Introduction
Thyroid cancer is a common endocrine malignant
tumor, accounting for 1% of human cancers. These tumors have been
classified as well-differentiated thyroid carcinomas, including the
papillary (PTC) and follicular (FTC) types, which account for more
than 95% of thyroid cancers, or anaplastic thyroid carcinomas
(ATC), which account for just 1–5% of thyroid malignancies.
Differentiated thyroid cancers such as PTC and FTC usually grow
slowly and are highly curable using a combination of surgery,
radioiodine ablation and thyroid-stimulating hormone
(TSH)-suppressive therapy. However, ATC is a lethal disease with a
median survival period of 6 months subsequent to diagnosis
(1–4). ATC is a malignant undifferentiated
neoplasm, without the thyroid differentiations. This malignant
tumor is usually well-advanced by the time of diagnosis, with an
average tumor size of 8 cm. Ninety percent of patients with ATC
have extraglandular spread at the time of diagnosis, while 75% of
them develop distant metastases (5,6).
Consequently, ATC cases are staged as stage IV in the American
Joint Commission on Cancer system (7). Whether primary
chemotherapy/radiotherapy results in a longer survival period
compared to the outcomes of primary surgical intervention remains
controversial. Nevertheless, no effective therapeutic regimen has
been identified for ATC as yet. This may be partly due to the
rarity of this carcinoma; however, it also reflects the inadequacy
of the available treatment options and suggests an urgent need for
the development of novel treatment strategies (8).
Pulsatilla koreana is a perennial plant that
grows around Korea and China and is used in traditional Chinese
herbal medicine. It has been used to treat amoebic dysentery,
malaria and internal hemorrhoids (9). Pulsatilla koreana extract
(PKE) contains various bioactive compounds; some of these are
capable of lowering the blood pressure, and also demonstrate
anti-inflammatory effects and anti-acne activities against aerobic
bacteria and fungi (10). In
addition, several studies have reported that the compounds in PKE
have anticancer effects in human melanoma, colon and lung cancers
(11). PKE has also recently been
reported to show anticancer effects in hepatocellular carcinoma
(12). Although ATC is the most
lethal disease among thyroid cancers, research on ATC treatment is
insufficient. Therefore, this study aimed to investigate the
anticancer activity of PKE in ATC, and the mechanism whereby PKE
affects apoptosis and angiogenesis, as previously described, with
regard to the pathogenesis of cancer.
Materials and methods
Extraction of PKE
The powdered roots of Pulsatilla koreana were
extracted as described in our previous study (12). Briefly, they were extracted using
50% ethanol, while the final extracts were concentrated in
vacuo to yield a light brown residue. The residue was suspended
in acetone, then centrifuged, and the resulting supernatant was
removed to obtain a brown precipitate. The precipitate was poured
into water and subsequently filtered to remove the insoluble
portion. The filtrate was concentrated into a brown mass.
Cells and materials
Human ATC cell line 8505c was purchased from the
Japanese Collection of Research Bioresources (JCRB, Shinjuku,
Japan), while SNU-80 was purchased from the Korean Cell Line Bank
(Seoul, Korea). 8505c cells were cultured in minimum essential
medium Eagle (MEM) (Gibco-BRL, Carlsbad, CA, USA), whereas SNU-80
cells were cultured in RPMI-1640 (Gibco-BRL), supplemented with 10%
fetal bovine serum (FBS, Gibco-BRL) and 1% penicillin/streptomycin.
Cultures were maintained at 37°C in a CO2 incubator with
a controlled humidified atmosphere composed of 95% air and 5%
CO2. Human umbilical vein endothelial cells (HUVECs)
were grown in 0.2% gelatin-coated 75-cm2 flasks in
endothelial cell growth medium (ECGM) 2, containing its supplement
mixture at 37°C. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) and proteinase K were purchased from
Sigma-Aldrich (St. Louis, MO, USA). RNase A was purchased from
Qiagen (Valencia, CA, USA).
Cell viability assay
Cell viability was performed through an MTT assay.
Briefly, 8505c and SNU-80 cells were plated at a density of
7×103 cells/well on 96-well plates overnight. The media
were removed, and cells were treated with either saline as a
control or various concentrations of PKE followed by incubation for
48 h. After that, MTT solutions were added to each well and
incubated for 4 h at 37°C. The formazan crystals that formed were
dissolved in dimethyl sulfoxide (DMSO). Absorbance was measured
using a microplate reader at 540 nm. Three replicate wells were
used for each analysis.
Western blot analysis
The cells were washed with ice-cold
phosphate-buffered saline (PBS), then lysed with TNN buffer
containing 1% Triton X-100, 1% Nonidet P-40, as well as the
following protease and phosphatase inhibitors: aprotinin (10
mg/ml), leupeptin (10 mg/ml) (ICN Biomedicals, Inc., Asse-Relegem,
Belgium), phenylmethylsulfonyl fluoride (1.72 mM), NaF (100 mM),
NaVO3 (500 mM) and
Na4P2O7 (500 mg/ml)
(Sigma-Aldrich). Equal amounts of protein were separated by
SDS-PAGE then transferred onto PVDF. Immunostaining of the blots
was performed using the primary antibodies, followed by the
secondary antibodies conjugated to horseradish peroxidase and
detection by enhanced chemiluminescence reagent (ELPS, Seoul,
Korea). The primary antibodies were monoclonal antibodies:
anti-HIF-1α (BD Biosciences, San Jose, CA, USA), anti-vascular
endothelial growth factor (VEGF) (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), anti-Bax, anti-Bcl-2 (Santa Cruz
Biotechnology, Inc.), anti-cleaved caspase-3 and anti-cleaved poly
ADP-ribose polymerase (PARP; Cell Signaling Technology, Inc.,
Danvers, MA, USA). The secondary antibodies were purchased from
Amersham Biosciences, Inc., (Piscataway, NJ, USA) The bands were
visualized with the ECL Plus system (Amersham Pharmacia Biotech,
Inc., Piscataway, NJ, USA).
DAPI staining and terminal
deoxynucleotidyltransferase-mediated nick end labeling (TUNEL)
assay
8505c cells were plated onto 18-mm cover glasses in
MEM medium at ~70% confluence for 24 h. The cells were then treated
with PKE at 100 μg/ml for 24 h. They were fixed in 2% ice-cold
paraformaldehyde (PFA), washed with PBS, then stained with 2 μg/ml
of 4,6-diamidino-2-phenylindole (DAPI) for 20 min at 37°C. The
DAPI-stained cells were examined under a fluorescent microscope
analyzing nuclear fragmentation. TUNEL was performed following the
manufacturer’s instructions for TUNEL kit (Chemicon, Temecula, CA,
USA).
Tube formation assay
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
was polymerized for 30 min at 37°C. HUVECs were suspended in ECGM2
medium, containing 50 ng/ml VEGF at a density of 3×104
cells/ml, and 0.2 ml of cell suspension was added to each
Matrigel-coated well, with or without the concentrations of PKE
indicated for 10 h. The morphological changes of the tube formation
were observed under a phase-contrast microscope and photographed at
magnification, ×200.
Migration assay
HUVECs, plated on culture dishes of 60 mm diameter
at 90% confluence, were wounded with a 2-mm razor blade and marked
at the injury line. Subsequently, the cells that peeled off were
removed with PBS and the wounded HUVECs were incubated in media
with 50 ng/ml VEGF, 1 mM thymidine (Sigma-Aldrich) and/or PKE.
HUVECs were allowed to migrate for 16 h and were then rinsed with
PBS, followed by fixation with methanol.
Immunofluorescence
8505c cells and HUVECs were seeded on 18-mm glass
plates in growth medium at ~70% confluence for 24 h. The cells were
treated with CoCl2 for 1 h. Subsequently, PKE was added
to the medium and incubated for 6 h. They were fixed in 2% ice-cold
PFA, and washed with PBS. Immunostaining of the cells was performed
using the primary antibodies, followed by the secondary antibodies
conjugated to FITC (Vector Laboratories, Burlingame, CA, USA) or
TRITC (Vector Laboratories) then stained with 2 μg/ml of DAPI for
20 min at 37°C. Each slide was observed using a confocal laser
scanning microscope (Olympus, Tokyo, Japan).
Tumor xenograft study
Male nude mice were obtained from the Central Animal
Laboratory, Inc. (Seoul, Korea). Animal care and experimental
procedures were in accordance with the approval and guidelines of
the Inha Institutional Animal Care and Use Committee (INHA IACUC)
of the Medical School of Inha University (Incheon, Korea). The
animals were fed standard rat chow and tap water ad libitum,
and were kept under 12 h dark/light cycle at 21°C. Male nude mice
(6 weeks; weight, 20–22 g) were randomized to three groups
(control, PKE 125 and PKE 250 mg/kg). 8505c cells were harvested
and mixed with PBS (200 μl/mouse), and then inoculated into one
flank of each nude mouse (1×107 of 8505c cells). When
the tumors had reached a volume of ~50 mm3, the mice
were given a daily intraperitoneal injection of PKE (125 and 250
mg/kg, treated group) or vehicle (200 μl PBS, control group) for 28
days. The tumor dimensions were measured twice a week using a
digital caliper, while the tumor volume was calculated using the
formula: V= length × width2 × 0.5. At the end of the
experiment, the mice were sacrificed and the tumors were excised
and weighed.
Statistical analysis
Data were expressed as the mean ± SD, and
statistical analysis was performed using ANOVA and an unpaired
Student’s t-test. P≤0.05 was considered to indicate a statistically
significant difference. Statistical calculations were performed
using SPSS software for Windows operating system (version 10.0;
SPSS, Chicago, IL, USA).
Results
Inhibition of ATC cell growth by PKE
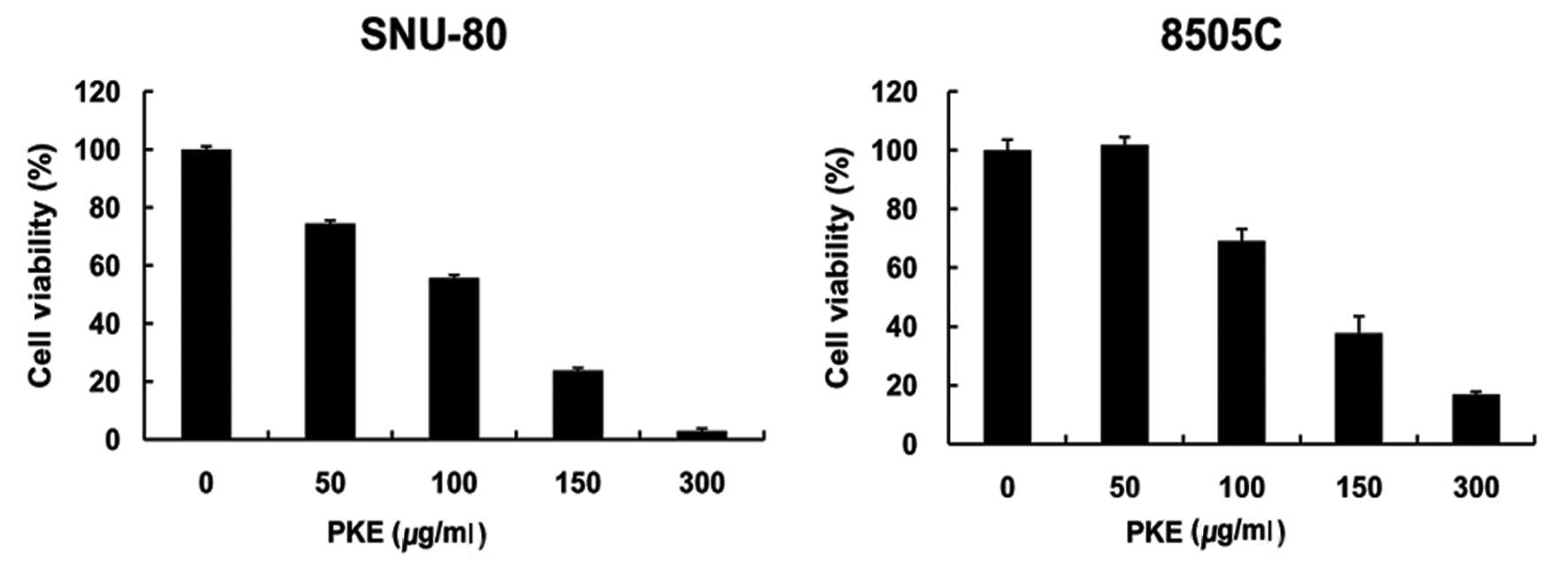
The effect of PKE on the viability of two ATC cell
lines (8505c and SNU-80) was examined. ATC cells were incubated in
media containing 50–300 μg/ml of PKE for 48 h. The results showed
that cell growth was inhibited by PKE treatment in a dose-dependent
manner (Fig. 1). The
IC50 for growth inhibition was 120 μg/ml on SNU-80 and
140 μg/ml on 8505c ATC cells.
Effects of PKE on apoptotic cell death in
8505c ATC cells
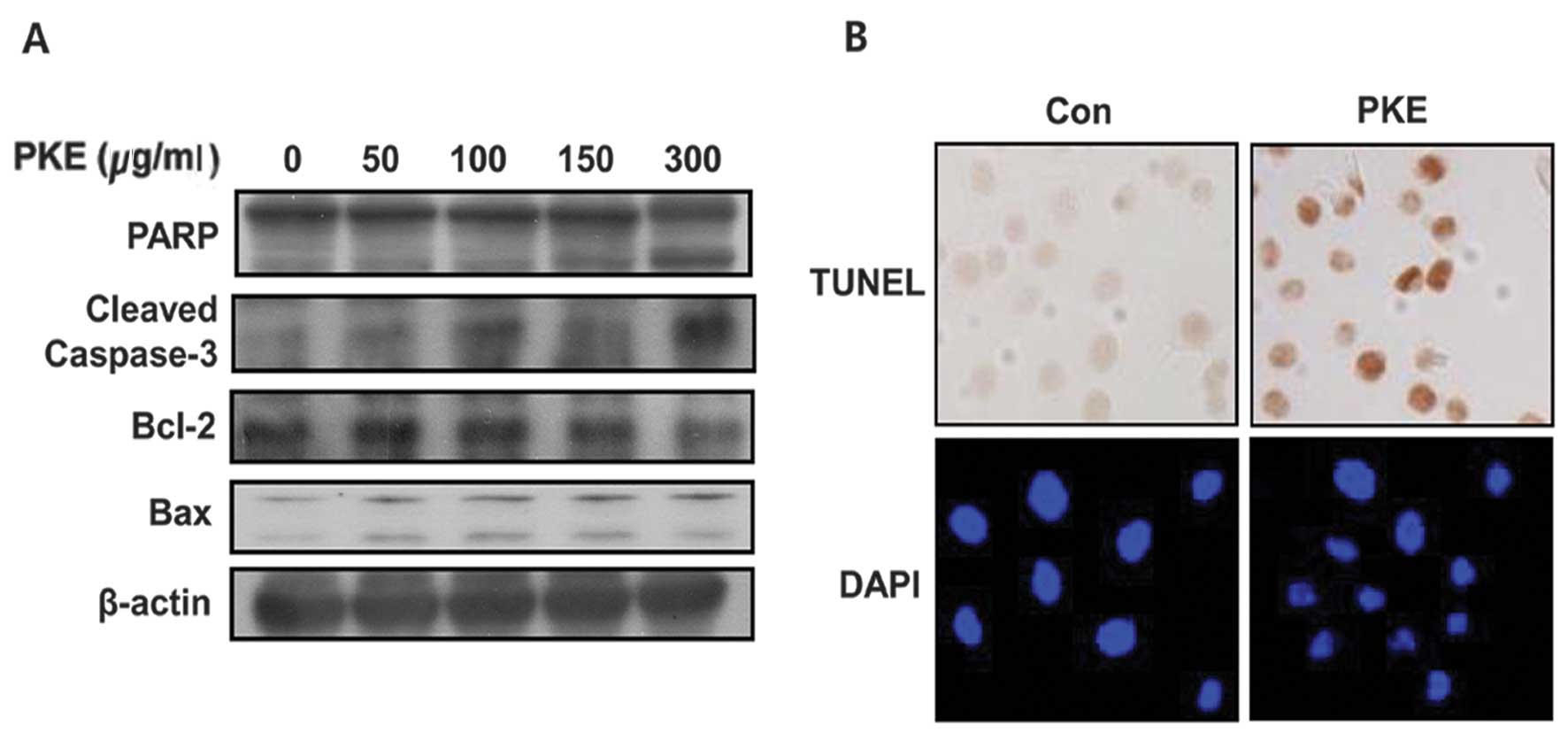
To identify the apoptotic effect of PKE in 8505c ATC
cells, the expression of Bax and Bcl-2, as well as the cleavage of
PARP and caspase-3 was measured by western blot analysis with PKE
for 48 h. PKE was found to lead to the upregulation of Bax and
cleaved PARP and caspase-3 and a downregulation of Bcl-2 in 8505c
ATC cells in a dose-dependent manner (Fig. 2A). These results showed that PKE
induced cell apoptosis in 8505c ATC cells. When treated with PKE
(100 μg/ml), the 8505c ATC cells presented the morphological
features of apoptotic cells, such as DNA fragmentation and
perinuclear apoptotic bodies by DAPI (Fig. 2B). The results of the TUNEL also
exhibited PKE-induced apoptosis by causing DNA strand breaks.
Effects of PKE on angiogenesis
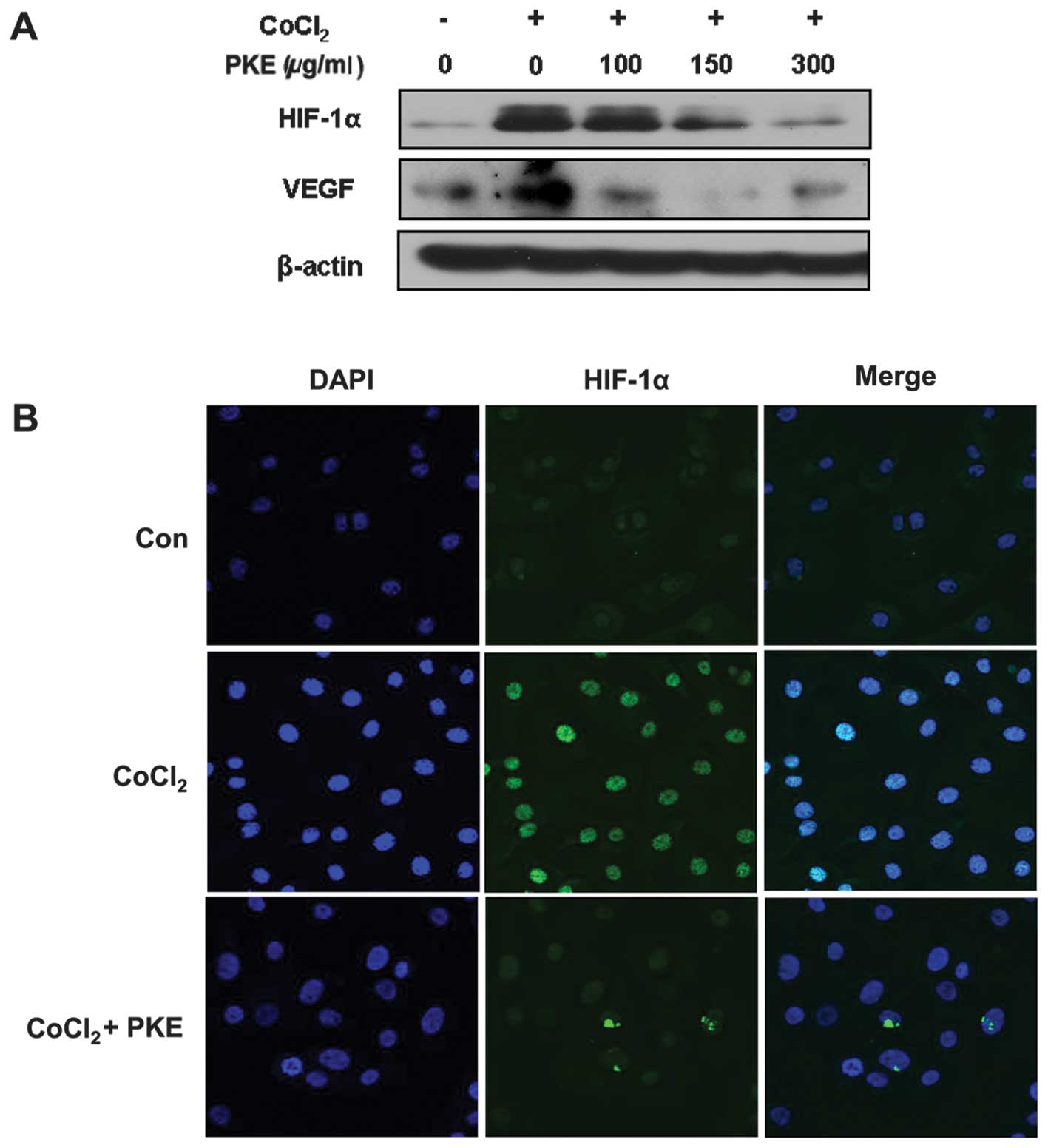
HIF-1α and VEGF are important angiogenic factors in
tumor progression. Thus, the effect of PKE on the hypoxia-induced
HIF-1α and VEGF expressions was examined. The cells were treated
with varying concentrations of PKE under hypoxia-like conditions
induced by CoCl2 (100 μM) for 18 h. As shown in Fig. 3A, the HIF-1α expression was
increased under hypoxic conditions, whereas PKE treatment at
100–300 μg/ml inhibited the hypoxia-induced HIF-1α expression in a
dose-dependent manner. Additionally, VEGF expression was increased
under the hypoxia-like conditions, while PKE inhibited VEGF
expression at a dose of 100–300 μg/ml. Consistent with the results
of the western blot analysis, immunofluorescence using a confocal
microscope also showed that PKE inhibited HIF-1α expression
(Fig. 3B). In addition, the
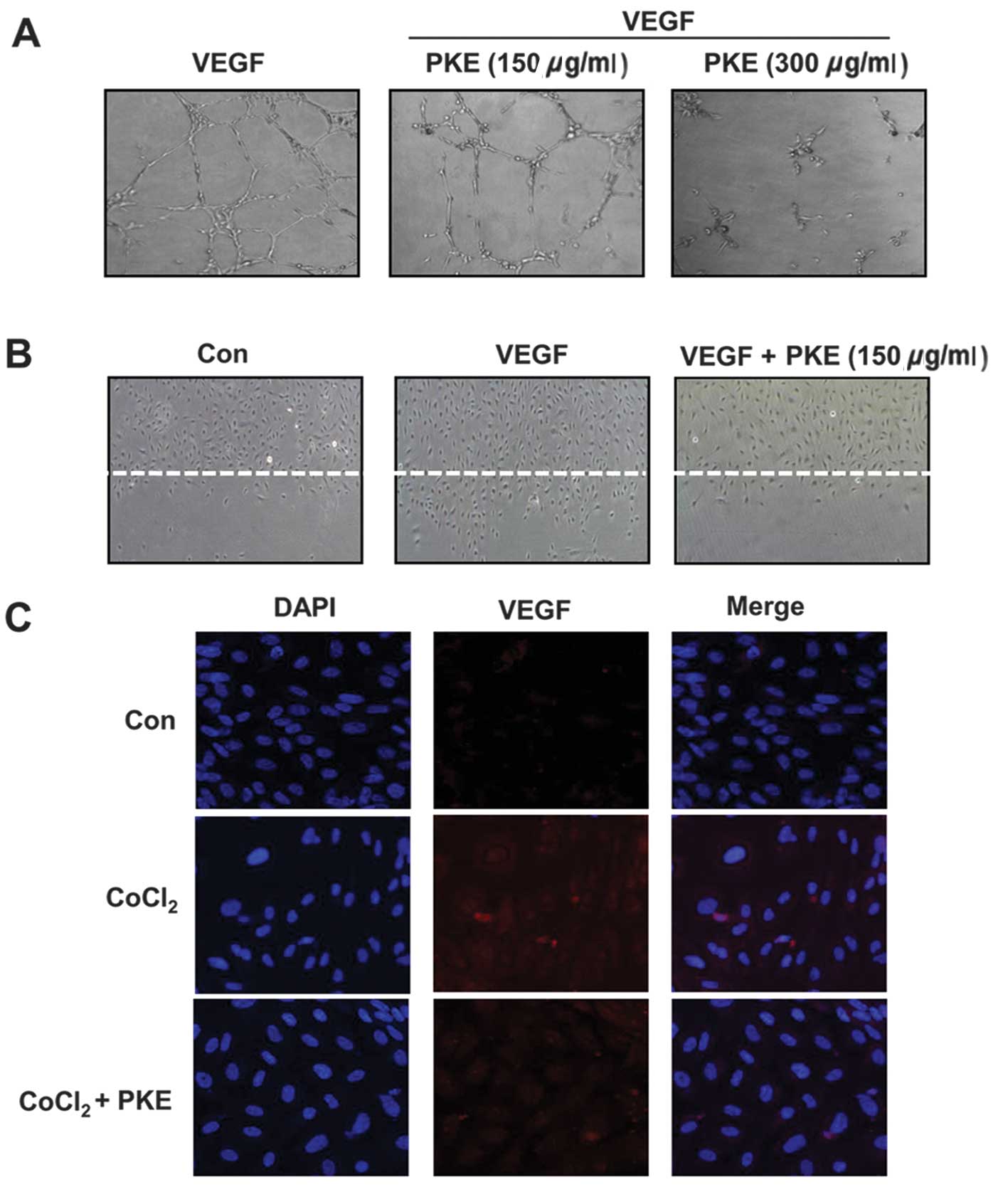
anti-angiogenic potential of PKE was examined using HUVECs. During
an in vitro tube formation assay, PKE was observed to have
inhibited the formation of vessel-like structures, comprising the
elongation and alignment of the cells at the indicated
concentrations (Fig. 4A). Cell
migration is critical for endothelia cells to form blood vessels in
angiogenesis and is necessary for tumor growth and metastasis.
Thus, a wound migration assay was carried out to examine the effect
of PKE on cell migration. Notably, when the endothelial cells were
wounded and incubated in media with 50 ng/ml VEGF and 1 mM
thymidine in the presence of PKE (100 μg/ml) for 16 h, subsequent
to PKE treatment the wound was unable to heal (Fig. 4B). In addition, since VEGF is one
of the critical factors of the tube formation and migration of
HUVECs, we investigated whether PKE affects the expression of VEGF
under hypoxic conditions in HUVECs using immunofluorescence. As
expected, PKE decreased VEGF expression under hypoxia (Fig. 4C). These results showed that PKE
prevented the tube formation and migration of endothelial cells
while inhibiting the VEGF expression, suggesting that PKE had a
potent anti-angiogenic property.
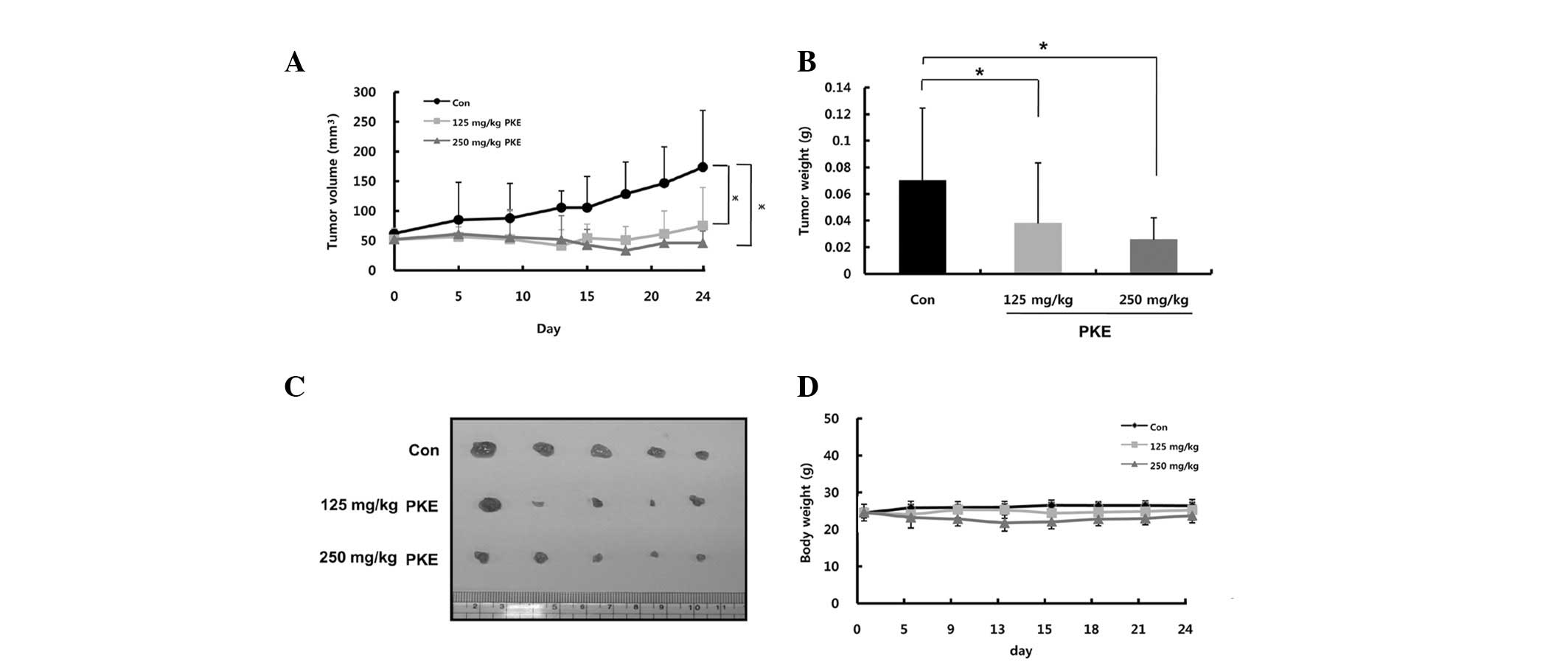
Inhibition of tumor growth by PKE in a
mouse xenograft model
Based on these findings demonstrating a strong
efficacy of PKE against 8505c ATC cells, the in vivo
efficacy of PKE against the 8505c ATC cells was next examined in a
nude mouse xenograft. As shown in Fig.
5A, PKE induced dose-dependent tumor growth inhibition at the
doses of 125 or 250 mg/kg for 24 days, when compared to the control
group. PKE administration at the doses of 125 and 250 mg/kg
resulted in a significant reduction of the tumor volume (57 and
74%, respectively) in nude mice. Consistent with this finding, the
tumor weight isolated from PKE-treated groups was decreased by 54
and 36% at the PKE doses of 125 and 250 mg/kg, respectively,
compared to the control (Fig. 5B and
C, P<0.05). No difference in the body weight of
PKE-treatment groups was observed, compared to the control group
(Fig. 5D), indicating that PKE had
a low toxicity in mice at curative doses. These results
demonstrated the in vivo antitumor efficacy of PKE against
ATC without any apparent sign of toxicity.
Discussion
Thyroid cancer, particularly ATC, is an
undifferentiated, fast-growing malignancy, for which novel
therapeutic approaches are needed. Several studies are currently
being conducted with a view to investigating the anticancer effects
of plants or their components, given their lower toxic properties
as natural products (13). In
cancer therapy, approximately 70% of the effective drugs are
products of natural origin or may be traced back to their
pharmachophores (14). In this
study, therefore, the anticancer efficacy of PKE and its mechanism
in ATC were investigated. The results showed that PKE inhibited
tumor growth and induced apoptosis both in vitro and in
vivo. PKE also suppressed angiogenesis by decreasing the
expression of HIF-1α and VEGF.
Apoptosis, which is also known as programmed cell
death, plays a critical role in treating cancer (15). Caspases are a conserved family of
enzymes that bring cells to apoptosis. Of these, caspase-3 is one
of the key components of apoptosis, being responsible either
partially or completely for the proteolytic cleavage of various key
proteins, such as PARP, a protein repairing DNA and maintaining
genomic DNA integrity (16,17).
Thus, the anticancer effects of PKE were first investigated through
the mechanism of apoptosis in 8505c cells. In this study, we
observed that PKE increased the expression of cleaved caspase-3 and
PARP, leading to apoptotic cell death. These apoptotic effects of
PKE were confirmed by the results of TUNEL and DAPI staining. The
cells keep the homeostasis of the anti-apoptotic regulators,
including Bcl-2, and pro-apoptotic regulators, such as Bax, to
maintain the proper survival and turnover. In this study, a
dose-dependent increase of Bax and decrease of Bcl-2 were observed
in PKE-treated 8505c cells. These results implied that PKE-induced
apoptosis is likely to be an important factor in the suppression of
tumor growth.
Angiogenesis is a prerequisite for tumor growth, as
well as metastatic spread, and involves the recruitment of blood
vessels by growing primary tumors or metastases. It is initiated by
angiogenic factors, such as VEGF (18,19).
HIF-1α, an upstream signal molecule of VEGF, has been targeted as a
major regulator of angiogenesis in various types of cancers
(20). Since VEGF may be inhibited
through the inactivation of HIF-1α, the HIF-1α/VEGF pathway may be
a candidate target of therapeutic strategy for ATC. These results
showed that PKE inhibited HIF-1α and VEGF expression under
CoCl2-induced hypoxia conditions in 8505c cells.
Additionally, extracts of natural plants, such as green tea or
Ginkgo biloba have been reported to show anti-angiogenic
effects through the inhibition of VEGF. Moreover, the in
vitro anti-angiogenic effect of PKE was supported by the
inhibition of HUVEC cell migration and tube formation, indicating
that PKE inhibited angiogenesis through VEGF, as well as through
targeting endothelial cells directly. Consequently, being a natural
product, PKE has great potential as an anticancer agent.
In conclusion, although the anticancer activity of
PKE and its mechanism have not been investigated in thyroid cancer,
findings of the present study demonstrate the anticancer effects of
PKE in ATC cells involved in the induction of apoptosis, as well as
of anti-angiogenesis by inhibition of VEGF via HIF-1α suppression.
These findings suggest that PKE may be a potential candidate for
cancer therapy against ATC.
Acknowledgements
This study was supported by the Inha University
Grant and the Korean Health Technology R&D Project (A120266),
Ministry of Health & Welfare, and the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (NRF 2012-0002988, 2012R1A2A2A01045602).
References
|
1
|
Catalano MG, Poli R, Pugliese M, Fortunati
N and Boccuzzi G: Emerging molecular therapies of advanced thyroid
cancer. Mol Aspects Med. 31:215–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fassnacht M, Kreissl MC, Weismann D and
Allolio B: New targets and therapeutic approaches for endocrine
malignancies. Pharmacol Ther. 123:117–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel KN and Shaha AR: Poorly
differentiated and anaplastic thyroid cancer. Cancer Control.
13:119–128. 2006.PubMed/NCBI
|
|
4
|
Sakorafas GH, Sampanis D and Safioleas M:
Cervical lymph node dissection in papillary thyroid cancer: current
trends, persisting controversies, and unclarified uncertainties.
Surg Oncol. 19:e57–e70. 2010. View Article : Google Scholar
|
|
5
|
Shimaoka K, Schoenfeld DA, DeWys WD,
Creech RH and DeConti R: A randomized trial of doxorubicin versus
doxorubicin plus cisplatin in patients with advanced thyroid
carcinoma. Cancer. 56:2155–2160. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahuja S and Ernst H: Chemotherapy of
thyroid carcinoma. J Endocrinol Invest. 10:303–310. 1987.
View Article : Google Scholar
|
|
7
|
Fleming ID, Phillips JL, Menck HR, Murphy
GP and Winchester DP: The National Cancer Data Base report on
recent hospital cancer program progress toward complete American
Joint Committee on Cancer/TNM staging. Cancer. 80:2305–2310. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herbst RS: ZD1839: targeting the epidermal
growth factor receptor in cancer therapy. Expert Opin Investig
Drugs. 11:837–849. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bae K: The medicinal plants of Korea.
Kyo-Hak Press; Seoul: 1999
|
|
10
|
Lee HS, Beon MS and Kim MK: Selective
growth inhibitor toward human intestinal bacteria derived from
Pulsatilla cernua root. J Agric Food Chem. 49:4656–4661. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bang SC, Lee JH, Song GY, Kim DH, Yoon MY
and Ahn BZ: Antitumor activity of Pulsatilla koreana
saponins and their structure-activity relationship. Chem Pharm Bull
(Tokyo). 53:1451–1454. 2005.PubMed/NCBI
|
|
12
|
Hong SW, Jung KH, Lee HS, et al: Apoptotic
and anti-angiogenic effects of Pulsatilla koreana extract on
hepatocellular carcinoma. Int J Oncol. 40:452–460. 2012.PubMed/NCBI
|
|
13
|
Yin F, Giuliano AE and Van Herle AJ:
Growth inhibitory effects of flavonoids in human thyroid cancer
cell lines. Thyroid. 9:369–376. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cragg GM, Newman DJ and Yang SS: Natural
product extracts of plant and marine origin having antileukemia
potential. The NCI experience. J Nat Prod. 69:488–498. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O’Connor L, Huang DC, O’Reilly LA and
Strasser A: Apoptosis and cell division. Curr Opin Cell Biol.
12:257–263. 2000.
|
|
16
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krishnakumar R and Kraus WL: The PARP side
of the nucleus: molecular actions, physiological outcomes, and
clinical targets. Mol Cell. 39:8–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bolat F, Haberal N, Tunali N, Aslan E, Bal
N and Tuncer I: Expression of vascular endothelial growth factor
(VEGF), hypoxia inducible factor 1 alpha (HIF-1alpha), and
transforming growth factors beta1 (TGFbeta1) and beta3 (TGFbeta3)
in gestational trophoblastic disease. Pathol Res Pract. 206:19–23.
2010. View Article : Google Scholar
|