Introduction
Mandibular defects remain a major challenge for
dentists. Traditionally, augmentation of bony defects is performed
using allografts, xenografts and autogenous bone (1). More recently, biomaterials, including
commercially available Bio-Oss, have been utilized for restoration
of mandibular defects (2). Bio-Oss
is an inorganic sterilized bovine bone, composed of a
calcium-deficient carbonate apatite (3). Previous studies have reported
promising clinical outcomes for implantation of Bio-Oss in
intra-bony periodontal and mandibular defects associated with
dental implants (4). Bio-Oss is
considered to exhibit superior biocompatibility and is highly
suitable for use as a scaffold for osteogenesis and osteogenic
cells. However, studies have also demonstrated that Bio-Oss has
poor osteoinduction (5).
Bone marrow stromal cells (BMSCs) are a subset of
plastic adherent nonhematopoietic stem cells, characterized by
their ability to self-renew and differentiate into multiple cell
types, including osteoblasts, adipocytes and chondrocytes (6). It has been widely accepted that
tissue repair of bone defects is advanced by bone marrow stem cells
that migrate to the site of damage and undergo differentiation
promoting structural and functional repair (7). BMSCs are induced to differentiate
into osteoblasts and restore bone defects. A previous study
demonstrated that intravenous delivery of BMSCs led to migration to
the injury site for restoration of bone or cartilage fracture
(8). In particular, when combined
with tridimensional scaffolds, BMSCs differentiate into osteoblasts
and deposit extracellular matrix on the ceramic surface, promoting
formation of new bones. However, currently this process is limited
by poor proliferation of BMSCs (9).
Basic fibroblast growth factor (bFGF) is a potent
mitogen for fibroblasts and other mesoderm-derived cells, including
osteoblasts and vascular endothelial cells (10). Previously, bFGF was identified to
induce bone formation by stimulating proliferation and
differentiation of bone marrow stromal cells (BMSCs), enhancing
chondrogenic and osteogenic differentiation of BMSCs and
stimulating these cells to deposit new mineralized bone (11,12).
To date, a limited number of studies have analyzed
the potential of bFGF and BMSCs combined with Bio-Oss scaffolds for
roles in the repair of mandibular defects. In the present study, we
hypothesized that bFGF is likely to promote BMSC osteogenesis in
conjunction with Bio-Oss scaffolds. To test the hypothesis, the
potential effects of bFGF on the proliferation and differentiation
of BMSCs were addressed in vitro. The effect of bFGF on the
attachment of BMSCs to Bio-Oss scaffolds was also investigated
in vitro. Furthermore, we examined whether bFGF enhanced
osteogenesis of BMSCs attached to scaffolds in vivo.
Materials and methods
Animal experiments
All animal experiments were approved by the Animal
Care and Use Committee of the Second Affiliated Hospital of Harbin
Medical University (Harbin, China) and were performed in compliance
with the NIH ‘Guide for the Care and Use of Laboratory
Animals’.
Isolation and culture of BMSCs
BMSCs were isolated by flushing the femurs and
tibias of 9-month-old New Zealand white rabbits (Cyagen
Biosciences, Guangzhou, China). Total bone marrow was washed,
triturated using a 20-gauge needle and passed through a 40-μm nylon
mesh cell strainer (Becton Dickinson, Franklin Lakes, NJ, USA) to
produce a single cell suspension in phosphate-buffered saline
(PBS). These cells were cultured in α-MEM (Gibco-BRL, Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL,
Grand Island, NY, USA). Isolation was based on the plastic
adherence and rapid proliferation of BMSCs. Following 48 h culture,
nonadherent cells were removed by washing with PBS and adherent
cells were maintained in fresh medium for propagation. Medium was
changed every 2–3 days. Cells at passage 3 were used for the
experiments. Culture was performed in a humidified incubator at
37°C in a 5% CO2 atmosphere.
Construction and transfection of the
pVAX1-bFGF plasmid
Total RNA was isolated from BMSCs using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and cDNA
was synthesized from 2 μg of purified total RNA using Moloney
murine leukemia virus reverse transcriptase (Promega, Madison, WI,
USA). Primer sequences used to amplify the target rabbit bFGF gene
(XM_002717238) were as follows: forward,
5′-GAATTCATGGCAGCCGGGAGCATCA-3′ and reverse,
5′-GGATTCTCAGCTCTTAGCAGACATTGG-3′. The amplified cDNA fragment was
ligated with the plasmid vector ligase and cloned into the pVAX1
vector (Invitrogen Life Technologies). The pVAX1-bFGF plasmid was
introduced into competent DH5α E. coli for replication and
later extracted and purified using an EndoFree kit (Qiagen, Hilden,
Germany). The recombinant plasmid was validated by restriction
enzyme digestion and sequencing. Recombinant pVAX1-bFGF and empty
pVAX1 vector were then transfected into BMSCs using Lipofectamine
2000 (Invitrogen Life Technologies) according to the manufacturer’s
instructions. In brief, Lipofectamine 2000 was mixed with
recombinant pVAX1-bFGF plasmids at 10 μl:4 μg, diluted with DMEM
(Gibco-BRL) and incubated for 20 min at room temperature. BMSCs
were resuspended in DMEM. The mixture of Lipofectamine
2000/pVAX1-bFGF was added and cells were incubated at 37°C, 5%
CO2 for 4 h. Following this, DMEM containing 10% FBS
without antibiotics was added and the cells were incubated for 48
h. G418 (Roche Diagnostics, San Francisco, CA, USA) was used to
select for stably transfected BMSCs. Restriction enzyme digestion
and sequencing assays were used to test whether the transfection
was successfully performed. To confirm the expression of bFGF in
BMSCs, indirect immunofluorescence analysis was performed. Cells
were fixed in 2% paraformaldehyde, permeabilized in ice-cold
methanol and incubated with an antibody against bFGF (Cell
Signaling Technology, Danvers, MA, USA) overnight at 4°C, followed
by incubation with goat anti-mouse FITC secondary antibody and
mounting in medium containing DAPI. Cells were visualized with a
Zeiss immunofluorescence microscope.
Cell proliferation assay
Cell proliferation assays were performed using Cell
Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the
manufacturer’s instructions. Briefly, 2×103 cells/well
were plated in 96-well plates and cultured in growth medium. At the
indicated time points, medium was aspirated and 100 μl serum-free
DMEM and 10 μl
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H
tetrazolium, monosodium salt (WST-8) was added and incubated at
37°C for 1.5 h. Absorbance was measured at 450 nm with a reference
wavelength of 630 nm on a spectrophotometer (Molecular Devices,
Sunnyvale, CA, USA). All experiments were performed 5 times.
Alkaline phosphatase (ALP) activity
BMSCs transfected with pVAX1-bFGF or empty vector
were plated into 96-well culture plates (2×104
cells/well) and cultured for 24 h. Following this, cells were
subjected to culture medium containing 1 mmol/l β-sodium phosphate,
50 μg/l L-ascorbic acid (both obtained from InterGen, Burlington,
MA, USA) and 10 nmol/l dexamethasone (Sigma-Aldrich, St. Louis, MO,
USA) for osteogenic induction. The culture medium was renewed twice
a week. ALP activity was measured at days 1, 7, 14, 21 and 28 using
an ALP assay kit (BioAssay Systems, Hayward, CA, USA) according to
the manufacturer’s instructions.
Seeding of BMSCs into Bio-Oss
collagen
Bovine spongious bone collagen graft (Bio-Oss
collagen; Geistlich Pharma AG, Wolhusen, Switzerland) was cut to
6×4×2 mm under sterile conditions and prepared as scaffolds. Two
groups of BMSCs, BMSCs/bFGF and BMSCs/pVAX1 were trypsinized and
suspended in culture medium containing gelatum (20 mg/ml) and
immediately loaded into Bio-Oss scaffolds (2×105
BMSCs/scaffold) using a pipette. Scaffolds were incubated in
osteoblast-induction medium under standard cell culture conditions
for 18 days prior to implantation. The surface morphological
characteristics of Bio-Oss were investigated at day 9 using
scanning electron microscopy.
Scanning electron microscopy
analysis
BMSCs/bFGF or BMSCs/pVAX1 cells loaded in Bio-Oss
were morphologically analyzed following incubation for 9 days using
a scanning electron microscope (S2300; Hitachi, Tokyo, Japan).
Samples were fixed with 2.5% glutaraldehyde for 2 h and washed 3
times with PBS. Osmium tetroxide (1%) was used for secondary
fixation. Following washing, dehydration of the samples was
performed for 30 min through a graded ethanol series (50, 70, 90
and 100% ethanol, each for 15 min). Then, samples were subjected to
sputter coating with platinum and examined by scanning electron
microscope at 10 kV.
Surgery and implantation of Bio-Oss
Six New Zealand white rabbits (9–12 months old;
weight, ~2 kg; Cyagen Biosciences) were used for this study.
Bio-Oss scaffolds groups were as follows (n=6 each group): A
(seeded with BMSCs/bFGF); B (seeded with BMSCs/pVAX1); and C
(cell-free). Animals were acclimatized for 1 week and maintained
throughout at standard conditions: 25±2°C temperature, 40–60%
relative humidity and 12-h light/dark cycle. Prior to surgery,
animals were anesthetized with 3% pentobarbital sodium (1 ml/kg
body weight). Three mandibular defects (2.5×3 mm) were induced in
the lower border of the bilateral mandibular ramus using a
micromotor drill. Each rabbit recieved a scaffold from each group,
which was implanted into mandibular defects under sterile
conditions. Following this, muscles, soft tissues and skin were
carefully repositioned and wounds were closed with 30 nylon
sutures. Animals were sacrificed by anesthesia overdose 12 weeks
following surgery and the scaffolds were extracted for
analysis.
Bone mineral density (BMD) analysis
Extracted scaffolds were examined for bone
mineralization by X-ray. BMD (g/cm2) was measured using
dual-energy X-ray absorptiometry (DEXA; Lunar Prodigy, GE
Healthcare Biosciences, Pittsburgh, PA, USA). Scaffolds were
scanned and analyzed using a specific animal program provided by
the manufacturer. All experimental data were sampled 3 times.
Histological analysis
Samples for histological analysis were prepared by
placing scaffolds in 4% paraformaldehyde solution for 24 h,
followed by decalcification in a 10% EDTA solution for 3 weeks.
Samples were dehydrated and embedded in paraffin and then cut into
5-μm sections and transferred to silicon-coated slides. For
hematoxylin and eosin staining, sections were dewaxed, rehydrated
in an ethanol gradient and rinsed in water. Sections were stained
with hematoxylin and eosin for 10 and 5 min, respectively, then
dehydrated by rinsing in an ethanol gradient. Sections were
coverslipped and examined by light microscopy.
Statistical analysis
SPSS version 17.0 software (SPSS Inc., Chicago, IL,
USA) was used for all statistical analyses. Data are expressed as
mean ± SEM. Statistical analysis was performed using standard
one-way ANOVA followed by LSD post hoc test. Bonferroni’s
correction was used to adjust for multiple comparisons. A
two-tailed Student’s paired t-test was also used to compare the
difference in values between 2 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Ectopic expression of bFGF stimulates
BMSC proliferation and osteogenic differentiation in vitro
Stable bFGF-expressing and negative control pVAX1
(empty vector) BMSC lines were successfully established and
confirmed by restriction enzyme digestion and sequencing. In
addition, indirect immunofluorescence results revealed green
fluorescence on the membrane of PVAX1-bFGF- but not
PVAX1-transfected cells (Fig. 1A),
indicating that bFGF was expressed in BMSCs. To investigate the
effects of bFGF expression on BMSC cell growth, BMSCs transfected
with recombinant plasmid pVAX1-bFGF or empty vector and
untransfected BMSCs were plated and cultured with growth medium for
specified times (1–6 days), followed by WST-8 assay. As
demonstrated in Fig. 1B, bFGF was
found to significantly stimulate growth of BMSCs, while no
statistical significance between BMSCs and vector transfected cells
BMSCs/pVAX1 was identified. Analysis of ALP activity, an early
marker of osteoblast differentiation (13), demonstrated that bFGF enhanced ALP
activity in BMSCs (Fig. 1C).
Significant differences between BMSCs/bFGF and BMSCs/pVAX1 cells
were apparent at day 14 and the peak value of ALP activity appeared
at 32 h, following this, ALP activity decreased (Fig. 1C). Results indicate that osteoblast
differentiation of BMSCs is stimulated by bFGF in vitro.
 | Figure 1Effect of ectopic bFGF expression on
proliferation and differentiation of BMSCs. (A) Confirmation of
bFGF expression in BMSCs by indirect immunofluorescence. BMSCs
transfected with (left, green) pVAX1-bFGF or (right, red) pVAX1
empty vector. (B) Ectopic bFGF expression promotes cellular growth
of BMSCs. Cell proliferation was assessed by WST-8 assay, data are
presented as the mean ± SEM of each time point from 5 samples.
*P<0.05 and #P<0.01, vs. control BMSCs
or BMSCs/pVAX1 cells. (C) Ectopic bFGF expression enhances ALP
activity of BMSCs. ALP activity of BMSC transfected with bFGF or
empty vector was measured at various days, data are presented as
the mean ± SEM of each time point from 3 samples.
*P<0.05 and #P<0.01, vs. control
BMSCs/pVAX1 cells. BMSCs, bone marrow stromal cells; bFGF, basic
fibroblast growth factor; WST-8,
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H
tetrazolium, monosodium salt; ALP, alkaline phosphatase. |
Surface morphology of the Bio-Oss
Morphology and microstructures of the cells seeded
on the surfaces of Bio-Oss were examined by scanning electron
microscopy. Images of the 2 groups seeded with BMSCs/bFGF or
BMSCs/pVAX1 cells are presented in Fig. 2. At day 9, scanning electron
microscopy revealed that the cells formed a dense multilayered
arrangement. No difference in surface morphology between the 2
groups was observed.
Analysis of Bio-Oss implantations
BMD measurements
Following animal sacrifice at week 12, scaffolds
were extracted and BMD (g/cm2) was measured using DEXA.
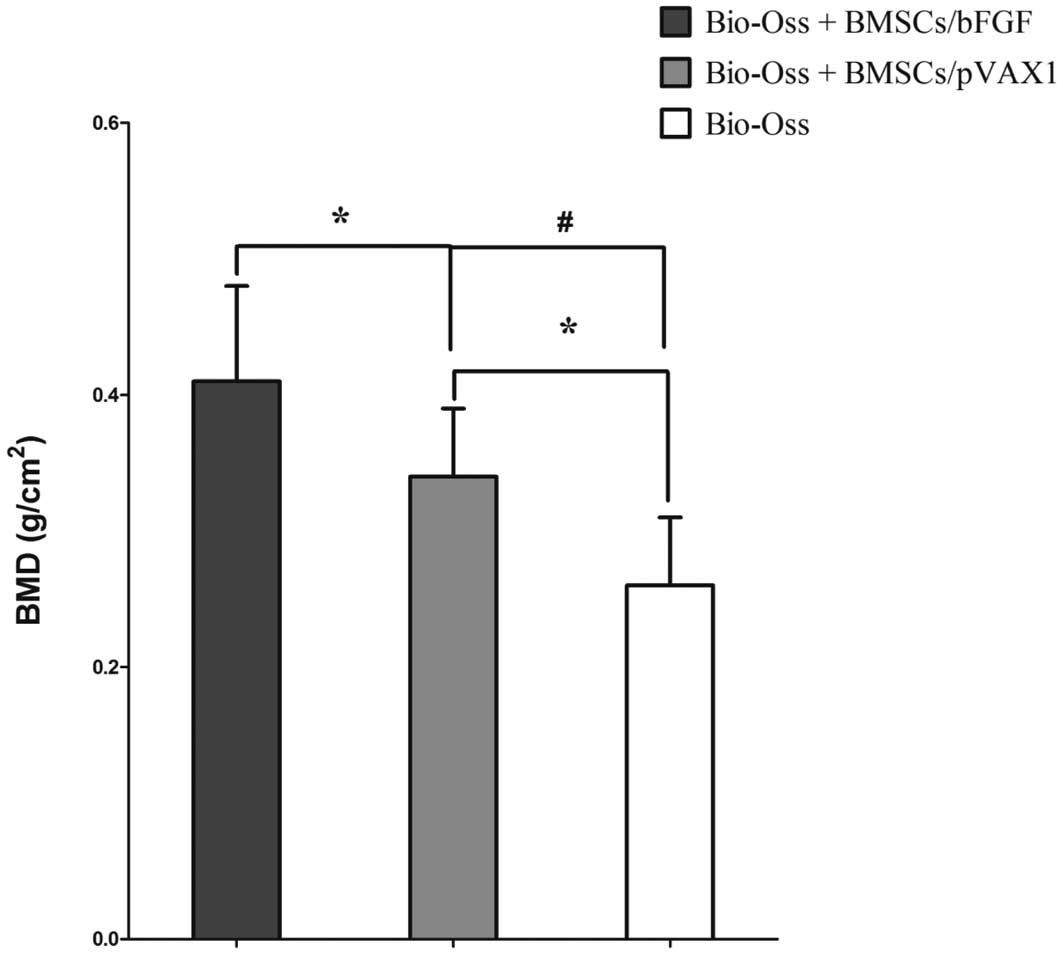
As demonstrated in Fig. 3,
compared with the sham control cell-free scaffold group C, BMD in
experimental groups A (BMSCs/bFGF) and B (BMSCs/pVAX1) was found to
be significantly higher (P<0.05 and P<0.01, respectively). In
addition, BMD in group A was markedly higher compared with B
(P<0.05). Results indicate that bFGF promotes mineralization of
BMSCs in Bio-Oss scaffolds.
Histological analysis
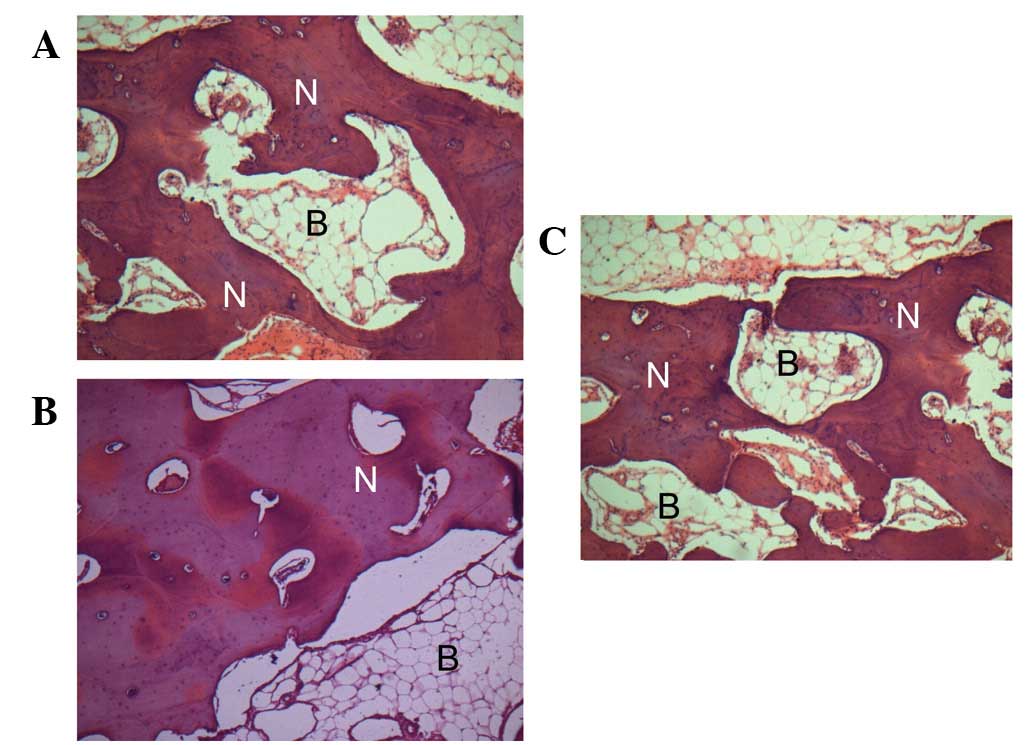
In addition, scaffolds were extracted for and
underwent histological analysis. As revealed in Fig. 4, in groups A and B, new bone was
observed to be growing towards and amalgamating with the Bio-Oss
granules. By contrast, more compact mineralized areas were observed
by toluidine blue staining in group A compared with B and C. In
group C implants without seeded cells were used as controls,
formation of a vascularized loose connective tissue was oberved,
however, newly deposited bone was rare (Fig. 4C). Results indicate that bFGF
enhanced the in vivo osteogenic potential of BMSCs.
Discussion
In the present study, BMSCs transfected with bFGF
exhibited increased proliferation and differentiation compared with
controls in vitro. Following seeding into Bio-Oss scaffolds
and transplantation into experimental mandibular defects of
rabbits, BMSCs transfected with bFGF loaded in Bio-Oss markedly
accelerated bone regeneration. Results indicate that bFGF increased
osteogenesis of BMSCs and Bio-Oss loaded with BMSCs transfected
with bFGF may prove to be a valid option for repairing mandibular
defects.
BMSCs are osteogenic cell sources containing a
phenotypically and functionally heterogeneous population of
mesenchymal precursors which contribute to the physiological
regeneration of bone, cartilage, adipose, muscle and other
connective tissues (6,14). The osteogenic potential of BMSCs to
produce bone-like mineralized tissue has been widely demonstrated
in vitro and in vivo(15,16).
BMSCs have been used in several in vivo animal model systems
to generate bone tissue and have been demonstrated to enhance the
ability of demineralized bone matrices to promote bone formation
within defect sites (17). bFGF is
considered a growth factor with high osteoinductive activity in the
FGF family (18). A previous study
identified that the commitment of rat BMSCs to mineralization is
enhanced by cytokine bFGF that affect the proliferation and
differentiation of cells (19).
bFGF has been demonstrated to upregulate markers of the mature
osteoblastic phenotype, including ALP activity (20). In the present study, proliferation
rate was observed to be significantly higher in BMSCs transfected
with bFGF compared with untransfected BMSCs during various culture
periods. Moreover, bFGF significantly stimulated ALP activity of
BMSCs. ALP activity is widely used as a marker of early
differentiation of osteoblast-like cells (13), indicating that bFGF stimulated
differentiation of BMSCs in vitro. ALP activity in BMSCs
transfected with or without bFGF was markedly decreased on day 28
compared with day 21, indicating that ALP activity peaked prior to
initiation of calcification. These observations demonstrate the key
role of bFGF in enhancing proliferation and differentiation of
BMSCs.
Mandibular defects remain a major challenge in
orthopedic surgery. Traditionally, autologous bone transplantation
was considered the gold standard treatment method, however, damaged
donor sites and insufficient donor tissue have prevented the
large-scale application of this approach (21). In addition, allogeneic bone grafts
are restricted in clinical applications due to immunological
reactions in recipients (22). At
present, a number of biodegradable polymer scaffolds have been used
for experimental transplantation of BMSCs to regenerate bones
(23). BMSCs are easily obtained
from bone marrow and are therefore good candidates for innovative
clinical applications when cultured with biomaterials. Bio-Oss is
recognized to exhibit superior biocompatibility and high
suitability for use as a osteogenesis scaffold, however, it is also
known to exhibit poor osteoinductive properties (24). In addition, nutrient supply and
cell viability at the centre of the scaffold remains a key problem
in the use of Bio-Oss to restore bone defects (25). BMSCs are known to exhibit reduced
proliferative capacities (26) and
the maintenance of this ability is important for tissue engineering
in Bio-Oss scaffolds. Therefore, specific pro-growth factors must
be included in the Bio-Oss scaffold system. In the current study,
bFGF was used to overcome reduced proliferative capacity and we
investigated whether Bio-Oss, derived from bovine bone matrix,
supported the osteogenic differentiation and bone-forming capacity
of BMSCs in vivo. Results demonstrate that bFGF-transfected
BMSCs seeded in Bio-Oss significantly accelerated bone regeneration
compared with Bio-Oss alone or loaded with BMSCs transfected with
empty vectors, indicating that bFGF may enhance bone regeneration
of BMSCs in vivo. As the Bio-Oss scaffold is surrounded by
fibrous tissues and macrophages appear, the material undergoes
degradation and absorption and new bone grows and fills the entire
space, indicating that Bio-Oss scaffold favors bone conductibility
and biodegradability for BMSCs transfected with bFGF. Currently,
bFGF is hypothesized to promote bone regeneration by accelerating
BMSC proliferation. These cells are likely to differentiate into
progenitor cells in implanted sites, by stimulating proliferation
of osteoblasts and periodontal fibroblasts directly and by
stimulating quiescent endothelial cells to induce morphogenesis and
proliferation (27). In addition,
bFGF has been reported to be important for wound healing by
inducing angiogenesis, which in turn accelerates bone regeneration
(28). However, the exact
mechanism remains unknown and must be elucidated by further
studies.
In the current study, the main constituents of the
Bio-Oss scaffolds were calf decalcified bone with interspaces
representative of the structure of human cancellous bones. A number
of clinical studies have reported that Bio-Oss bone block is highly
biocompatible and produces a desired synostosis by combining with
the host bone (29,30). Therefore, this form of composite
artificial bone is consistent with the organism bone in
constituents and structure, in accordance with physiological
requirements. In the present study, no inflammatory cell
infiltration around the implanted material was observed during
development. The material combined closely with the new bone
tissues and the process of osteogenesis was rapid without
development of surrounding fibrous tissue, demonstrating further
that Bio-Oss composed of bFGF-BMSCs has excellent biocompatibility.
Histological analysis demonstrated that osteogenesis was based on
marked bone conductibility of the Bio-Oss bone block and the
efficient bone inductivity of bFGF. Its bone conductibility mainly
depends on the growth of new bones around the host bone, which
takes the specific gap structure of Bio-Oss bone block as its
support at any time in each group. The remaining space will be
occupied by new bones as well, which develops a coordinating
relationship. However, the effective existence of bone inductivity
can be observed from the aggregation and differentiation of
mesenchymal cells in the experimental group at an early stage.
Moreover, it could keep the vigorous osteogenesis activity in long
term, and its mode of osteogenesis is always multicentric in
material exposure. This greatly accelerates the process of
osteogenesis and has a significant difference from the control
group. However, since the material used in this experiment is a
compound form of various biological materials, there are some
questions which are worthy of being discussed, such as what type of
composite material functions most effectively, what appropriate
proportion of each material should be used. These questions await
further studies.
In conclusion, the results of the present study
demonstrate that the bFGF gene was transfected into BMSCs and
expressed and proliferation and differentiation of BMSCs was
enhanced by bFGF in vitro.
In a mandibular defect model of rabbitts, the
implantation of bFGF-modified BMSCs associated with Bio-Oss
promoted new bone regeneration more effectively than traditional
methods. These observations are likely to be useful for future
studies on the use of BMSC-seeded Bio-Oss to repair bone defects,
which may improve therapies for clinical mandibula
reconstruction.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Heilongjiang Province of China (no.
D200935).
Abbreviations:
|
BMSCs
|
bone marrow stromal cells
|
|
bFGF
|
basic fibroblast growth factor
|
|
ALP
|
alkaline phosphatase
|
|
BMD
|
bone mineral density
|
References
|
1
|
Lacerda SA, Lanzoni JF, Bombonato-Prado
KF, Campos AA, Prata CA and Brentegani LG: Osteogenic potential of
autogenous bone associated with bone marrow osteoblastic cells in
bony defects: a histomorphometric study. Implant Dent. 18:521–529.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmagnola D, Berglundh T and Lindhe J:
The effect of a fibrin glue on the integration of Bio-Oss with bone
tissue. A experimental study in labrador dogs. J Clin Periodontol.
29:377–383. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haas R, Donath K, Fodinger M and Watzek G:
Bovine hydroxyapatite for maxillary sinus grafting: comparative
histomorphometric findings in sheep. Clin Oral Implants Res.
9:107–116. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zitzmann NU, Scharer P, Marinello CP,
Schupbach P and Berglundh T: Alveolar ridge augmentation with
Bio-Oss: a histologic study in humans. Int J Periodontics
Restorative Dent. 21:288–295. 2001.PubMed/NCBI
|
|
5
|
Tadjoedin ES, de Lange GL, Bronckers AL,
Lyaruu DM and Burger EH: Deproteinized cancellous bovine bone
(Bio-Oss) as bone substitute for sinus floor elevation. A
retrospective, histomorphometrical study of five cases. J Clin
Periodontol. 30:261–270. 2003. View Article : Google Scholar
|
|
6
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abkowitz JL, Robinson AE, Kale S, Long MW
and Chen J: Mobilization of hematopoietic stem cells during
homeostasis and after cytokine exposure. Blood. 102:1249–1253.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murphy JM, Fink DJ, Hunziker EB and Barry
FP: Stem cell therapy in a caprine model of osteoarthritis.
Arthritis Rheum. 48:3464–3474. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mauney JR, Sjostorm S, Blumberg J, et al:
Mechanical stimulation promotes osteogenic differentiation of human
bone marrow stromal cells on 3-D partially demineralized bone
scaffolds in vitro. Calcif Tissue Int. 74:458–468. 2004. View Article : Google Scholar
|
|
10
|
Ferrara N: Vascular endothelial growth
factor: basic science and clinical progress. Endocr Rev.
25:581–611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mastrogiacomo M, Cancedda R and Quarto R:
Effect of different growth factors on the chondrogenic potential of
human bone marrow stromal cells. Osteoarthritis Cartilage. 9(Suppl
1): S36–S40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bianchi G, Banfi A, Mastrogiacomo M, et
al: Ex vivo enrichment of mesenchymal cell progenitors by
fibroblast growth factor 2. Exp Cell Res. 287:98–105. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng SL, Yang JW, Rifas L, Zhang SF and
Avioli LV: Differentiation of human bone marrow osteogenic stromal
cells in vitro: induction of the osteoblast phenotype by
dexamethasone. Endocrinology. 134:277–286. 1994.PubMed/NCBI
|
|
14
|
Grassel S, Stockl S and Jenei-Lanzl Z:
Isolation, culture and osteogenic/chondrogenic differentiation of
bone marrow-derived mesenchymal stem cells. Methods Mol Biol.
879:203–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pham QP, Kasper FK, Scott Baggett L,
Raphael RM, Jansen JA and Mikos AG: The influence of an in vitro
generated bone-like extracellular matrix on osteoblastic gene
expression of marrow stromal cells. Biomaterials. 29:2729–2739.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marolt D, Augst A, Freed LE, et al: Bone
and cartilage tissue constructs grown using human bone marrow
stromal cells, silk scaffolds and rotating bioreactors.
Biomaterials. 27:6138–6149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Satomura K, Hiraiwa K and Nagayama M:
Mineralized nodule formation in rat bone marrow stromal cell
culture without beta-glycerophosphate. Bone Miner. 14:41–54. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scutt A and Bertram P: Basic fibroblast
growth factor in the presence of dexamethasone stimulates colony
formation, expansion and osteoblastic differentiation by rat bone
marrow stromal cells. Calcif Tissue Int. 64:69–77. 1999. View Article : Google Scholar
|
|
20
|
Chen M, Song K, Rao N, Huang M, Huang Z
and Cao Y: Roles of exogenously regulated bFGF expression in
angiogenesis and bone regeneration in rat calvarial defects. Int J
Mol Med. 27:545–553. 2011.PubMed/NCBI
|
|
21
|
Sharifi D, Khoushkerdar HR, Abedi G,
Asghari A and Hesaraki S: Mechanical properties of radial bone
defects treated with autogenous graft covered with hydroxyapatite
in rabbit. Acta Cir Bras. 27:256–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Santis E, Lang NP, Cesaretti G,
Mainetti T, Beolchini M and Botticelli D: Healing outcomes at
implants installed in sites augmented with particulate autologous
bone and xenografts An experimental study in dogs. Clin Oral
Implants Res. April 4–2012.(Epub ahead of print).
|
|
23
|
Costa-Pinto AR, Reis RL and Neves NM:
Scaffolds based bone tissue engineering: the role of chitosan.
Tissue Eng Part B Rev. 17:331–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeong KI, Kim SG, Kim YK, Oh JS, Jeong MA
and Park JJ: Clinical study of graft materials using autogenous
teeth in maxillary sinus augmentation. Implant Dent. 20:471–475.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Wu G and de Groot K: Biomimetic
coatings for bone tissue engineering of critical-sized defects. J R
Soc Interface. 7(Suppl 5): S631–S647. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krebsbach PH, Kuznetsov SA, Bianco P and
Robey PG: Bone marrow stromal cells: characterization and clinical
application. Crit Rev Oral Biol Med. 10:165–181. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan Z, Zhao Q, Gong P, et al: Research on
promoting periodontal regeneration with human basic fibroblast
growth factor-modified bone marrow mesenchymal stromal cell gene
therapy. Cytotherapy. 11:317–325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ai-Aql ZS, Alagl AS, Graves DT,
Gerstenfeld LC and Einhorn TA: Molecular mechanisms controlling
bone formation during fracture healing and distraction
osteogenesis. J Dent Res. 87:107–118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fontana F, Rocchietta I, Dellavia C,
Nevins M and Simion M: Biocompatibility and manageability of a new
fixable bone graft for the treatment of localized bone defects:
preliminary study in a dog model. Int J Periodontics Restorative
Dent. 28:601–607. 2008.PubMed/NCBI
|
|
30
|
Camelo M, Nevins ML, Lynch SE, Schenk RK,
Simion M and Nevins M: Periodontal regeneration with an autogenous
bone-Bio-Oss composite graft and a Bio-Gide membrane. Int J
Periodontics Restorative Dent. 21:109–119. 2001.PubMed/NCBI
|