonpolyposis colorectal cancer. J Clin Oncol.
20:3178–3179. 2002.
|
|
3
|
Fang Q, Naidu KA, Naidu KA, et al:
Ascorbyl stearate inhibits cell proliferation and tumor growth in
human ovarian carcinoma cells by targeting the PI3K/AKT pathway.
Anticancer Res. 26:203–209. 2006.PubMed/NCBI
|
|
4
|
Pan MH, Chen WJ, Lin-Shiau SY, Ho CT and
Lin JK: Tangeretin induces cell-cycle G1 arrest through inhibiting
Cyclin-dependent kinases 2 and 4 activities as well as elevating
Cdk inhibitors p21 and p27 in human colorectal carcinoma cells.
Carcinogenesis. 23:1677–1684. 2002. View Article : Google Scholar
|
|
5
|
Hirano T, Abe K, Gotoh M and Oka K: Citrus
flavone tangeretin inhibits leukaemic HL-60 cell growth partially
through induction of apoptosis with less cytotoxicity on normal
nymphocytes. Br J Cancer. 72:1380–1388. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Carlo G, Mascolo N, Izzo AA and Capasso
F: Flavonoids: old and new aspects of a class of natural
therapeutic drugs. Life Sci. 65:337–353. 1999.PubMed/NCBI
|
|
7
|
Razina TG, Udintsev SN, Tiutrin II,
Borovskaia TG and Iaremenko KV: The role of thrombocyte aggregation
function in the mechanism of the antimetastatic action of an
extract of Baikal skullcap. Vopr Onkol. 35:331–335. 1989.(In
Russian).
|
|
8
|
Konoshima T, Kokumai M, Kozuka M, et al:
Studies on inhibitors of skin tumor promotion. XI Inhibitory
effects of flavonoids from Scutellaria baicalensis on
Epstein-Barr virus activation and their anti-tumor-promoting
activities. Chem Pharm Bull (Tokyo). 40:531–533. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kubo M, Kimura Y, Odani T, Tani T and
Namba K: Studies on Scutellariae radix. Part II: The
antibacterial substance. Planta Med. 43:194–201. 1981.
|
|
10
|
Kubo M, Matsuda H, Tanaka M, et al:
Studies on Scutellariae radix. VII Anti-arthritic and
anti-inflammatory actions of methanolic extract and flavonoid
components from Scutellariae radix. Chem Pharm Bull (Tokyo).
32:2724–2729. 1984.
|
|
11
|
Mahmood N, Pizza C, Aquino R, et al:
Inhibition of HIV infection by flavanoids. Antiviral Res.
22:189–199. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kyo R, Nakahata N, Sakakibara I, Kubo M
and Ohizumi Y: Baicalin and baicalein, constituents of an important
medicinal plant, inhibit intracellular Ca2+ elevation by
reducing phospholipase C activity in C6 rat glioma cells. J Pharm
Pharmacol. 50:1179–1182. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma Z, Otsuyama K, Liu S, et al: Baicalein,
a component of Scutellaria radix from Huang-Lian-Jie-Du-Tang
(HLJDT), leads to suppression of proliferation and induction of
apoptosis in human myeloma cells. Blood. 105:3312–3318.
2005.PubMed/NCBI
|
|
14
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zamaraeva MV, Sabirov RZ, Maeno E,
Ando-Akatsuka Y, Bessonova SV and Okada Y: Cells die with increased
cytosolic ATP during apoptosis: a bioluminescence study with
intracellular luciferase. Cell Death Differ. 12:1390–1397. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao D, Vogel V and Singh SV: Benzyl
isothiocyanate-induced apoptosis in human breast cancer cells is
initiated by reactive oxygen species and regulated by Bax and Bak.
Mol Cancer Ther. 5:2931–2945. 2006. View Article : Google Scholar
|
|
18
|
Zhang M, Guo R, Zhai Y and Yang D: LIGHT
sensitizes IFNgamma-mediated apoptosis of MDA-MB-231 breast cancer
cells leading to down-regulation of anti-apoptosis Bcl-2 family
members. Cancer Lett. 195:201–210. 2003. View Article : Google Scholar
|
|
19
|
Jin CY, Moon DO, Lee JD, et al:
Sulforaphane sensitizes tumor necrosis factor-related
apoptosis-inducing ligand-mediated apoptosis through
down-regulation of ERK and Akt in lung adenocarcinoma A549 cells.
Carcinogenesis. 28:1058–1066. 2007. View Article : Google Scholar
|
|
20
|
McDonald PC, Oloumi A, Mills J, et al:
Rictor and integrin-linked kinase interact and regulate Akt
phosphorylation and cancer cell survival. Cancer Res. 68:1618–1624.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim S, Kang J, Qiao J, Thomas RP, Evers BM
and Chung DH: Phosphatidylinositol 3-kinase inhibition
down-regulates survivin and facilitates TRAIL-mediated apoptosis in
neuroblastomas. J Pediatr Surg. 39:516–521. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su RY, Chao Y, Chen TY, Huang DY and Lin
WW: 5-Aminoimidazole-4-carboxamide riboside sensitizes TRAIL- and
TNF{alpha}-induced cytotoxicity in colon cancer cells through
AMP-activated protein kinase signaling. Mol Cancer Ther.
6:1562–1571. 2007.PubMed/NCBI
|
|
23
|
Hwang JT, Ha J, Park IJ, et al: Apoptotic
effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway.
Cancer Lett. 247:115–121. 2007. View Article : Google Scholar : PubMed/NCBI
|