Introduction
Hepatic fibrosis can be considered as a
wound-healing response to chronic liver injury, and oxidative
stress plays a significant role in the pathogenesis of liver
diseases. Hepatocyte apoptosis is caused by hydrogen peroxide
(H2O2), which is attributed to heightened
production of reactive oxygen species (ROS) and defects in the
cellular antioxidant systems. ROS overproduction may cause
hepatocyte apoptosis, which plays a central role in liver fibrosis
(1–3). It is expected that certain natural
antioxidants may act as potential anti-fibrotic agents that protect
hepatocytes against ROS.
Cordyceps polysaccharide (CPS) is a major aqueous
extract component of the Chinese herb summer grass and winter worm
(Cordyceps militaris), which has been widely used as a tonic
for longevity, endurance and vitality (4). Previous studies have shown that
polysaccharides from various Cordyceps species have many useful
biological activities including antitumor (5,6),
anti-influenza virus (7),
immunopotentiation (8),
hypoglycemic (9),
hypocholesterolemic (10) and
anti-oxidant effects (11–13). Multiple studies have shown that CPS
is capable of increasing the activities of antioxidant enzymes such
as catalase (CAT), superoxide dismutase (SOD) and glutathione
peroxide (GPx), and effectively scavenges free radicals such as
hydroxyl and superoxide anion radicals, which are byproducts of the
mitochondrial electron transfer chain (ETC) (14).
Mitochondria play many pivotal functions, such as
being the site of election transfer chain, oxidative
phosphorylation and ATP synthesis, and mainly participate in cell
apoptosis regulation (15).
Mitochondria are also primary sites for ROS production, which gives
rise to mitochondrial dysfunction. High ROS levels in cells lead to
depolarization of mitochondrial membrane potential (MMP) and the
loss of MMP subsequently triggers Cyt C release from mitochondria
to the cytosol (16). The release
of Cyt C impacts functions of respiratory chain complexes III and
IV, which interrupts cellular electron flow, subsequently
suppressing ATP generation and promoting cell apoptosis (17,18).
Mitochondria are considered the pacemakers of tissue diseases due
to the continuous production of oxygen free radicals, nitrogen free
radicals and related reactive species, and the selective oxidative
damage that leads to mitochondrial dysfunction. However, the
hepatocyte protective effects of CPS on
H2O2-induced mitochondrial dysfunction remain
unknown. In the present study, the aim was to evaluate whether CPS
elicits protective actions against HL-7702 cell apoptosis induced
by H2O2 through focusing on mitochondrial
dysfunction.
Materials and methods
Materials
HL-7702 cells were purchased from the Institute of
Biochemistry and Cell Biology, China Academy of Science, Shanghai.
Cordyceps polysaccharide, obtained from Shanghai University of
Traditional Chinese Medicine, was dissolved in sterile distilled
water. 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium
bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO,
USA). Dimethlysulfoxide (DMSO) was obtained from Shenggong Biology
(Shanghai, China). Mitochondrial membrane potential detection kit,
ATP detection kit and Hoechst 33258 were purchased from Beyotime
(Jiangsu, China). Dulbecco’s modified Eagle’s medium (DMEM)
supplement was obtained from Gibco Invitrogen Co. (Gaithersburg,
MD, USA). The fluorescent dye 2′,7′-dichlorodihydro-fluorescein
diacetate (H2DCF-DA) was purchased from Invitrogen. Antibodies
against Cyt C were from Cell Signaling Technology (Beverly, MA,
USA) and Epitomics (Burlingame, CA, USA). Antibodies against Bcl-2
and Bax were purchased from Epitomics. All the other chemicals were
of the highest grade of purity available commercially.
Cell culture and treatment
HL-7702 cells were routinely cultured in DMEM
(Gibco) containing 10% fetal calf serum (FBS; Gibco), 100 U/ml
penicillin and 100 mg/ml streptomycin and maintained in a
humidified 5% CO2 atmosphere at 37°C. The medium was
changed every two days. Cells were incubated with 400 μM
H2O2 for 2 h to induce cell apoptosis. In
order to study the effects of CPS, cells were pre-incubated with
various concentrations of CPS for 2 h, and then
H2O2 was added to the medium for another 2
h.
Isolation and purification of CPS
The dried powder of cultured cordyceps mycelia was
purchased from Traditional Chinese Medicine Limited Liability
Company (Jiangxi, China) and was defatted with various
concentrations of ethanol. The extract was boiled twelve times in
water for 2 h and centrifuged; the supernatant was concentrated and
treated with 15 times volume of ethanol for precipitation. The
precipitate was suspended in water, dialyzed and lyophilized to
yield the crude polysaccharide-enriched fraction. The extraction
ratio of crude polysaccharide was 15% and the purity was above 99%.
The crude polysaccharide verified by Fehling Reagent, mainly
contained reductive sugars such as mannose, galactose glucose,
cordycepin, adenosine, arabinose, xylose and fucose, and these
monosaccharides composed the polysaccharide.
Preparation of CPS stock solution
The stock solution of CPS was prepared by dissolving
10 mg CPS using 10 ml sterile distilled water and was stored at
−70°C for future use.
Determination of cell viability
Cell viability was measured by conventional MTT
reduction assay. The cultured cells were seeded at an initial
density of 5×104 cells/ml in a 96-well plate for 24 h
and pre-incubated with 400, 500 and 600 μM CPS for 2 h and exposed
to 400 μM H2O2 for 2 h. Following incubation,
20 μl MTT stock solution (5 mg/ml) was added into each well at a
final concentration of 0.5 mg/ml for another 4 h. The resulting
formazan was dissolved in 150 μl DMSO and measured with a
microtiter plate reader at a wavelength of 492 nm.
Detection of ROS
The intracellular ROS was detected by H2DCF-DA, an
oxidation-sensitive fluorescent probe. Cells were pre-incubated
with CPS for 2 h and exposed to 400 μM H2O2
for 2 h. The medium was removed; cells were washed twice with
serum-free medium and incubated with H2DCF-DA (10 μM) for 20 min at
37°C in the dark. The fluorescence intensity was monitored on an
automatic fluorescence microplate reader with an excitation
wavelength of 488 nm and an emission wavelength of 525 nm and
observed under a fluorescence microscope. Data were expressed as a
percentage of the control.
Detection of ATP levels
Intracellular ATP levels were measured by a
firefly-luciferase-based ATP detection kit (Beyotime) according to
the manufacturer’s instructions. Briefly, cells were seeded into
the 24-well plates and washed with phosphate-buffered saline (PBS)
three times. The supernatant of samples was collected immediately
on ice and measured with an illuminometer. ATP levels were
calculated according to an ATP standard curve. Intracellular ATP
levels were analyzed as the percentage of the control group.
Measurement of MMP
The intracellular MMP was evaluated by use of the
fluorescent, lipophilic and cationic probe,
5,5′,6,6′-tetrachloro-1,1′,3,3′-iodide (JC-1) (Beyotime) according
to the manufacturer’s instructions. Briefly, following treatment,
the cells were loaded with JC-1 staining solution for 20 min at
37°C and washed three times with JC-1 staining buffer. The
fluorescence intensity was measured by a CytoFluor multiwell plate
reader with 514 nm for excitation and 529 nm for emission of green
(monomer form) fluorescence, and 585 nm for excitation and 590 nm
for emission for red (aggregate form) fluorescence. The MMP of
cells in each group was evaluated as the fluorescence ratio of red
to green and observed by a fluorescence microscope. The data were
expressed as the percentage of the control.
Immunofluorescence
Cells were placed on cover slips in 24-well plates
and pretreated with CPS for 2 h prior to exposure to 400 μM
H2O2 for 2 h. After washing with PBS, the
slides were fixed in 4% paraformaldehyde for 10 min at room
temperature, washed three times with PBS, permeabilized with 0.1%
saponin, blocked with 10% normal goat serum and incubated overnight
at 4°C with anti-Cyt C. The slides were incubated with
FITC-conjugated goat anti-rabbit immunoglobulin (Sigma-Aldrich) for
2 h. Nuclei were stained with Hoechst 33258 (Beyotime). Cover slips
were observed under a fluorescence microscope (Leica Microsystems,
Wetzlar, Germany) equipped with a Leica DFC420 camera.
Western blot analysis
Cells were lysed in SDS buffer supplemented with a
mixture of protease inhibitors, 1 μg/ml aprotinin and 100 μg/ml
phenylmethylsulfonyl fluorides. The cell suspension was incubated
on ice for 30 min then centrifuged at 20,000 × g for 15 min at 4°C.
The supernatant was collected for further analysis. The protein
concentrations of the supernatants were determined using the
Bradford method. Cell lysates were denatured for 15 min in 5X
sample buffer and separated by SDS-polyacrylamide gel
electrophoresis. For western blot analysis, the gel was transferred
onto nitrocellulose membranes using a tank transfer system. Blotted
membranes were placed in a blocking solution of 5% nonfat milk in
Tris-buffered saline Tween-20 (TBS-T). For immunodetection,
membranes were incubated for 1 h at room temperature and then
incubated overnight at 4°C with the relevant primary antibodies,
followed by washing with TBST and incubation with the appropriate
horseradish peroxidase-conjugated secondary antibodies.
Immunocomplexes were visualized using a commercially available
enhanced chemiluminescence kit with exposure of the transfer
membrane to X-ray film. The following antibodies were used:
anti-Bcl-2, anti-Bax and anti-glyceraldehyde-3-phosphate
dehydrogenase (GAPDH).
Statistical analysis
Data were expressed as the means ± SEM from at least
three independent experiments. Statistical significance analysis
was carried out using Student’s t-test or ANOVA. Mean values were
considered to be statistically significant at P<0.05 or
P<0.01.
Results
CPS prevented
H2O2-induced cell apoptosis
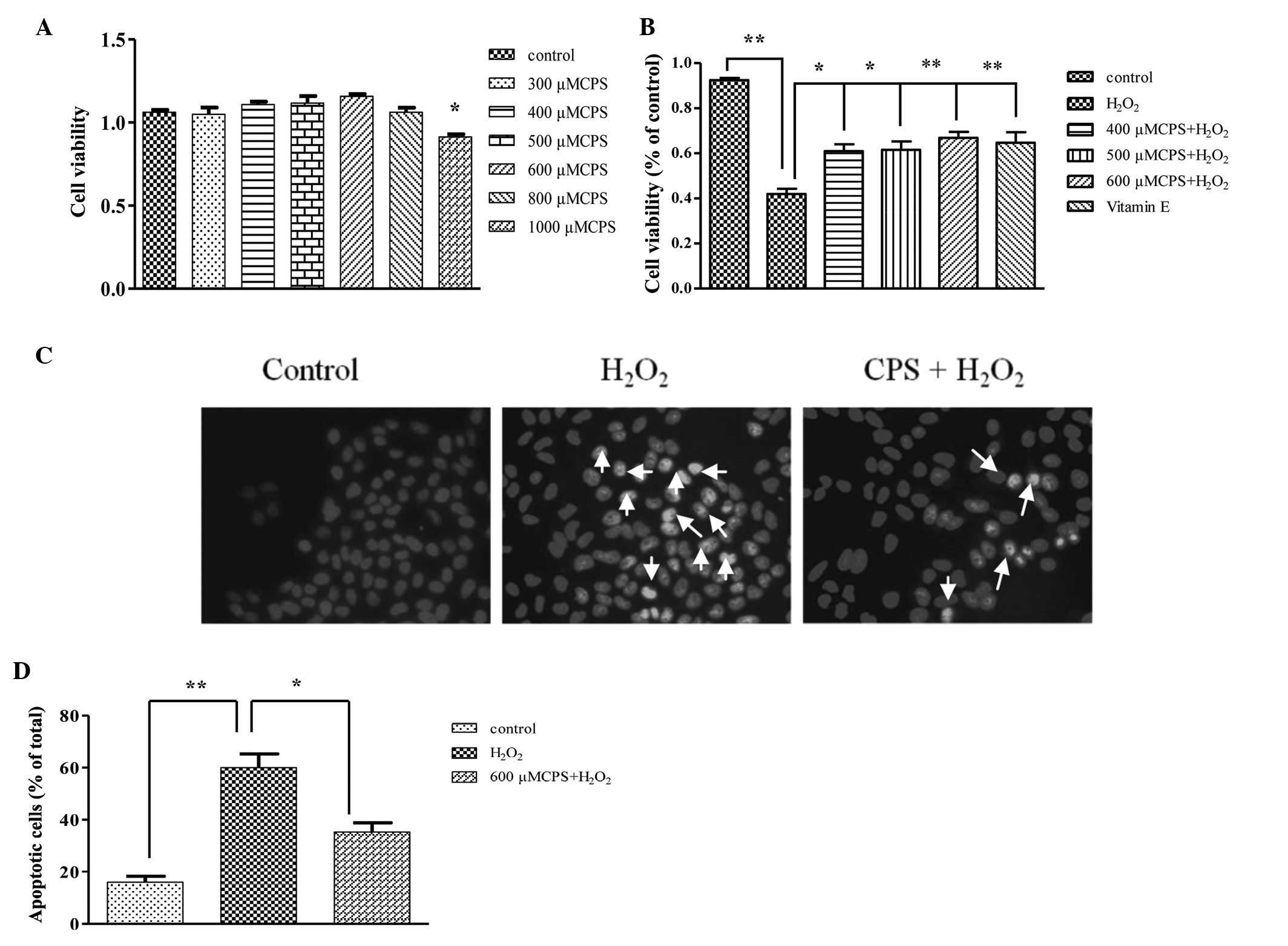
Firstly, in order to investigate the effect of CPS
on HL-7702 cells, an MTT assay was used to evaluate the
cytotoxicity of cells treated with various concentrations of CPS.
As shown in Fig. 1A, CPS at a
concentration range from 300 to 800 μM had no significant
cytotoxicity on HL-7702 cells and the cytotoxicity was observed
when the concentration of CPS increased to 1000 μM. Then, to
examine the protective effect of CPS on
H2O2-induced cell apoptosis, cells were
incubated with 400 μM H2O2 for 2 h with or
without different concentrations of CPS. The findings showed that
cell viability decreased to 43.3% after treatment with
H2O2 for 2 h compared with the control group.
However, pretreatment with CPS significantly enhanced cell
viability from 43.3 to 58.5, 52.1 and 54.9%, respectively (Fig. 1B), while Vitamin E (VE) serving as
a control showed a protection of 56.2%. In addition, the effects
were also observed under Hoechst 33258 staining, which revealed
contracted nucleus and condensed chromatin fragments. Following
treatment with H2O2 for 2 h, the number of
apoptotic cells increased compared to the control. However,
pretreatment with CPS significantly decreased the number of
apoptotic cells (Fig. 1C and D).
These results suggested that CPS inhibits cell apoptosis induced by
H2O2, which may be correlated with scavenging
free radicals. Therefore, we further measured the eliminating
capacity of CPS.
CPS ameliorated
H2O2-induced oxidative stress
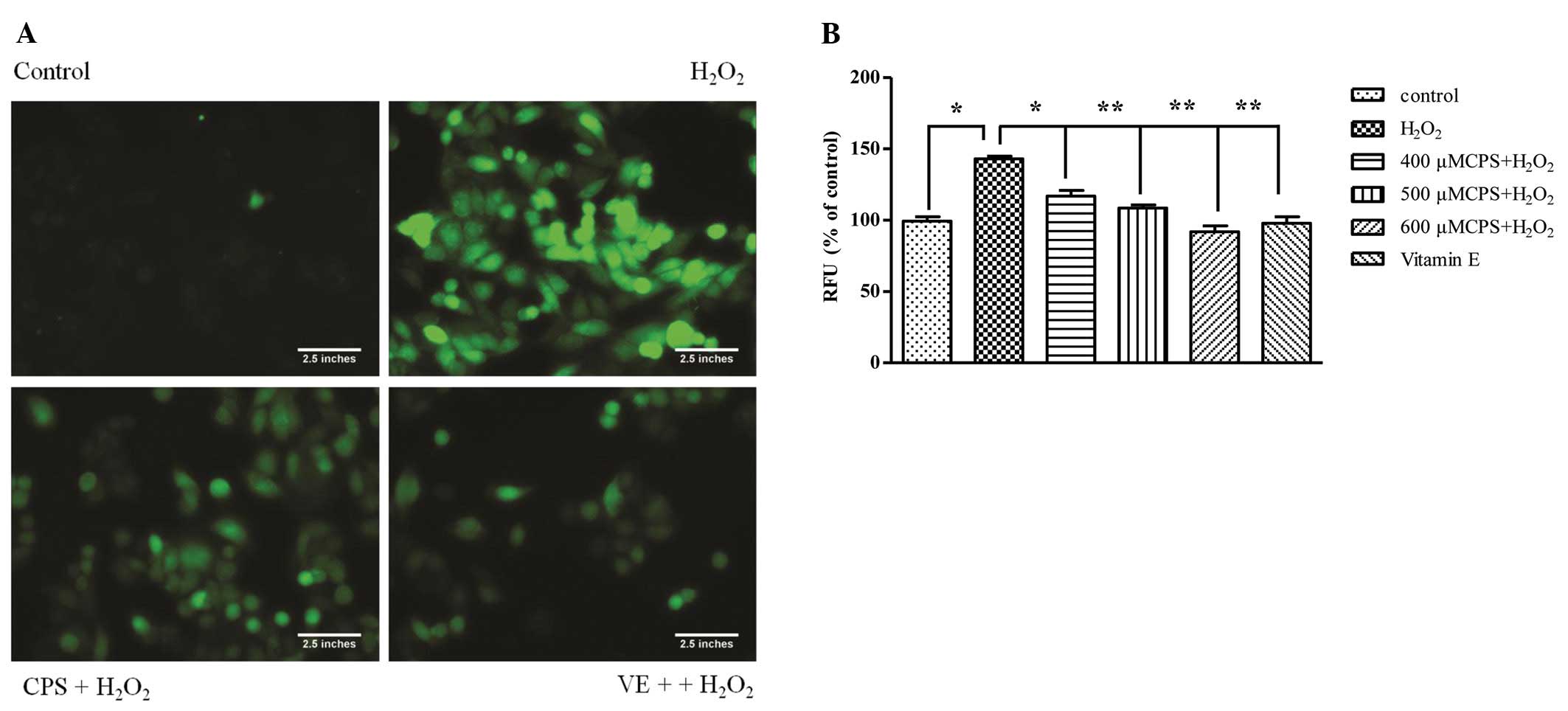
ROS generation is an important indicator of
oxidative stress-induced mitochondrial dysfunction. In order to
detect the capability of CPS scavenging free radicals, the H2DCF-DA
assay was used to detect the generation of intracellular ROS
induced by 400 μM H2O2 for 2 h. The
fluorescence images showed that green fluorescence intensity
markedly increased when HL-7702 cells were incubated with 400 μM
H2O2 for 2 h. However, pretreatment with CPS
reduced the green fluorescence intensity (Fig. 2A). Fig. 2B shows the same result: exposure of
HL-7702 cells to H2O2 led to an increase of
the intracellular ROS levels, which was approximately 1.39-fold
relative to that of control cells. Pretreatment with CPS and VE
inhibited the intracellular ROS level. These results suggested that
CPS restrains H2O2-induced cell apoptosis by
eliminating intracellular ROS, and mitochondrial membranes are key
action sites of ROS. Therefore, we further detected the effect of
CPS on mitochondrial membrane potential and energy synthesis.
CPS improved MMP
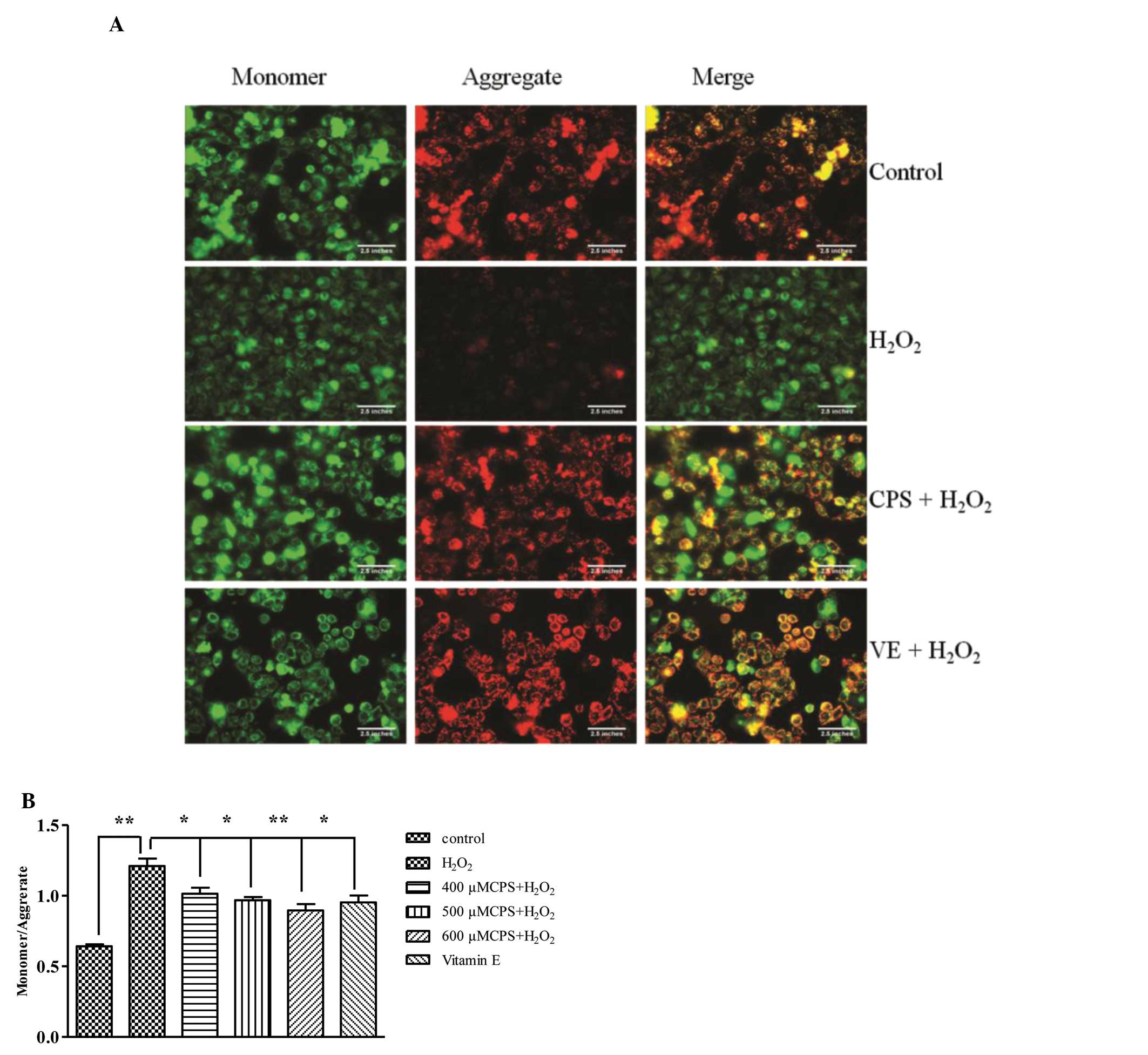
Dissipation of mitochondrial integrity is one of the
early events leading to apoptosis (19). To assess whether CPS affects the
function of mitochondria, the changes of MMP were analyzed by
employing mitochondria fluorescence dye, JC-1, which stains
mitochondria in a membrane potential-dependent manner. As shown in
Fig. 3A, cells exposed to 400 μM
H2O2 for 2 h resulted in a significant
decrease in aggregate and increase in monomer forms; however,
pretreatment with CPS prevented the loss of aggregate and the
increase of the monomer forms. In addition, the effects of CPS on
H2O2-induced MMP disruption were also
confirmed by an automatic fluorescence microplate reader. Exposure
to H2O2 for 2 h gave rise to a decrease in
the ratio of aggregate to monomer, and 400, 500 and 600 μM CPS
prevented the decrease of the ratio between aggregate and monomer
in a concentration-dependent manner. VE also increased the ratio
between aggregate and monomer forms (Fig. 3B). These results implied that CPS
attenuated H2O2-induced MMP dissipation.
Effect of CPS on intracellular ATP level
in H2O2-induced HL-7702 cells
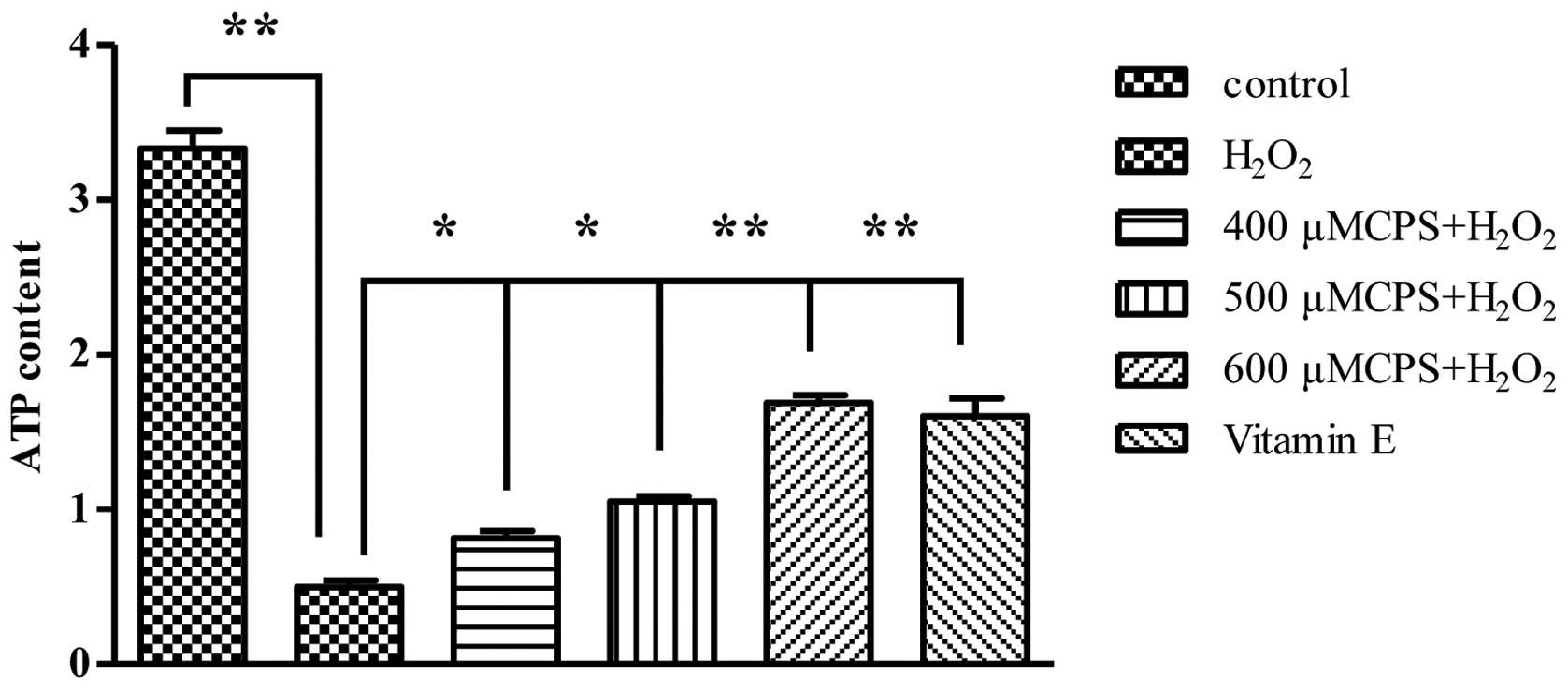
In order to determine whether the dysfunction of
mitochondrial energy generated occurred in
H2O2-treated cells, we investigated the
changes of intracellular ATP content in the
H2O2-treated cells with and without various
concentrations of CPS (400–600 μM). As shown in Fig. 4A, when HL-7702 cells were treated
with 400 μM H2O2 for 2 h, ATP concentration
markedly decreased to 0.465 μM (the concentration of control group
was 4.537 μM). However, pretreatment with CPS (400, 500 and 600 μM)
increased the ATP concentrations from 0.456 to 0.799, 1.085 and
1.722 μM, respectively, and VE inhibited the decrease of ATP levels
induced by H2O2. Our data implied that CPS
avoids the mitochondrial energy dysfunction induced by
H2O2.
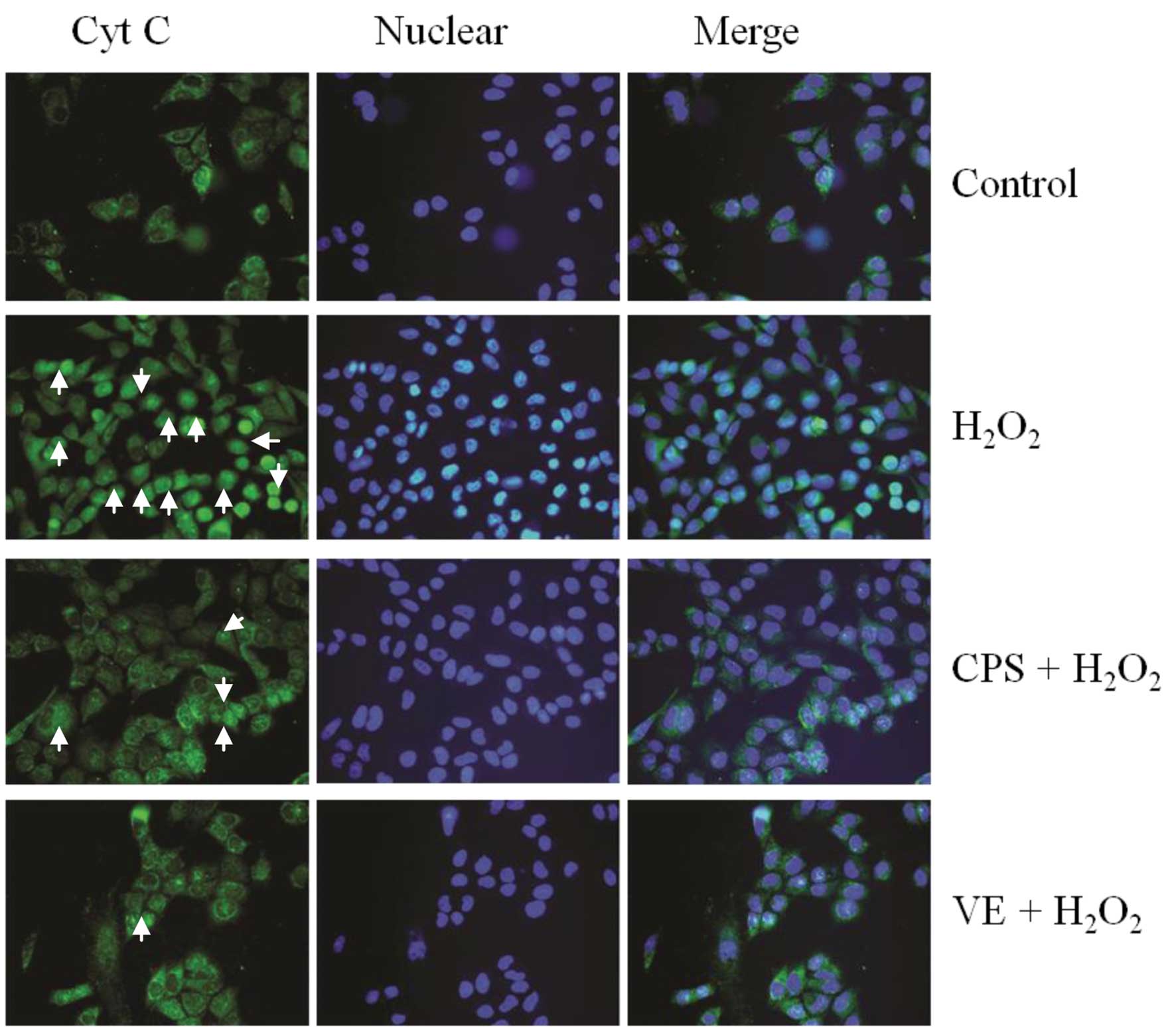
Effect of CPS on
H2O2-induced Cyt C release
Cyt C release from the mitochondria to the cytosol
is a critical event in the mitochondrial dysfunction induced by
various types of cell stress. We therefore examined whether CPS has
a significant role in regulating the release of Cyt C using
immunofluorescence staining. As shown in Fig. 5, following treatment with 400 μM
H2O2 for 2 h, diffuse cytoplasmic staining
was detected, implying that Cyt C was released from the
mitochondria to the cytosol. Moreover, pretreatment with 600 μM CPS
prevented the Cyt C release from the mitochondria to the cytosol
caused by H2O2. The data demonstrated that
Cyt C is a key apoptotic factor in the mitochondrial-dependent
apoptotic pathway, and CPS inhibits cell apoptosis by regulating
mitochondrial apoptotic factors, such as Cyt C. Consequently, we
further observed whether CPS was capable of accommodating other
apoptosis-related factors.
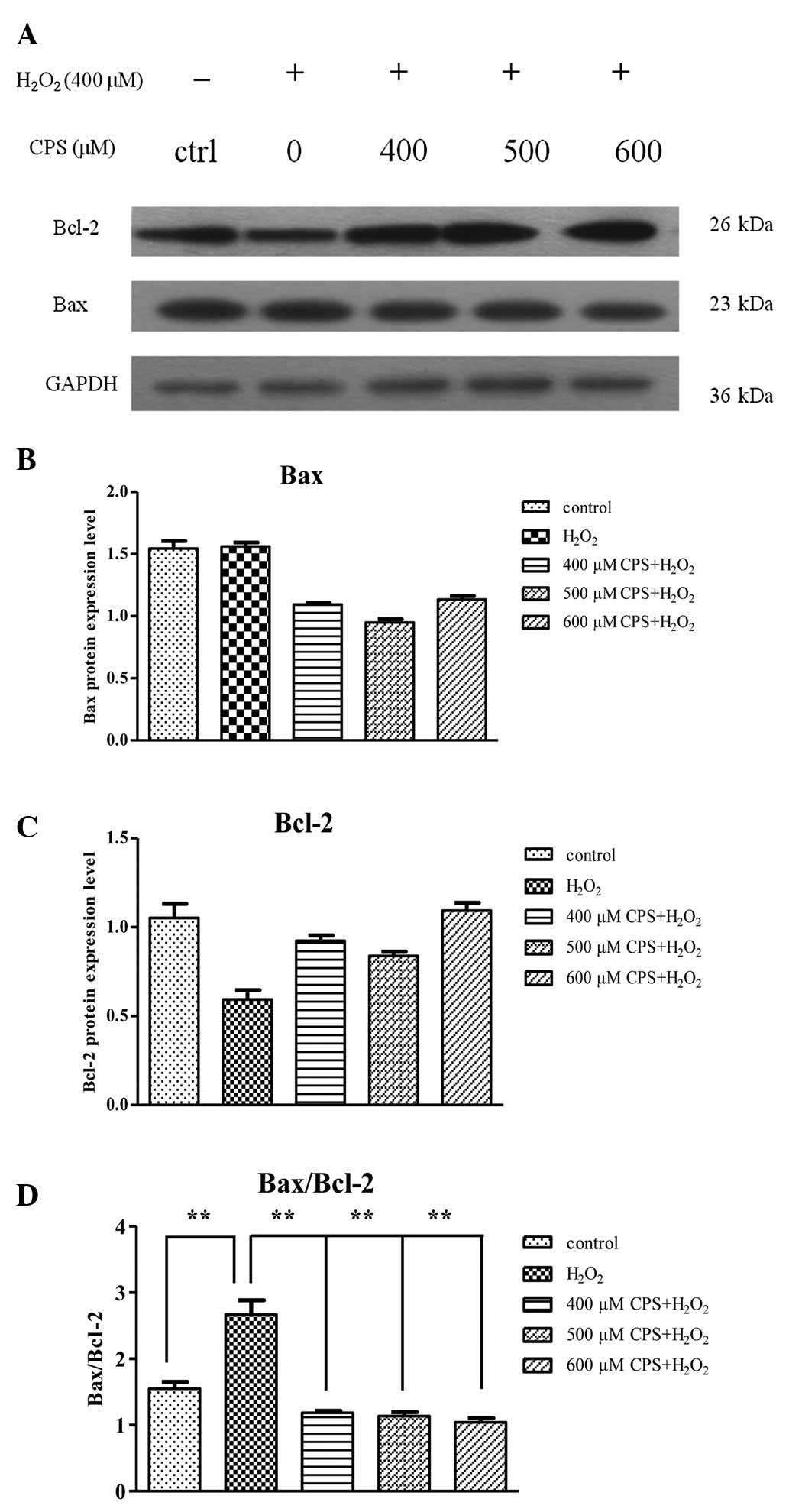
Effect of CPS on the expression of Bcl-2
family proteins in H2O2-induced HL-7702
cells
Bcl-2 family membranes play critical roles in
maintaining mitochondrial integrity and mitochondria-initiated Cyt
C release. Previous studies have reported that the ratio of the
pro-apoptotic protein Bax to the anti-apoptotic Bcl-2 was
correlated with cell apoptosis (20). Our results showed that the protein
level of Bax displayed little change and there was a prominent
decrease in the protein expression of Bcl-2 after HL-7702 cell
treatment with 400 μM H2O2, and there was an
approximate 1.9-fold increase in the ratio of Bax/Bcl-2 in the
H2O2 treatment group compared with the
control group, as shown by western blot analysis. However, CPS
inhibited the H2O2-induced increase of
Bax/Bcl-2 ratio in a concentration-dependent manner (Fig. 6A–D). The effect of CPS on
H2O2-induced apoptosis may be, at least in
part, mediated by the regulation of Bax and Bcl-2 expression. CPS
decreased the ratio of Bax/Bcl-2 and suggested that it may suppress
the mitochondrial-dependent apoptosis induced by
H2O2.
Discussion
H2O2 is extensively used as an
indicator of oxidative stress inducing cell injury in a number of
in vitro models. It is now well known that
H2O2 is able to react with intracellular
metal ions (iron or copper) creating highly toxic hydroxyl radicals
that cause cell damage (21). The
H2O2-induced cytotoxicity has been reported
to be attenuated by antioxidants and free radical scavengers
(22,23). The present study provides evidence
that CPS significantly prevented H2O2-induced
hepatocyte injury in HL-7702 cells, potentially through antioxidant
and anti-apoptotic mechanisms. Several studies have reported that
CPS displays potent antioxidant properties through increasing the
activities of CAT, SOD and GPx (14,24).
The present results showed that H2O2 induced
a decrease in cell viability, whereas different concentrations of
CPS were able to significantly inhibit cell injury by increasing
cell viability (Fig. 1). Taken
together, these results demonstrated that
H2O2 is capable of leading to HL-7702 cell
injury and CPS has a hepatoprotective effect against
H2O2-induced HL-7702 cell injury.
Powerful evidence showed that oxidative damage leads
to mitochondrial dysfunction, which plays critical roles in
hepatocyte toxicity (25). ROS are
chiefly produced in the mitochondria and contribute to
intracellular signaling processes and then regulate various
biological activities including cell apoptosis (26). The present study demonstrated that
H2O2 induces intracellular ROS generation,
whereas CPS is capable of inhibiting cell apoptosis by scavenging
intracellular ROS. Excessive ROS are known to induce the collapse
of MMP, which is an important event in mitochondrial dysfunction
(27). CPS restrained the loss of
MMP induced by H2O2 and the results showed
that CPS protects HL-7702 cells against
H2O2-induced mitochondrial dysfunction. ROS
overproduction lead to free radical attack of mitochondrial
membrane phospholipids following the reduction of MMP, which caused
the release of pro-apoptotic factors, such as Cyt C, from the
mitochondrial intermembrane space to the cytosol, and induced cell
apoptosis by activating downstream factor caspase-3 (28). In accordance with these theories,
the present study showed that CPS prevented the release of Cyt C
from the mitochondria to the cytosol induced by
H2O2. It is well known that mitochondria are
the key site of ATP cellular energy metabolism, oxidative
phosphorylation is the major ATP synthetic pathway, and complexes
I-IV constitute the respiratory chain. When a H+
gradient was established across the mitochondrial double membrane,
complex IV derived ATP synthesis occurred (29). Our results showed that cells
treated with H2O2 showed a decrease in ATP
level; however, pretreatment with CPS may elevate intracellular ATP
content. The potent antioxidant of CPS protects HL-7702 cells
against mitochondrial dysfunction.
The Bcl-2 family of proteins are important
participants in the mitochondrial apoptotic pathway, and play a
critical role in regulating the interaction between pro-apoptotic
proteins and anti-apoptotic proteins to determine the life or death
of cells (30,31). In normal cells, anti-apoptotic
proteins, such as Bcl-2, are mainly located in the mitochondrial
outer membrane while pro-apoptotic proteins, such as Bax, primarily
exist in the cytoplasm (32).
Apoptotic factors acting on the mitochondria triggered Bax to
translocate to the mitochondrial outer membrane and homodimerize,
resulting in the release of apoptosis-inducing factors (33). Bax then translocates to the
mitochondrial membrane where it interacts with the anti-apoptotic
protein Bcl-2, inhibits Bcl-2 ability and promotes apoptosis.
Therefore, alteration of the ratio of Bax and Bcl-2 influences cell
apoptosis (34). Our data
demonstrated that HL-7702 cells treated with
H2O2 increased the ratio of Bax/Bcl-2;
however, CPS decreased the ratio of Bax/Bcl-2. These results
suggested that CPS inhibited HL-7702 cell apoptosis induced by
H2O2 by modulating the Bcl-2 family
proteins.
In conclusion, the results of the current study
demonstrate that CPS is able to ameliorate the mitochondrial
dysfunction and oxidative stress induced by
H2O2 in HL-7702 hepatocytes through
increasing cell viability, attenuating intracellular ROS levels,
preventing the loss of MMP, enhancing ATP content, inhibiting Cyt C
release from mitochondria to cytosol and decreasing the ratio of
Bax/Bcl-2. Although more detailed mechanistic investigations should
be undertaken to clarify the mitochondrial protection of CPS, these
results imply that the antioxidant CPS has promising potential to
be used in treating hepatic diseases that involve free radical and
oxidative injury.
Acknowledgements
This study was supported by National Science and
Technology Major Project of China (2009ZX09311-003).
References
|
1
|
Friedman SL: Liver fibrosis - from bench
to bedside. J Hepatol. 38(Suppl 1): S38–53. 2003. View Article : Google Scholar
|
|
2
|
Koek GH, Liedorp PR and Bast A: The role
of oxidative stress in non-alcoholic steatohepatitis. Clin Chim
Acta. 412:1297–1305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan X, Zhou T, Tao Y, Wang Q, Liu P and
Liu C: Salvianolic acid B attenuates hepatocyte apoptosis by
regulating mediators in death receptor and mitochondrial pathways.
Exp Biol Med (Maywood). 235:623–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park NS, Lee KS, Sohn HD, Kim DH, Lee SM,
Park E, Kim I, Je YH and Jin BR: Molecular cloning, expression, and
characterization of the Cu,Zn superoxide dismutase (SOD1) gene from
the entomopathogenic fungus Cordyceps militaris. Mycologia.
97:130–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YJ, Shiao MS, Lee SS and Wang SY:
Effect of Cordyceps sinensis on the proliferation and
differentiation of human leukemic U937 cells. Life Sci.
60:2349–2359. 1997.
|
|
6
|
Xiao JH, Liang ZQ and Liu AY: Advances of
polysaccharides research and exploitation of anamorph and its
related fungi from Cordyceps. Yao Xue Xue Bao. 37:589–592. 2002.(In
Chinese).
|
|
7
|
Ohta Y, Lee JB, Hayashi K, Fujita A, Park
DK and Hayashi T: In vivo anti-influenza virus activity of an
immunomodulatory acidic polysaccharide isolated from Cordyceps
militaris grown on germinated soybeans. J Agric Food Chem.
55:10194–10199. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura K, Yamaguchi Y, Kagota S,
Shinozuka K and Kunitomo M: Activation of in vivo Kupffer cell
function by oral administration of Cordyceps sinensis in rats. Jpn
J Pharmacol. 79:505–508. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiho T, Ookubo K, Usui S, Ukai S and
Hirano K: Structural features and hypoglycemic activity of a
polysaccharide (CS-F10) from the cultured mycelium of Cordyceps
sinensis. Biol Pharm Bull. 22:966–970. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koh JH, Kim JM, Chang UJ and Suh HJ:
Hypocholesterolemic effect of hot-water extract from mycelia of
Cordyceps sinensis. Biol Pharm Bull. 26:84–87. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamaguchi Y, Kagota S, Nakamura K,
Shinozuka K and Kunitomo M: Antioxidant activity of the extracts
from fruiting bodies of cultured Cordyceps sinensis.
Phytother Res. 14:647–649. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li SP, Su ZR, Dong TT and Tsim KW: The
fruiting body and its caterpillar host of Cordyceps sinensis
show close resemblance in main constituents and anti-oxidation
activity. Phytomedicine. 9:319–324. 2002.PubMed/NCBI
|
|
13
|
Li SP, Zhao KJ, Ji ZN, Song ZH, Dong TT,
Lo CK, Cheung JK, Zhu SQ and Tsim KW: A polysaccharide isolated
from Cordyceps sinensis, a traditional Chinese medicine,
protects PC12 cells against hydrogen peroxide-induced injury. Life
Sci. 73:2503–2513. 2003.
|
|
14
|
Li XT, Li HC, Li CB, Dou DQ and Gao MB:
Protective effects on mitochondria and anti-aging activity of
polysaccharides from cultivated fruiting bodies of Cordyceps
militaris. Am J Chin Med. 38:1093–1106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zamzami N, Marchetti P, Castedo M, Zanin
C, Vayssiere JL, Petit PX and Kroemer G: Reduction in mitochondrial
potential constitutes an early irreversible step of programmed
lymphocyte death in vivo. J Exp Med. 181:1661–1672. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Preston TJ, Abadi A, Wilson L and Singh G:
Mitochondrial contributions to cancer cell physiology: potential
for drug development. Adv Drug Deliv Rev. 49:45–61. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cadenas E and Davies KJ: Mitochondrial
free radical generation, oxidative stress, and aging. Free Radic
Biol Med. 29:222–230. 2000.PubMed/NCBI
|
|
18
|
Raha S and Robinson BH: Mitochondria,
oxygen free radicals, disease and ageing. Trends Biochem Sci.
25:502–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rasola A and Bernardi P: The mitochondrial
permeability transition pore and its involvement in cell death and
in disease pathogenesis. Apoptosis. 12:815–833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Xu Y, Yan J, Zhao X, Sun X, Zhang
Y, Guo J and Zhu C: Acteoside protects human neuroblastoma SH-SY5Y
cells against beta-amyloid-induced cell injury. Brain Res.
1283:139–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Naval MV, Gomez-Serranillos MP, Carretero
ME and Villar AM: Neuroprotective effect of a ginseng (Panax
ginseng) root extract on astrocytes primary culture. J
Ethnopharmacol. 112:262–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hong H and Liu GQ: Protection against
hydrogen peroxide-induced cytotoxicity in PC12 cells by
scutellarin. Life Sci. 74:2959–2973. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu CS, Chen NH and Zhang JT: Protection
of PC12 cells from hydrogen peroxide-induced cytotoxicity by
salvianolic acid B, a new compound isolated from Radix Salviae
miltiorrhizae. Phytomedicine. 14:492–497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guizani N, Waly MI, Ali A, Al-Saidi G,
Singh V, Bhatt N and Rahman MS: Papaya epicarp extract protects
against hydrogen peroxide-induced oxidative stress in human SH-SY5Y
neuronal cells. Exp Biol Med (Maywood). 236:1205–1210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang JM, Cho JS, Kim TH and Lee YI:
Ellagic acid protects hepatocytes from damage by inhibiting
mitochondrial production of reactive oxygen species. Biomed
Pharmacother. 64:264–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rhee SG, Bae YS, Lee SR and Kwon J:
Hydrogen peroxide: a key messenger that modulates protein
phosphorylation through cysteine oxidation. Sci STKE.
2000:pe12000.PubMed/NCBI
|
|
27
|
Park MT, Kim MJ, Kang YH, Choi SY, Lee JH,
Choi JA, Kang CM, Cho CK, Kang S, Bae S, Lee YS, Chung HY and Lee
SJ: Phytosphingosine in combination with ionizing radiation
enhances apoptotic cell death in radiation-resistant cancer cells
through ROS-dependent and -independent AIF release. Blood.
105:1724–1733. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bras M, Queenan B and Susin SA: Programmed
cell death via mitochondria: different modes of dying. Biochemistry
(Mosc). 70:231–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Loeffler M and Kroemer G: The
mitochondrion in cell death control: certainties and incognita. Exp
Cell Res. 256:19–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Allen RT, Pai J, Bovard K, Hunter WJ 3rd
and Agrawal DK: Immunogold staining for Bcl-xL and morphological
analysis of rat and human vascular smooth muscle cells undergoing
apoptosis induced by c-myc or staurosporine. Scanning. 20:207–208.
1998.
|
|
31
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song XD, Zhang JJ, Wang MR, Liu WB, Gu XB
and Lv CJ: Astaxanthin induces mitochondria-mediated apoptosis in
rat hepatocellular carcinoma CBRH-7919 cells. Biol Pharm Bull.
34:839–844. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang SH, Chien CM, Lu MC, Lin YH, Hu XW
and Lin SR: Up-regulation of Bax and endonuclease G, and
down-modulation of Bcl-XL involved in cardiotoxin III-induced
apoptosis in K562 cells. Exp Mol Med. 38:435–444. 2006. View Article : Google Scholar : PubMed/NCBI
|