Introduction
As the numbers of cases of cardiovascular disease
steadily grow, grafting as a therapeutic method has been
demonstrated to be effective, and millions of tissues and organs
are demanded (1). However, as
there is a shortage of donor tissues and organs and low graft
efficiency, the clinical application of aorta grafting has been
restrained (2–4). Since the 1980s, a variety of
substitutes have been used for aorta grafting. Artificial
materials, biomaterials and composites have been tested as
substitutes for blood vessels in practical applications and
experiments (5–7). Due to rejection, thrombosis,
endothelial cell hyperplasia and calcification, they are not very
suitable for blood vessels with a diameter <1 cm (8). Thus, at present, substitutes are not
able to completely replace freeze-banked human tissues and
organs.
Three requirements for grafting are reduced
immunogenicity, preserved vessel structure and mechanical
properties, and elevated histocompatibility (9–12).
Given the variety of fatal diseases that may infect through blood,
including AIDS and SARS, the tissue or organ graft should be
pathogen-free.
Cryopreservation technologies represent potentially
long-term and minimally damaging methods for the preservation of
tissues. The conventional cryopreservation of allogeneic blood
vessels involving freezing is currently being used clinically, but
in vivo studies using these grafts have exhibited poor
long-term patency rates due to tissue structure injury and changes
in mechanical properties (13). A
suitable method of tissue preservation is urgently required.
With highly structural and functional homology,
porcine tissues and organs are used as replacements for human ones,
which, to some extent, has eased the situation of a deficit of
tissues and organs. Due to calcification and high immunogenicity,
the clinical application of porcine material has been limited
(14). A new method of reducing
immunogenicity and elevating histocompatibility is required for
porcine xenografts.
The current study introduces a process of vacuum
freeze-drying combined with radiation treatment for the pre-grafted
aorta, aiming at reducing immunogenicity, preserving vessel
structure and mechanical properties, elevating histocompatibility
and removing pathogenic microorganisms from the allogenetic aorta
graft. The tissue structure, mechanical properties, pathogen
content and immunogenicity of the graft are measured and
discussed.
Materials and methods
Reagents and instruments
D-Hanks’ solution and PBS solution were acquired
from Sangon (Shanghai, China). The experimental instruments used
were as follows: S450 scanning electron microscope (Hitachi, Tokyo,
Japan), optical microscope (Olympus, Tokyo, Japan), texture
analyzer (Trapezium Lite, Hong Kong, China), freeze drier (SP
Industries, Inc., Warminster, PA, USA) and ABI Prism 7000 real-time
PCR system (Beckman-Coulter, Miami, FL, USA).
Animals and sampling
Young pigs weighing 10–15 kg were supplied by a
Shanghai pig farm. All animal experiments were carried out in
accordance with the guide for Care and Use of Laboratory Animals
(the ‘NIH Guide’). The protocols for the use of animals were
approved by the Department of Laboratory Animal Sciences from
Shanghai Jiao Tong University School of Medicine.
Vacuum freeze-drying combined with
radiation treatment
The aortas, ~2 cm in length, were dissected out from
the pigs under anesthesia. The isolated aortas were washed with
D-Hanks’ solution and PBS solution, once each. The aortas were
applied to a programmed cooling system as described in our previous
study (15). Briefly, the aortas
were cooled to -43°C at a cooling rate of 1°C per minute. The
aortas were positioned vertically and kept at -43°C for 1 h. The
aortas were then dried at -20°C for 2 h and at 15°C for 4 h, while
the pressure was kept at 3–10 Pa. The aortas were then put into a
sealed and sterile apparatus, irradiated with 20 kGy
60Co rays for 8 h and preserved at room temperature.
Prior to performing follow-up experiments, the dried aortas were
immersed in physiological saline for 2 h.
Histological observation
The aortas were fixed in 40 g/l formaldehyde
solution for 48 h. Following dehydration using ethanol and xylene,
the samples were embedded in paraffin and sectioned at 5 μm.
Subsequently, hematoxylin and eosin (H&E) staining was
performed.
Immunohistochemistry
The aorta (1 cm in length) was dissected out and
then cut into frozen sections at a thickness of 5 μm. The sections
were fixed in formaldehyde solution for 1 min. After washing once
with PBS solution, the sections were placed into a humidor. The
primary antibody (MHC) at a dilution of 1:100 was added to the
sections, and PBS solution replaced the primary antibody in the
negative controls. The slides were maintained at 37°C for 1 h.
After extensive washing with PBS solution, the secondary antibody
(FITC-conjugated rabbit anti-mouse IgG) at a dilution of 1:100 was
added to the sections, which were then incubated at 37°C for
another 1 h. Factor VIII staining was performed according to the
instructions provided with the factor VIII immunochemistry staining
kit (Beijing TCT Medical Technology, Beijing, China). For CD68 and
CD3 staining, the two antibodies and SP staining kit were purchased
from Zhongshanjingqiao Biotechnical Co. Ltd (Beijing, China). The
sample embedded in paraffin was deparaffinized and hydrated,
followed by SP staining according to the instructions provided with
the staining kit, and PBS was used as a negative control.
PCR detection
For the detection of the porcine endogenous
retrovirus, primers were designed according to the porcine
endogenous retrovirus gag protein sequence (GeneBank accession no.
AY265811.1). The forward primer was 5′-GCGACCCACGCAGTTGCATA-3′ and
the reverse primer was 5′-CAGTTCCT TGCCCAGTGTCCTT-3′. The length of
the target fragment was 662 bp. The PCR conditions were: 95°C for 1
min, annealing at 55°C for 1 min and extension at 72°C for 1 min.
The PCR product (5 μl) was analyzed on a 15 g/l agarose gel and
visualized by ethidium bromide staining with UV illumination.
Measurement of mechanical properties
The puncture stress (PT) was evaluated and tensile
stress determined by stretching the tissue at a rate of 1 mm/min
using a texture analyzer with a 500 N load cell.
Aorta grafting
The pig was subjected to intravenous-inhalation
anesthesia prior to surgery. The graft was implanted into the
descending aorta. The incision was performed at the fourth
intercostal space. Subsequently, two intercostal aortas were
ligatured. The blood was blocked between the proximal and distal
ends of the descending aorta, and a section of descending aorta was
removed, ~2 cm in length. The reconstituted aorta was transplanted
by continuous suturing. Following the exhaustion of air, the
hemostatic forceps were released. The wound was then staunched and
the thorax and lung were washed. Two months after the operation,
the implanted blood vessel was dissected out using a similar method
to that previously described and the pig was sacrificed.
Statistical analyses
Data are presented as the mean ± SD. Statistical
analysis was performed using SPSS 11.0.0, 2001 (SPSS Inc., Chicago,
IL, USA). The Student’s t-test was performed for the analysis of
differences. P<0.05 was considered to indicate a statistically
significant result.
Results
Histological observation
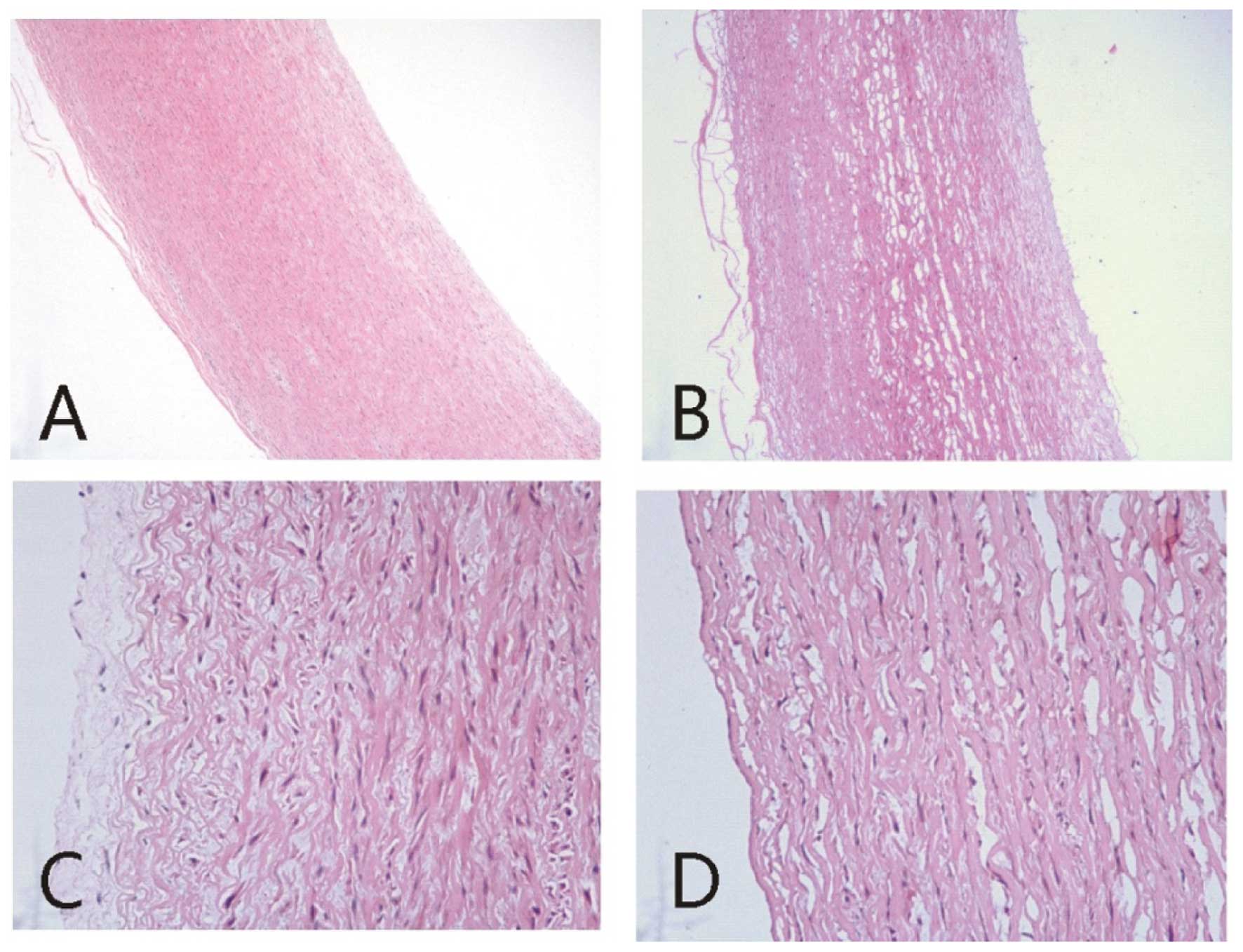
We histologically examined the reconstituted aorta
treated by the vacuum freeze-drying and radiation method. Compared
with the normal aorta which had a regular cell arrangement and well
structured intima, the graft exhibited numerous small holes with a
fundamentally complete media structure and loosely structured
spandex fibres, but lacked intima structure (Fig. 1).
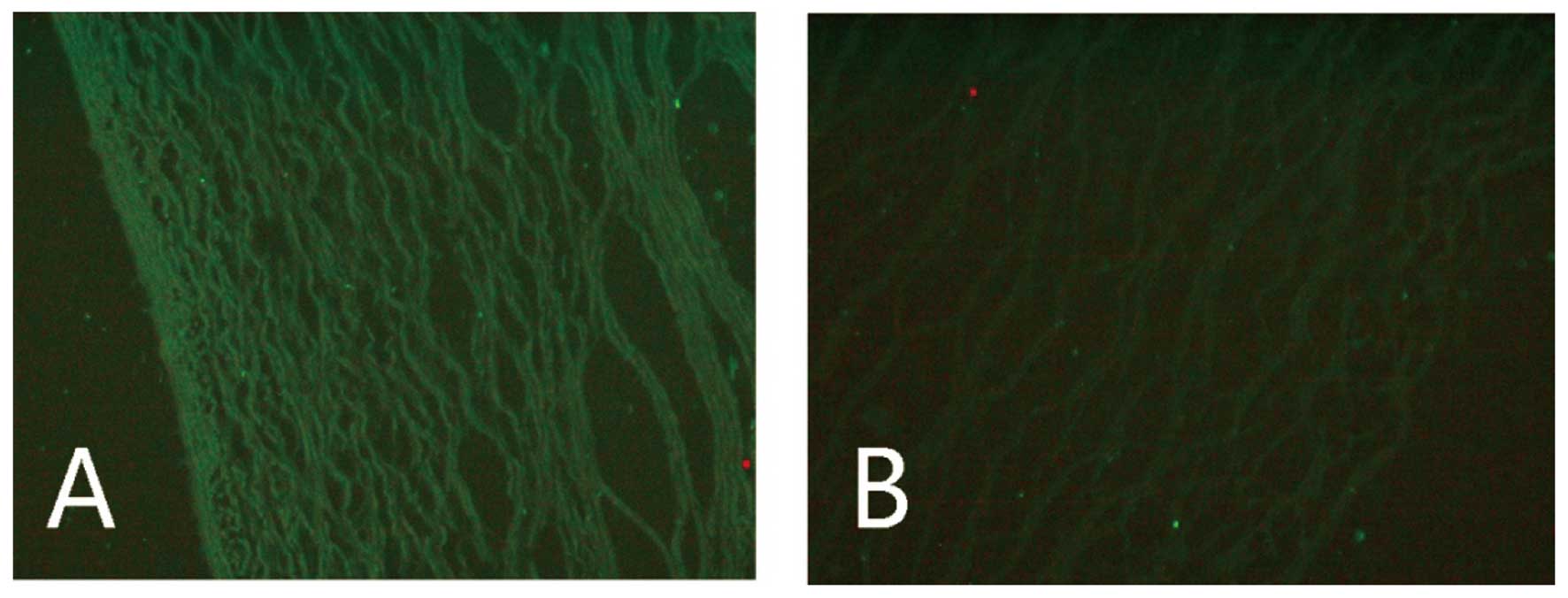
We also examined the immunogenicity of the
reconstituted aorta. A comparison of the signal strengths of the
MHC II-positive cells in the normal (0.2984±0.0121) and
reconstituted aorta (0.0748±0.0087) revealed that the reconstituted
graft had significantly lower immunogenicity than normal aorta
(P<0.01; Fig. 2).
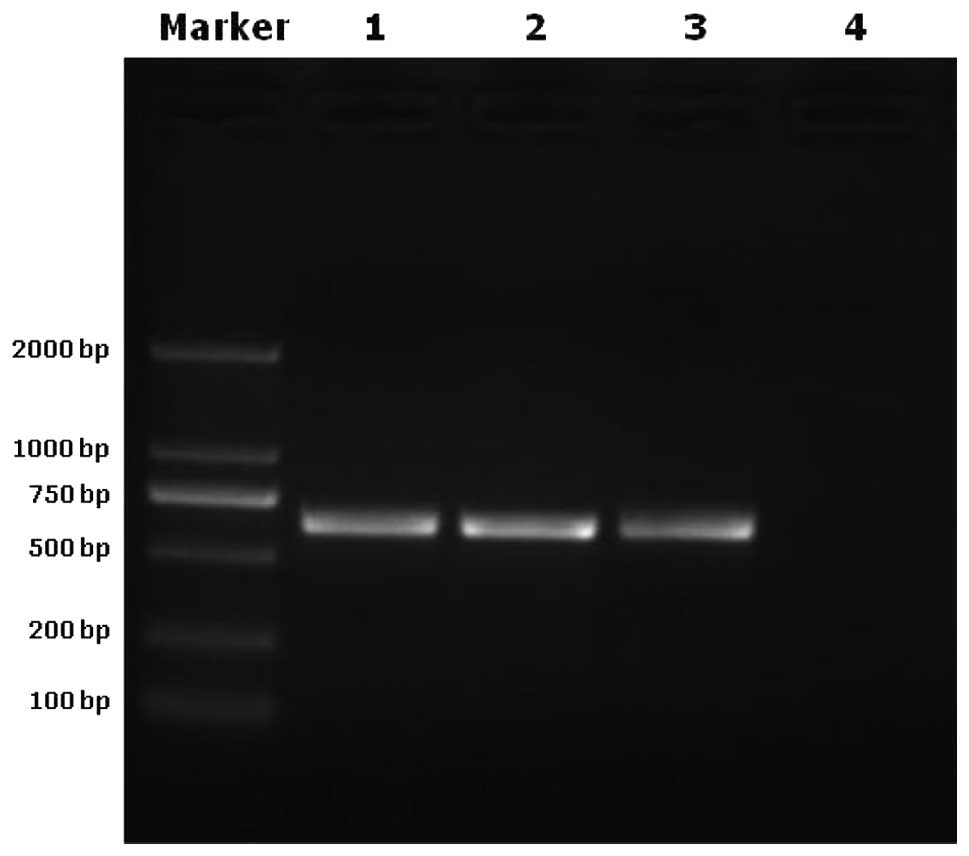
To investigate the endogenous retrovirus
deactivating effect of the vacuum freeze-drying and radiation
method, we analyzed the samples by PCR. Pre-viral DNA was
undetectable in the vacuum freeze-drying and radiation treatment
group, but was detected in the normal aorta and vacuum freeze
drying treatment groups and in the porcine endogenous retrovirus C
genome which served as a positive control (Fig. 3).
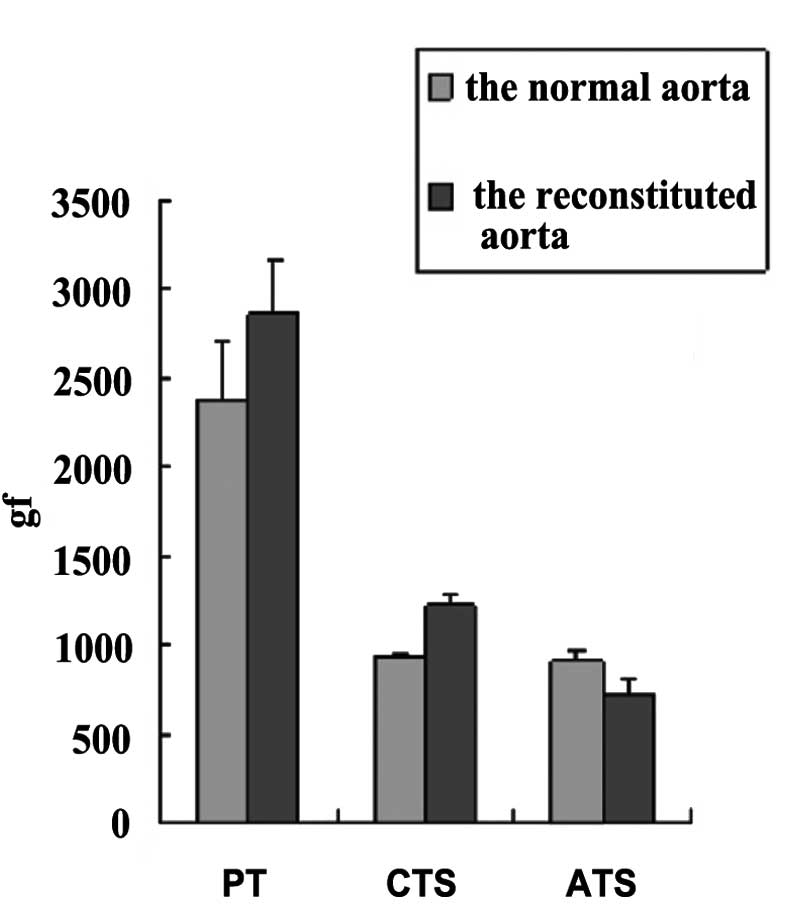
Next, we measured the mechanical properties of the
reconstituted aorta. Compared with normal aorta, the maximum axial
tensile stress (ATS) decreased by 20%, the maximum circumferential
tensile stress (CTS) increased by 30% and the maximum PT decreased
by 20% (Fig. 4).
To investigate the validity of the vacuum
freeze-drying and radiation method, aorta grafting was carried out.
After the surgery, without using any drugs, abnormal behavior and
disease were not detected. Two months after the operation, when the
grafts were dissected out, no particular abnormalities were
observed. Histologically, clear intima, media and adventitia had
formed, endothelial cells had almost covered the inner wall of
vessel, and a great number of host cells had migrated into the
intima and adventitia in which a small number of inflammatory cells
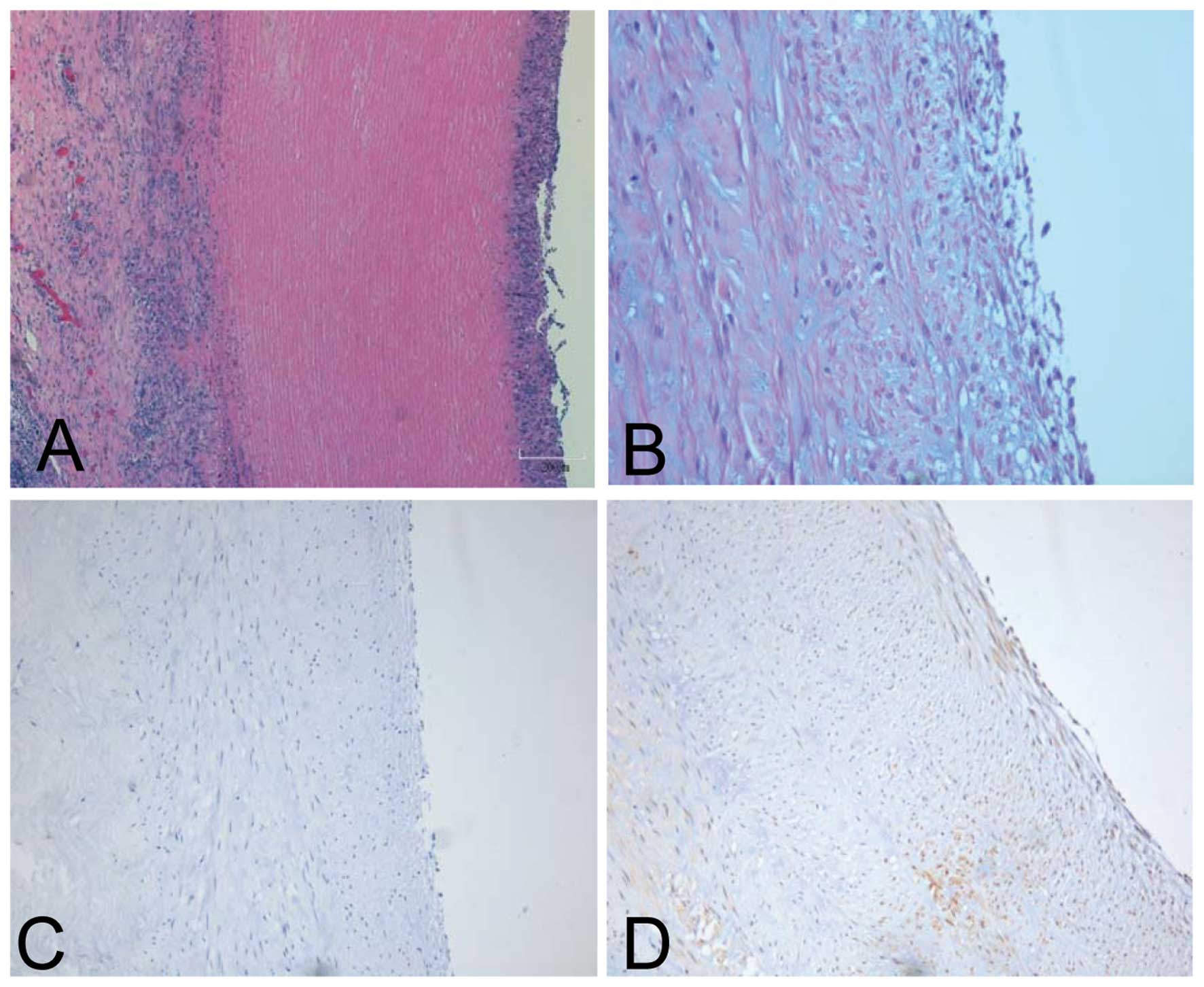
also had migrated (Fig. 5A).
Factor VIII staining was performed to distinctly visualize the
well-developing host endothelialization (Fig. 5B). Staining for CD68 (a marker for
macrophages) and CD3 (a marker for T lymphocytes) was performed to
measure the inflammatory reaction level. The CD68 and CD3 positive
lymphocytes were present at very low levels within the adventitia,
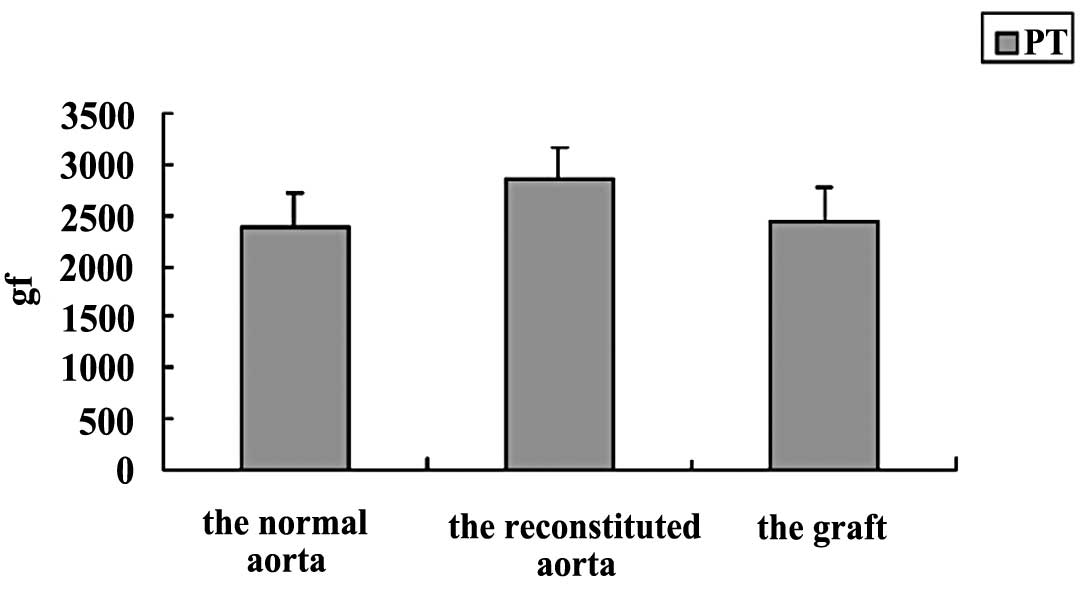
indicating that the graft lacked immunogenicity (Fig. 5C and D). The PT of the grafts was
similar to that of normal aortas (Fig.
6).
Discussion
The antigenicity of the blood vessel exists in the
endothelial cells which are the first point of contact between the
recipient and the donor tissue and the first to be recognized by
the immune system (16,17). It has been reported that certain
parts of the MHC antigenic determinant may be blocked by
lyophilization treatment, reducing immunogenicity (18). A murine grafting study revealed
that the levels of MHC I and MHC II antigens decreased by 70%
following lyophilization (19).
The results of the current study reveal that the vacuum
freeze-drying and radiation treatment reduced the MHC II level by
75%. Furthermore, two months after the grafting procedure, only a
small number of CD68 or CD3-positive cells were visualized,
revealing a low level of inflammatory reaction. This indicates that
the vacuum freeze-drying and radiation method may serve as an
effective tool for reducing immunogenicity.
It is not clear whether that the intima was required
in grafting. In a previous study, the intima of the human heart
valve has been demonstrated to be unnecessary in allografts
(20). Our study demonstrated that
although the intima could not be identified in the reconstituted
aorta, two months after the grafting the intima had
re-endothelialized.
Although the reconstituted aorta was in a loose
condition, and its mechanical properties had altered, two months
after grafting the cells of the graft had rearranged; new intima,
media and adventitia had formed and endothelial cells had almost
covered the inner wall of the vessel. The structure had nearly
returned to that of normal aorta. Moreover, the PT of the graft had
already returned to a normal level.
During the two months of the experiment, abnormal
behavior and diseases were not detected. No marked size change was
observed by the unaided eye when the graft was withdrawn. Hence,
the reconstituted aorta may meet the requirements for grafting.
The effects of thermal stress on blood vessels
during cryopreservation have already been carefully studied
(21–23). Since a fast cooling rate generates
higher thermal stresses, the cooling rate greatly affects the
thermal stress in the blood vessel during the cooling process.
Therefore, a low rate cooling process is required during
cryopreservation. In this study, we developed a new cooling process
suitable for vessel grafts.
Decades ago, the porcine aorta was first utilized as
a replacement for human aorta, but the immunogenicity of the graft
and the presence of the porcine endogenous virus limited its
clinical application (24,25). The porcine endogenous retrovirus
has been reported to have the ability to infect various human cell
lines (26), therefore our study
aimed to provide a new perspective on xenografts.
In conclusion, we have introduced a vacuum
freeze-drying and radiation method for the pre-grafting treatment
of porcine aorta, aimed at reducing immunogenicity, preserving
vessel structure and mechanical properties, elevating
histocompatibility and removing pathogenic microorganisms. In
further studies, we propose to combine a decellularizing technique
with the vacuum freeze-drying and radiation method to remove the
cell debris which may cause calcification in xenografts (27).
Acknowledgements
This study was financially supported by the Shanghai
Municipal Education Commission Innovation Project (no. 2012zz101)
and Shanghai Jiaotong University Academic Project (no. YZ1012).
References
|
1
|
Hua ZZ, Xu HY, Zhou GY, Liu JF, Huang HM
and Ding WX: Analyses of thermal stress and fracture during
cryopreservation of blood vessel. Sci China Ser E-Tech Sci.
31:123–127. 2001.
|
|
2
|
Dahl SL, Koh J, Prabhakar V and Niklason
LE: Decellularized native and engineered arterial scaffolds for
transplantation. Cell Transplant. 12:659–666. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gui L, Muto A, Chan SA, Breuer CK and
Niklason LE: Development of decellularized human umbilical arteries
as small-diameter vascular grafts. Tissue Eng Part A. 15:2665–2676.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frota AC, Lima Filho AA, Dias AB, Lourenço
AC, Antecka E and Burnier MN Jr: Freeze-drying as an alternative
method of human sclera preservation. Arq Bras Oftalmol. 71:137–141.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gherardini G, Haegerstrand A, Matarasso A,
Gurlek A, Evans GR and Lundeberg T: Cell adhesion and short-term
patency in human endothelium preseeded 1.5-mm
polytetrafluoroethylene vascular grafts: an experimental study.
Plast Reconstr Surg. 99:472–478. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weinberg CB and Bell E: A blood vessel
model constructed from collagen and cultured vascular cells.
Science. 231:397–400. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuda T, Kitamura T, Iwata H, Takano H
and Akutsu T: A hybrid artificial vascular graft based upon an
organ reconstruction model. Significance and design criteria of an
artificial basement membrane. ASAIO Trans. 34:640–643. 1988.
|
|
8
|
Ratcliffe A: Tissue engineering of
vascular grafts. Matrix Biol. 19:353–357. 2000. View Article : Google Scholar
|
|
9
|
Guymer RH and Mandel TE: A comparison of
corneal, pancreas, and skin grafts in mice. A study of the
determinants of tissue immunogenicity. Transplantation.
57:1251–1262. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Umscheid T and Stelter WJ: Time-related
alterations in shape, position, and structure of self-expanding,
modular aortic stent-grafts: a 4-year single-center follow-up. J
Endovasc Surg. 6:17–32. 1999.PubMed/NCBI
|
|
11
|
Hiles MC, Badylak SF, Lantz GC, Kokini K,
Geddes LA and Morff RJ: Mechanical properties of xenogeneic
small-intestinal submucosa when used as an aortic graft in the dog.
J Biomed Mater Res. 29:883–891. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bacon LD and Motta J: Skin-graft
histocompatibility within Regional Poultry Research Laboratory
inbred chicken lines. Poult Sci. 61:218–220. 1982. View Article : Google Scholar
|
|
13
|
Chang C and Zhang J: The analysis of
relationship between fetal stress and blood dynamics in fetal
vessels and placental bed vessels. Zhonghua Fu Chan Ke Za Zhi.
31:15–17. 1996.(In Chinese).
|
|
14
|
Rose AG, Forman R and Bowen RM:
Calcification of glutaraldehyde-fixed porcine xenograft. Thorax.
33:111–114. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin M, Liu MF, Cao Q, Wu JQ and Tao LR:
Mechanical properties of porcine aorta after vacuum freeze-drying
processes. J Clin Rehab Tissue Eng Res. 14:9539–9544. 2010.
|
|
16
|
Timaran CH, Stevens SL, Freeman MB and
Goldman MH: Infrainguinal bypass grafting using lyophilized
saphenous vein allografts for limb salvage. Cardiovasc Surg.
10:315–319. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cooper DK: Clinical survey of heart
transplantation between ABO blood group-incompatible recipients and
donors. J Heart Transplant. 9:376–381. 1990.PubMed/NCBI
|
|
18
|
Vesely I, Gonzalez-Lavin L, Graf D and
Boughner D: Mechanical testing of cryopreserved aortic allografts.
Comparison with xenografts and fresh tissue. J Thorac Cardiovasc
Surg. 99:119–123. 1990.PubMed/NCBI
|
|
19
|
Cattral MS, Warnock GL, Kneteman NM,
Halloran PF and Rajotte RV: The effect of cryopreservation on the
survival and MHC antigen expression of murine islet allografts.
Transplantation. 55:159–163. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gavin JB, Herdson PB, Monro JL and
Barratt-Boyes BG: Pathology of antibiotic-treated human heart valve
allografts. Thorax. 28:473–481. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao G, Liu ZF, Zhang AL, Zhang HF and
Cheng SX: Theoretical analyses of thermal stress of blood vessel
during cryopreservation. Cryo Letters. 26:239–250. 2005.PubMed/NCBI
|
|
22
|
Pegg DE, Wusteman MC and Boylan S:
Fractures in cryopreserved elastic arteries. Cryobiology.
34:183–192. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bujan J, Pascual G, Lopez R, et al:
Gradual thawing improves the preservation of cryopreserved
arteries. Cryobiology. 42:256–265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zuhdi N, Hawley W, Voehl V, Hancock W,
Carey J and Greer A: Porcine aortic valves as replacements for
human heart valves. Ann Thorac Surg. 17:479–491. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sawyer PN: Experimental observations on
antithrombotic biophysical phenomena in normal and atherosclerotic
human aorta and porcine aortic grafts. Ann NY Acad Sci.
149:628–642. 1968. View Article : Google Scholar
|
|
26
|
Leyh RG, Wilhelmi M, Walles T, et al:
Acellularized porcine heart valve scaffolds for heart valve tissue
engineering and the risk of cross-species transmission of porcine
endogenous retrovirus. J Thorac Cardiovasc Surg. 126:1000–1004.
2003. View Article : Google Scholar
|
|
27
|
Rieder E, Kasimir MT, Silberhumer G, et
al: Decellularization protocols of porcine heart valves differ
importantly in efficiency of cell removal and susceptibility of the
matrix to recellularization with human vascular cells. J Thorac
Cardiovasc Surg. 127:399–405. 2004. View Article : Google Scholar
|