Introduction
A number of studies have suggested that inflammation
of the blood vessel wall plays a pivotal role in the initiation and
maintenance of vascular diseases, including atherosclerosis,
transplant arteriopathy and restenosis following mechanical injury
(1). Monocyte chemoattractant
protein 1 (MCP-1) is an important chemokine that mediates monocyte
and macrophage infiltration and is largely responsible for the
recruitment of monocytes and macrophages to the vessel wall during
the early stages of atherogenesis. In addition, Boring et
al(2) found that the MCP-1
receptor, CC chemokine receptor (CCR2), markedly decreased lesion
formation in apolipoprotein E (ApoE)-deficient mice by inhibiting
macrophage infiltration. Furthermore, MCP-1 induces adhesion
molecule, pro-inflammatory cytokine, chemokine, matrix
metalloproteinase and tissue factor expression. Taken together,
these findings indicate that MCP-1 contributes to the initiation
and development of inflammation.
Curcumin is the active ingredient extracted from
turmeric, a curry spice, which is not only used in preparing Asian
curry dishes but is also a component of certain ancient herbal
remedies for various diseases (3).
Previous pharmacological studies have shown that curcumin has a
variety of health-beneficial effects, including anti-inflammatory,
antioxidant, anticarcinogenic, antithrombotic and cardiovascular
protective effects (4). Among
these biological activities, the anti-inflammatory effects of
curcumin have been assessed in various in vitro systems and
in experimental animal systems (5,6).
However, the cellular and molecular mechanisms of the
anti-inflammatory effects of curcumin have not yet been well
characterized.
In the present study, we monitored the inhibitory
effect of curcumin on lipopolysaccharide (LPS)-induced MCP-1
expression in macrophages and explored the cellular and molecular
mechanisms involved. Our results revealed that curcumin
significantly inhibited the LPS-induced increase in MCP-1
expression and enhanced heme oxygenase-1 (HO-1) expression in a
concentration-dependent manner. Additionally, HO-1 is a
rate-limiting enzyme that degrades heme to biliverdin, ferrous iron
and carbon monoxide (7). HO-1 and
its end products play a key role in protecting cells against
inflammatory responses and oxidative stress (8). Therefore, the present study was
designed to investigate whether the anti-inflammatory effects of
curcumin are partially dependent on HO-1-mediated reactive oxygen
species (ROS) reduction, subsequently suppresing LPS-induced MCP-1
production in macrophages.
Materials and methods
Cell culture and treatment
RAW264.7 cells (American Type Culture Collection)
were cultured in DMEM (Gibco-BRL, Carlsbad, CA, USA) containing 10%
FBS (Gibco-BRL), 100 U/ml penicillin and 100 μg/ml streptomycin at
37°C. The medium was changed to serum-free medium after the cells
had been grown to confluence, and the cells were incubated
overnight prior to the experiments. Before LPS was added to the
medium, the cells were pre-treated with curcumin (Sigma, St. Louis,
MO, USA). The macrophages were pre-treated with zinc protoporphyrin
(ZnPP; Sigma), N-acetylcysteine (NAC) (J&K Scientific, Ltd.,
Beijing, China), apocynin (Sigma), PD98059 (Beyotime Biotech,
Haimen, China), SB203580 (Beyotime Biotech) or SP600125 (Beyotime
Biotech) at 1 h before curcumin treatment.
Cell viability assay
The cells were plated with a variety of
concentrations of curcumin (0–80 μM) in 96-well microtiter plates
and were then cultured for 24 h at 37°C in a 5% CO2
incubator. Cell viability was determined using the conventional
methylthiazolyl tetrazolium (MTT) reduction assay. Following the
treatment of the cells with curcumin, MTT solution was added (final
concentration 5 mg/ml) and incubation was continued for 4 h at
37°C. The dark blue formazan crystals formed in the intact cells
were solubilized with DMSO and then the absorbance of the blue
color was measured at 490 nm using a microplate reader (Bio-Rad,
Hercules, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) and quantified by UV
absorption at 260 and 280 nm. RT-PCR was performed according to the
manufacturer’s instructions. According to GenBank, the RT-PCR
primers were designed as follows: HO-1 sense,
5′-GGGTGACAGAAGAGGCTAAGACC-3′ and antisense,
5′-AGATTCTCCCCTGCAGAGAGAAG-3′. The PCR conditions were as follows:
30 cycles of 94°C for 30 sec; 55°C for 30 sec; and 72°C for 45 sec.
The amplified products were visualized by 1.5% agarose gel
electrophoresis, stained with ethidium bromide and images were then
captured under ultraviolet light. Densitometric analysis of three
different observations was performed using Quantity One Software
(Bio-Rad). The quantity of each transcript was normalized to that
of GAPDH.
Western blot analysis
The cells were harvested and washed twice with PBS.
The harvested cells were then lysed and 50 μg total protein was
separated by SDS-PAGE on 10% polyacrylamide gels and transferred
onto nitrocellulose membranes. After blocking for 1 h with 8%
skimmed milk in TBS buffer (10 mM Tris, 150 mM NaCl), the membranes
were incubated with primary antibodies at 4°C overnight. The
membranes were then washed four times for 15 min each with TBST
buffer (10 mM Tris, 150 mM NaCl and 0.1% Tween-20) and incubated
with the appropriate HRP-conjugated secondary antibody at 37°C for
30 min. The protein bands were detected using an enhanced
chemiluminescene western blotting detection kit (Amersham,
Buckinghamshire, UK). The antibodies were all purchased from
Beyotime Biotech.
Measurement of ROS
Prior to the chemical treatment, the cells were
incubated in culture medium containing 30 μM
2′,7-dichlorofluorescein (DCF; a fluorescent dye, Beyotime Biotech)
for 30 min to establish a stable intracellular level of the probe.
Subsequently, the cells were washed with PBS, removed from the
Petri dishes by scraping and evaluated for DCF fluorescence
intensity, which was used as an index of the intracellular levels
of ROS. The fluorescent DCF was detected using a laser scanning
confocal microscope (Leica TCS-NT, Heidelberg, Germany) with
excitation and emission wavelengths of 488 and 520 nm, respectively
(9). The cell number in each
sample was counted and utilized to normalize the fluorescence
intensity of DCF.
Determination of MCP-1 levels by
enzyme-linked immunosorbent assay (ELISA)
The MCP-1 concentrations of the cell supernatants
were determined using a commercially available rat MCP-1 ELISA
development kit (Beyotime Biotech). The assays were performed using
the instructions provided by the manufacturer. All samples were
assayed in triplicate.
Statistical analysis
Data are expressed as the means ± SD of three
assays. The statistical analysis was conducted by one-way ANOVA.
P<0.05 was considered to indicate a statistically significant
difference.
Results
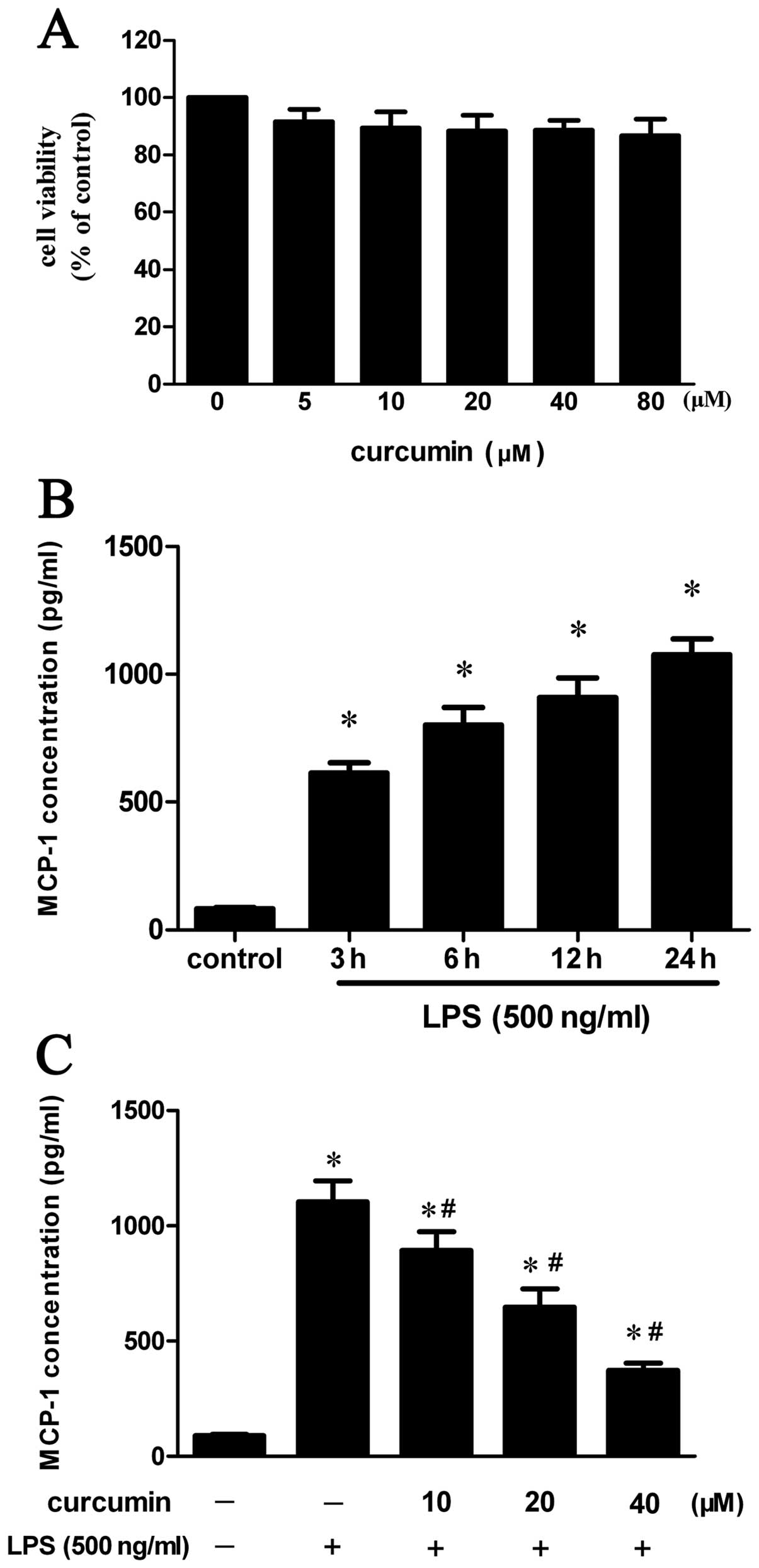
Assessment of cell toxicity of
curcumin
In order to exclude the possibility that reductions
in the levels of inflammatory cytokines in the cells were due to
the direct toxic effects of curcumin on the cells, we evaluated the
cytotoxicity of curcumin at various concentrations (0–80 μM) using
MTT assay. The results showed that the curcumin-induced
cytotoxicity was negligible at concentrations of 0–80 μM in
RAW264.7 macrophages (Fig.
1A).
Curcumin inhibits LPS-induced MCP-1
production in RAW264.7 macrophages
To investigate the inhibitory effect of curcumin on
MCP-1 expression in vitro, ELISA was performed. As expected,
treatment with 500 ng/ml LPS caused a time-dependent increase in
MCP-1 production in the RAW264.7 cells (Fig. 1B). In addition, when the RAW264.7
cells were pre-incubated with curcumin and then stimulated with
LPS, the curcumin pre-treatment significantly decreased the levels
of MCP-1 released by the LPS-stimulated RAW264.7 cells (Fig. 1C).
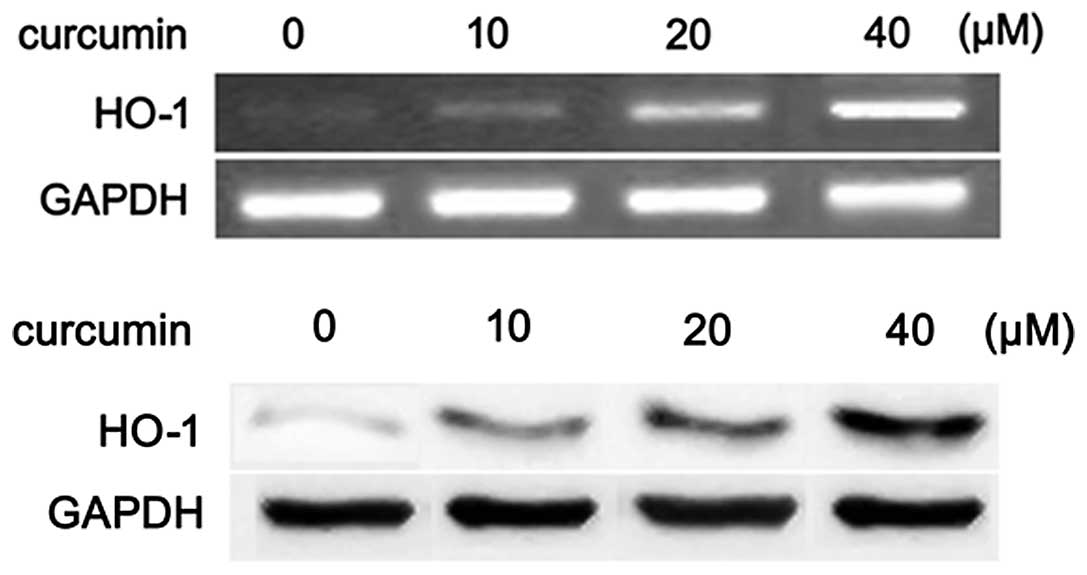
Curcumin upregulates HO-1 expression in a
concentration-dependent manner
To examine whether curcumin induces HO-1 expression,
RT-PCR and western blot analyses were performed to determined the
levels of HO-1 mRNA and protein. We found that curcumin upregulated
HO-1 expression in a concentration-dependent manner (Fig. 2).
HO-1 inhibitor, ZnPP, attenuates the
inhibitory effect of curcumin on LPS-induced MCP-1 expression
To further investigate whether HO-1 contributes to
the regulatory effect of curcumin on LPS-induced MCP-1 production,
the cells were pre-treated with ZnPP (a HO-1 inhibitor) and
curcumin prior to the addition of LPS. The results indicated that
the inhibitory effect of curcumin on LPS-induced MCP-1 production
was blocked by ZnPP (Fig. 3A).
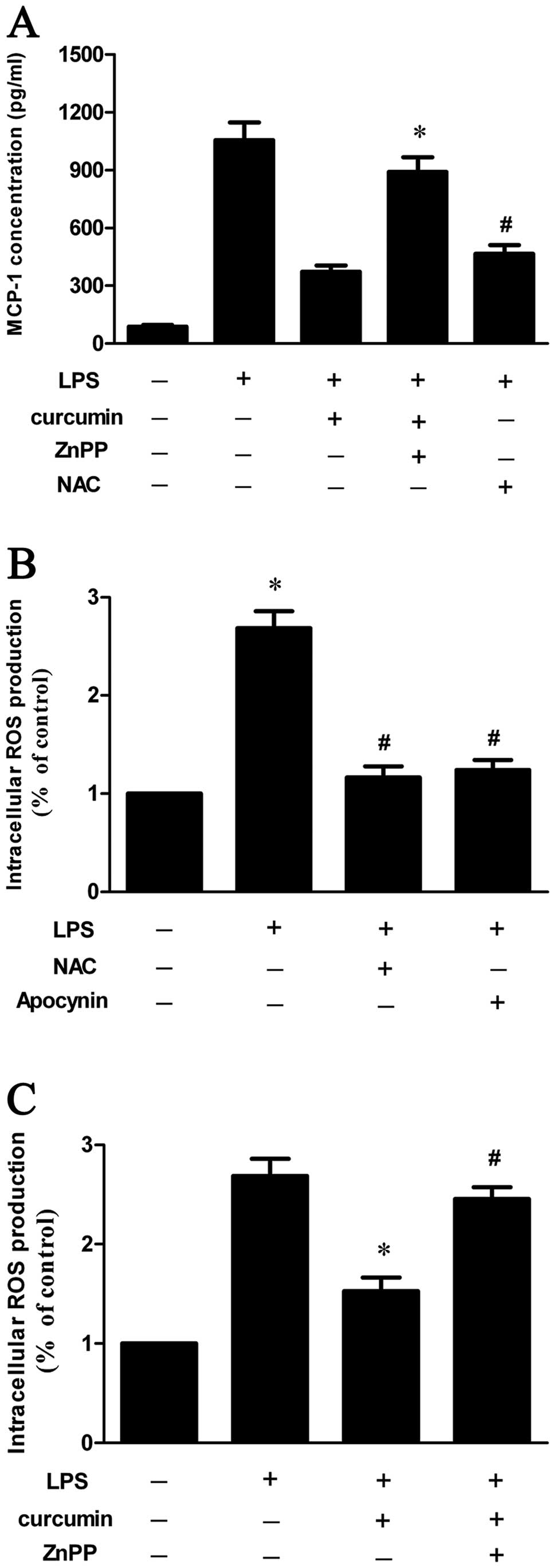 | Figure 3HO-1 inhibitor (ZnPP) blocked the
inhibitory effect of curcumin on LPS-induced MCP-1 expression and
ROS production. Data are the means ± SD of three independent
experiments. (A) Cells were pre-treated with curcumin (40 μM), ZnPP
(10 μM) or NAC (10 mM) for 30 min and cultured in the presence of
LPS (500 ng/ml) for 24 h. MCP-1 production was assayed by ELISA.
#P<0.05 vs. the LPS-treated group,
*P<0.05 vs. the LPS- and 40 μM curcumin-treated
group. (B) LPS-induced ROS production in RAW264.7 cells is
dependent on NADPH oxidase. Cells were pre-treated with NAC (10 mM)
or apocynin (1 mM) and cultured with LPS (500 ng/ml) for 2 h.
*P<0.05 compared with the control group.
#P<0.05 compared with the the LPS-treated group. (C)
ZnPP attenuates the inhibitory effect of curcumin on LPS-induced
ROS generation. Cells were pre-treated with curcumin and ZnPP (10
μM) for 30 min and cultured in the presence of LPS (500 ng/ml) for
2 h. Intracellular ROS levels were determined by DCF oxidation.
*P<0.05 compared with the LPS-treated group,
#P<0.05 compared with the LPS- and 40 μM
curcumin-treated group. Heme oxygenase-1, HO-1; ZnPP, zinc
protoporphyrin; LPS, lipopolysaccharide; MCP-1, monocyte
chemoattractant protein-1; ELISA, enzyme-linked immunosorbent
assay; ROS, reactive oxygen species; NAC, N-acetylcysteine. |
NAC (ROS scavenger) inhibits LPS-induced
MCP-1 production in RAW264.7 macrophages
Similarly, RAW264.7 cells were pre-incubated with
NAC and then stimulated with LPS. We found that NAC markedly
reduced the LPS-induced MCP-1 expression in RAW264.7 macrophages
(Fig. 3A).
HO-1 inhibitor (ZnPP) attenuates the
inhibitory effect of curcumin on LPS-induced ROS production
Increased ROS generation was observed in RAW264.7
cells stimulated with LPS, whereas the inhibitory effect was
significantly blocked by the ROS scavenger, NAC, and the specific
NADPH oxidase inhibitor, apocynin (Fig. 3B). In addition, LPS-mediated ROS
production was significantly inhibited by curcumin. However, when
the cells were pre-treated with ZnPP (a HO-1 inhibitor), the
inhibitory effect was effectively eliminated (Fig. 3C).
Discussion
Macrophages play important roles in inflammatory
diseases by producing multiple pro-inflammatory cytokines and
enzymes in response to various stimuli, including LPS, a bacterial
endotoxin (10). Activated
macrophages release multiple cytokines and play critical roles in
inflammatory processes. Among the numerous pro-inflammatory
mediators implicated in inflammatory processes, MCP-1 appears to be
one of the most significant chemokines that stimulates mononuclear
leukocytes and triggers their adhesiveness and transmigration
through the endothelial layer (11). It is widely expressed in
atherosclerotic lesions, including vascular endothelial cells,
smooth muscle cells and monocytes/macrophages. Therefore, methods
of inhibiting MCP-1 production during exposure to chronic or
long-term stimulation have been the subject of therapeutic
strategies for treatment of these diseases. In the present study,
we investigated the effect of curcumin on the LPS-stimulated
production of MCP-1. Subsequently, we demonstrated that curcumin
inhibited the LPS-induced MCP-1 production in RAW264.7 macrophages
and explored the possible mechanisms involved. To our knowledge, we
show for the first time that HO-1-mediated ROS reduction is
involved in the inhibitory effect of curcumin on LPS-induced MCP-1
expression in RAW264.7 macrophages. The present data extend our
knowledge of the effects of curcumin on macrophages.
HO-1, an inducible antioxidant enzyme, mediates the
degradation of heme into ferrous iron, carbon monoxide and
biliverdin. The products resulting from HO-1 activity have
antioxidant and anti-inflammatory effects (12). HO-1 is a cytoprotective protein
whose expression is consistently associated with therapeutic
benefits in a number of pathological conditions, including
atherosclerotic vascular disease and inflammation (13).
In the present study, we found that curcumin
significantly upregulates HO-1 mRNA and protein expression, which
is consistent with several previous studies (14–16).
Data from previous studies suggest that the induction of HO-1
enhances antioxidant and anti-inflammatory effects. It has been
reported that celastrol-induced HO-1 expression is responsible for
the suppression of IFN-γ-induced ICAM-1 expression and subsequent
monocyte adhesion in keratinocytes (17). Furthermore, celastrol attenuates
the hypertension-induced expression of inflammatory cytokines in
vascular smooth muscle cells via HO-1 induction (18). Chen et al also demonstrated
that HO-1 is involved the LPS-induced expression of
cyclooxygenase-2 and MCP-1 (19).
In the present study, our finding that curcumin inhibited
LPS-induced MCP-1 production in RAW264.7 macrophages is in
agreement with the findings of Panicker and Kartha (20), who revealed that curcumin
attenuates glucose-induced MCP-1 synthesis in aortic endothelial
cells through NF-κB. In addition, Lim and Kwon (21) identified that a possible
anti-inflammatory mechanism of curcumin may be the inhibition of
the secretion of the inflammatory MCP-1 chemokine. To investigate
whether the inhibitory effect of curcumin on LPS-induced MCP-1
expression is mediated via HO-1, we pre-treated cells with ZnPP (a
HO-1 inhibitor) and found that the inhibitory effect of curcumin
was partially, but not completely reversed in the presence of ZnPP.
Thus, our results demonstrate that the curcumin-induced HO-1
expression contributes, to a certain extent, to the MCP-1 reduction
in RAW264.7 macrophages stimulated with LPS.
Under physiological conditions, there is a balance
between the generation of ROS and detoxification by antioxidant
systems. In general, oxidative stress occurs when this balance is
disrupted, either directly by infectious agents or by cytokines
released from inflamed cells that may lead to increased ROS
generation and/or decreased antioxidant defense. Normally, ROS are
involved in signal transduction pathways which mediate certain
essential cellular functions, including host cell defense,
mitochondrial respiration, cytokine generation and cell
proliferation/apoptosis (22). LPS
and various inflammatory cytokines, including TNF-α, interleukin
(IL)-1 and IL-10, activate NADPH oxidase to generate significant,
occasionally toxic, amounts of ROS which propagate their signals
that activate transcription factors. In addition, accumulating
evidence indicates that ROS act as signaling molecules to trigger
pro-inflammatory cytokine production (23,24).
In our study, we found that ZnPP blocked the
inhibitory effect of curcumin on LPS-induced ROS production, which
demonstrated that curcumin-induced HO-1 expression is involved in
the reduction of ROS in RAW264.7 macrophages stimulated with LPS.
In addition, LPS-induced ROS production was suppressed by NAC and
apocynin, which suggests that LPS induces ROS production in
macrophages through NADPH oxidases. Moreover, the LPS-induced MCP-1
expression was also blocked by NAC (a ROS scavenger), which
indicates that ROS generation is involved in LPS-induced MCP-1
expression. Furthermore, we demonstrate that curcumin-induced HO-1
expression may contribute to a reduction in MCP-1 expression levels
in RAW264.7 macrophages stimulated with LPS. Taken together, our
results imply that the HO-1-mediated reduction of ROS is involved
in the inhibitory effect of curcumin on LPS-induced MCP-1
production.
Several intracellular signaling pathways are
involved in the regulation of the inflammatory reaction in
stimulated macrophages, including the MAPK pathway linked to the
activation of transcription factors. MAPKs may be activated by
various extracellular molecules and may induce the downstream
phosphorylation of a number of key signaling molecules related to
cell proliferation, inflammation and apoptosis (25,26).
In addition, numerous studies have implicated ROS as participants
in a variety of intracellular signaling pathways that include MAPK
members (27–29) and other signaling systems (30). It has been reported that curcumin
inhibits phorbol myristate acetate (PMA)-induced MCP-1 expression
by inhibiting ERK and NF-κB transcriptional activity (21). Takaya et al(31) found that albumin overload-induced
MCP-1 expression was regulated by the ERK pathway in mouse proximal
tubular cells. However, Hong et al(32) reported that the upregulation of
MCP-1 by PMA was inhibited by the blockade of the p38 MAPK, but not
by the blockade of the ERK and JNK pathways in human endothelial
ECV304 cells. These inconsistent observations may be due to diverse
experimental conditions and cell types.
Moreover, although the present study was conducted
in cell culture, these findings should provide some useful
molecular mechanisms to explain the beneficial anti-inflammatory
effects of curcumin. In the future, we will attempt to confirm
these observations with in vivo experiments.
In conclusion, this study provides evidence to
suggest that curcumin enhanced the expression of HO-1 to reduce the
LPS-induced production of ROS, which inhibited the expression of
MCP-1 in RAW264.7 macrophages.
References
|
1
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boring L, Gosling J, Cleary M and Charo
IF: Decreased lesion formation in CCR2−/− mice reveals a
role for chemokines in the initiation of atherosclerosis. Nature.
394:894–897. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Surh YJ: Anti-tumor promoting potential of
selected spice ingredients with antioxidative and anti-inflammatory
activities: a short review. Food Chem Toxicol. 40:1091–1097. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as ‘Curecumin’: from kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008.
|
|
5
|
Sumanont Y, Murakami Y, Tohda M,
Vajragupta O, Matsumoto K and Watanabe H: Evaluation of the nitric
oxide radical scavenging activity of manganese complexes of
curcumin and its derivative. Biol Pharm Bull. 27:170–173. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mahakunakorn P, Tohda M, Murakami Y,
Matsumoto K, Watanabe H and Vajaragupta O: Cytoprotective and
cytotoxic effects of curcumin: dual action on
H2O2-induced oxidative cell damage in
NG108–15 cells. Biol Pharm Bull. 26:725–728. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Montellano PR: The mechanism of heme
oxygenase. Curr Opin Chem Biol. 4:221–227. 2000. View Article : Google Scholar
|
|
8
|
Durante W: Heme oxygenase-1 in growth
control and its clinical application to vascular disease. J Cell
Physiol. 195:373–382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu JH, Kim KH, Kim DG and Kim H:
Diphenyleneiodonium suppresses apoptosis in cerulein-stimulated
pancreatic acinar cells. Int J Biochem Cell Biol. 39:2063–2075.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laskin DL and Pendino KJ: Macrophages and
inflammatory mediators in tissue injury. Annu Rev Pharmacol
Toxicol. 35:655–677. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baggiolini M, Dewald B and Moser B: Human
chemokines: an update. Annu Rev Immunol. 15:675–705. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ryter SW, Alam J and Choi AM: Heme
oxygenase-1/carbon monoxide: from basic science to therapeutic
applications. Physiol Rev. 86:583–650. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi T, Morita K, Akagi R and Sassa
S: Heme oxygenase-1: a novel therapeutic target in oxidative tissue
injuries. Curr Med Chem. 11:1545–1561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim AN, Jeon WK, Lee JJ and Kim BC:
Up-regulation of heme oxygenase-1 expression through
CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect
of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic
Biol Med. 49:323–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeong SO, Oh GS, Ha HY, et al:
Dimethoxycurcumin, a synthetic curcumin analogue, induces heme
oxygenase-1 expression through Nrf2 activation in RAW264.7
macrophages. J Clin Biochem Nutr. 44:79–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KM, Pae HO, Zhung M, et al:
Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory
effect of curcumin on the expression of pro-inflammatory inducible
nitric oxide synthase in RAW264.7 macrophages. Biomed Pharmacother.
62:630–636. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seo WY, Ju SM, Song HY, et al: Celastrol
suppresses IFN-gamma-induced ICAM-1 expression and subsequent
monocyte adhesiveness via the induction of heme oxygenase-1 in the
HaCaT cells. Biochem Biophys Res Commun. 398:140–145. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu X, Tao W, Jiang F, Li C, Lin J and Liu
C: Celastrol attenuates hypertension-induced inflammation and
oxidative stress in vascular smooth muscle cells via induction of
heme oxygenase-1. Am J Hypertens. 23:895–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen YC, Chen CH, Ko WS, Cheng CY, Sue YM
and Chen TH: Dipyridamole inhibits lipopolysaccharide-induced
cyclooxygenase-2 and monocyte chemoattractant protein-1 via heme
oxygenase-1-mediated reactive oxygen species reduction in rat
mesangial cells. Eur J Pharmacol. 650:445–450. 2011. View Article : Google Scholar
|
|
20
|
Panicker SR and Kartha CC: Curcumin
attenuates glucose-induced monocyte chemoattractant protein-1
synthesis in aortic endothelial cells by modulating the nuclear
factor-kappaB pathway. Pharmacology. 85:18–26. 2010. View Article : Google Scholar
|
|
21
|
Lim JH and Kwon TK: Curcumin inhibits
phorbol myristate acetate (PMA)-induced MCP-1 expression by
inhibiting ERK and NF-kappaB transcriptional activity. Food Chem
Toxicol. 48:47–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Halliwell B: Mechanisms involved in the
generation of free radicals. Pathol Biol (Paris). 44:6–13.
1996.PubMed/NCBI
|
|
23
|
Nakahira K, Haspel JA, Rathinam VA, et al:
Autophagy proteins regulate innate immune responses by inhibiting
the release of mitochondrial DNA mediated by the NALP3
inflammasome. Nat Immunol. 12:222–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou R, Yazdi AS, Menu P and Tschopp J: A
role for mitochondria in NLRP3 inflammasome activation. Nature.
469:221–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blüthgen N and Legewie S: Systems analysis
of MAPK signal transduction. Essays Biochem. 45:95–107. 2008.
|
|
26
|
Turjanski AG, Vaqué JP and Gutkind JS: MAP
kinases and the control of nuclear events. Oncogene. 26:3240–3253.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laderoute KR and Webster KA:
Hypoxia/reoxygenation stimulates Jun kinase activity through redox
signaling in cardiac myocytes. Circ Res. 80:336–344. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guyton KZ, Liu Y, Gorospe M, Xu Q and
Holbrook NJ: Activation of mitogen-activated protein kinase by
H2O2 Role in cell survival following oxidant
injury. J Biol Chem. 271:4138–4142. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ushio-Fukai M, Alexander RW, Akers M and
Griendling KK: p38 Mitogen-activated protein kinase is a critical
component of the redox-sensitive signaling pathways activated by
angiotensin II. Role in vascular smooth muscle cell hypertrophy. J
Biol Chem. 273:15022–15029. 1998. View Article : Google Scholar
|
|
30
|
Lander HM: An essential role for free
radicals and derived species in signal transduction. FASEB J.
11:118–124. 1997.PubMed/NCBI
|
|
31
|
Takaya K, Koya D, Isono M, et al:
Involvement of ERK pathway in albumin-induced MCP-1 expression in
mouse proximal tubular cells. Am J Physiol Renal Physiol.
284:1037–1045. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hong MH, Kim MH, Chang HJ, et al:
(-)-Epigallocatechin-3-gallate inhibits monocyte chemotactic
protein-1 expression in endothelial cells via blocking NF-kappaB
signaling. Life Sci. 80:1957–1965. 2007. View Article : Google Scholar : PubMed/NCBI
|