Introduction
Gastric carcinoma is a common disease with high
incidence rates in several Asian countries, particularly in Japan
and China. Lower incidence has been observed in certain Western
European countries and the United States (1,2).
Although the incidence of gastric carcinoma has decreased in recent
years, it remains the second cause of cancer-related death
worldwide (3). Due to the majority
of the cases being detected at advanced stages, the 5-year survival
rate in these cases is low (4).
Therefore, it is imperative to find new targets to improve
therapeutic or preventive strategies.
Inhibitor of DNA binding 1 (Id1) belongs to the
inhibitor of DNA binding/differentiation (Id) family, which lacks a
DNA-binding domain (5), so it acts
as a negative regulator of HLH transcription factors to inhibit
gene expression (6,7). Id1 was previously reported to
regulate various cell processes, including proliferation,
apoptosis, cell cycle, differentiation and angiogenesis (8–11).
The upregulation of Id1 may inhibit the ability to differentiate in
several cell models. Certain reports have suggested that cell
cycle-associated proteins, such as p16, p21, p27 and cyclin D1, are
transcriptionally inhibited by Id1; the upregulation of Id1 may
stimulate G1-S cell cycle transition (12–14).
The role of Id1 in cell proliferation or apoptosis showed different
effects in different cell types: the upregulation of Id1 induces
apoptosis in dense mammary epithelial cells and cardiac myocytes,
but promotes proliferation and tumor growth in lung cancer cells
(14–16). Id1 is regarded as a valuable marker
for both the diagnosis and prognosis of gastric carcinoma (17,18).
Although several reports have suggested that Id1 is involved in the
growth and migration of gastric cancer cells (19), the role of Id1 in the proliferation
and migration abilities of gastric cancer cells remains to be
determined.
In this study, we mainly investigated the role of
Id1 in the proliferation of SGC-7901 cells by knockdown and
overexpression techniques, and a possible mechanism was also found.
Our findings indicated that Id1 is involved in the growth and
migration abilities of gastric cancer cells.
Materials and methods
Cell culture
The SGC-7901 gastric cancer cell line was a gift
from Dr Yang Zhang (Department of Biochemistry and Molecular
Biology, Zhongshan Medical College, Sun Yat-Sen University, China)
(20). The cell line was cultured
in high-glucose DMEM (Gibco, BRL, Guangzhou, China) supplemented
with 10% fetal bovine serum at 37˚C with 5% CO2.
Id1 small interfering RNA (siRNA)
Id1-specific siRNA used for Id1 knockdown and the
control siRNA were synthesized by GenePharma (Shanghai GenePharma
Co., Ltd.). The sequences of siRNA targeting the Id1 coding region
were as follows: sense, 5′-CUCGGAAUCCGAAGUUGGADTDT-3′ and
antisense, 5′-UCCAACUUCGGAUUCCGAGDTDT-3′ (21). The siRNAs were then transfected
into the PC3 cells by Lipofectine 2000 (Invitrogen, USA), according
to the manufacturer's instructions.
Construction of the Id1 expressing
vector
The full-length Id1 cDNA was amplified from total
cDNA of SGC-7901 cells by PCR, and was then subcloned between the
KpnI and EcoRI sites of pcDNA3.1(+) vector. Purified
plasmids were sequence-verified by Invitrogen (Shanghai, China).
The plasmid was transfected into SGC-7901 cells by Lipofectine
2000. The primers used for PCR were as follows: forward,
5′-GATGGTACCATCATGAAAGTCGCCAGTG-3′ and reverse,
5′-GATGAATTCTCAGCGACACAAGATGCGA-3′.
MTT assay
SGC-7901 cells were seeded in 96-well plates at a
concentration of 5,000 cells/well in a volume of 150 μl of cell
culture medium. After 24 h, transfection was performed. The plates
were incubated at 37˚C with 5% CO2 for 48 and 72 h. MTT
solution (20 μl) (5 g/l, dissolved in PBS) was added to each well
and the plates were incubated at 37˚C for another 4 h.
Subsequently, the supernatant was discarded and l50 μl
dimethylsulfoxide was added to dissolve the insoluble MTT formazan.
The absorbance values at 570 nm were detected by a multi-well plate
reader (Tecan).
Flow cytometry assay
SGC-7901 cells were transfected with the
above-mentioned siRNA or plasmid for 72 h and were then presented
for flow cytometry assay. For cell cycle analysis, DNA labeling was
performed using the Cycletest Plus DNA Reagent kit (BD Biosciences
Pharmingen, USA), and the samples were analyzed using a flow
cytometer (Beckman Counter, USA). For the detection of apoptotic
cells, labeling tests involving both propidium iodide (PI) and
annexin-V were performed using an Annexin-V staining kit
(Invitrogen, USA), according to the manufacturer's instructions.
Briefly, at least 1×106 cells were harvested by
trypsinization, incubated with FITC-labeled annexin-V and PI stock
solutions for 10 min at room temperature and analyzed using a flow
cytometer (Beckman Counter).
Western blot analysis
SGC-7901 cells were transfected for 72 h, harvested
and lysed for total protein extraction. Protein concentration was
determined using the Bio-Rad protein assay kit (Bio-Rad, China).
Equal amounts of protein were separated by 15% SDS-PAGE and
transferred onto PVDF membranes. The membranes were rinsed with
TBST and incubated in blocking buffer (5% dried milk in PBS) for 1
h at 37˚C, followed by incubation with primary antibodies at 4˚C
overnight. The antibody against cyclin D1 used in western blotting
was purchased from Beyotime (China), antibody against β-actin was
purchased from PTG (USA), and other antibodies were purchased from
Santa Cruz (USA). After washing with TBST three times, the
membranes were incubated with their corresponding secondary
antibodies for 1 h. The blots were visualized by an enhanced
chemiluminescence detection system (Amersham). The expression of
β-actin was used as a normalization control for protein
loading.
Statistical analysis
Data were expressed as the means ± SD. Statistical
analyses were performed using Student's t-test. P<0.05 indicated
statistical significance.
Results
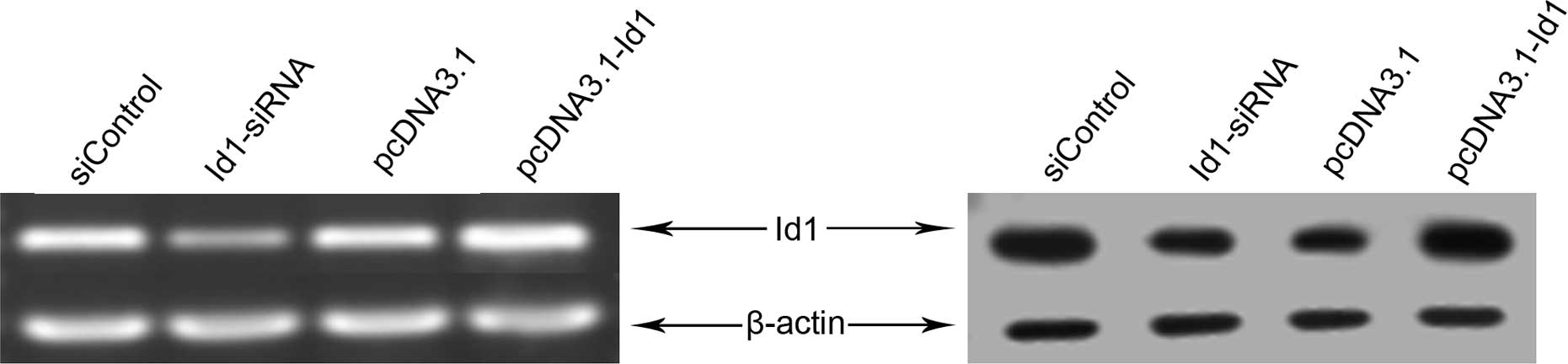
Effects of Id1 siRNA or pcDNA3.1-Id1 on
the levels of Id1 in SGC-7901 cells
The effect of siRNA or pcDNA3.1-Id1 on the levels of
Id1 was evaluated using both reverse transcriptase-PCR and western
blotting. As shown in Fig. 1,
significantly decreased Id1 mRNA and protein levels were detected
in siRNA transfected cells compared to the control group
(siControl), indicating that Id1 siRNA successfully downregulated
the Id1 gene in SGC-7901 cells. Furthermore, pcDNA3.1-Id1
upregulated the levels of Id1 in SGC-7901 cells.
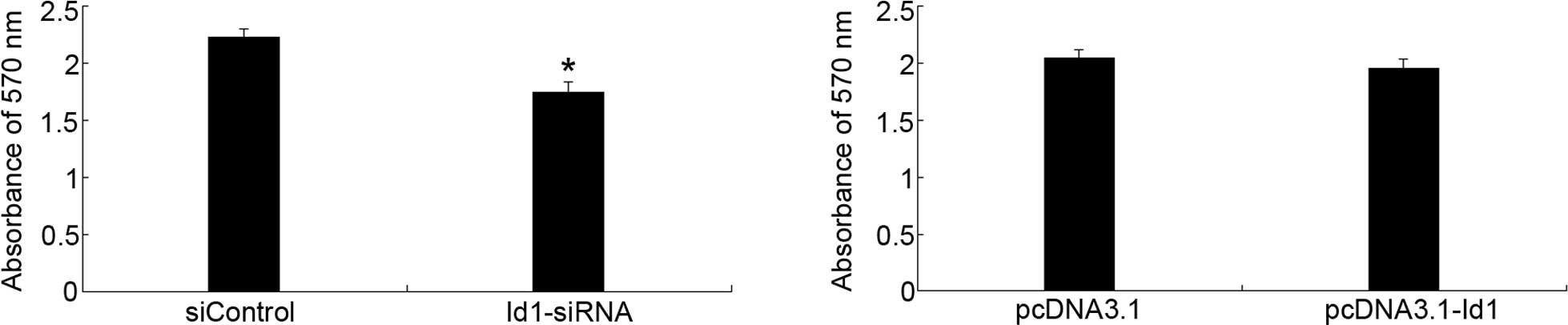
Regulation of SGC-7901 cell proliferation
by Id1 expression
To evaluate the effect of Id1 on the proliferation
of SGC-7901 cells, MTT assay was performed. As shown in Fig. 2, Id1 was involved in the
proliferation of SGC-7901 cells; the inhibitory effect was evident
after 72-h transfection with Id1-siRNA. However, the upregulation
of Id1 in SGC-7901 cells could not promote cell proliferation.
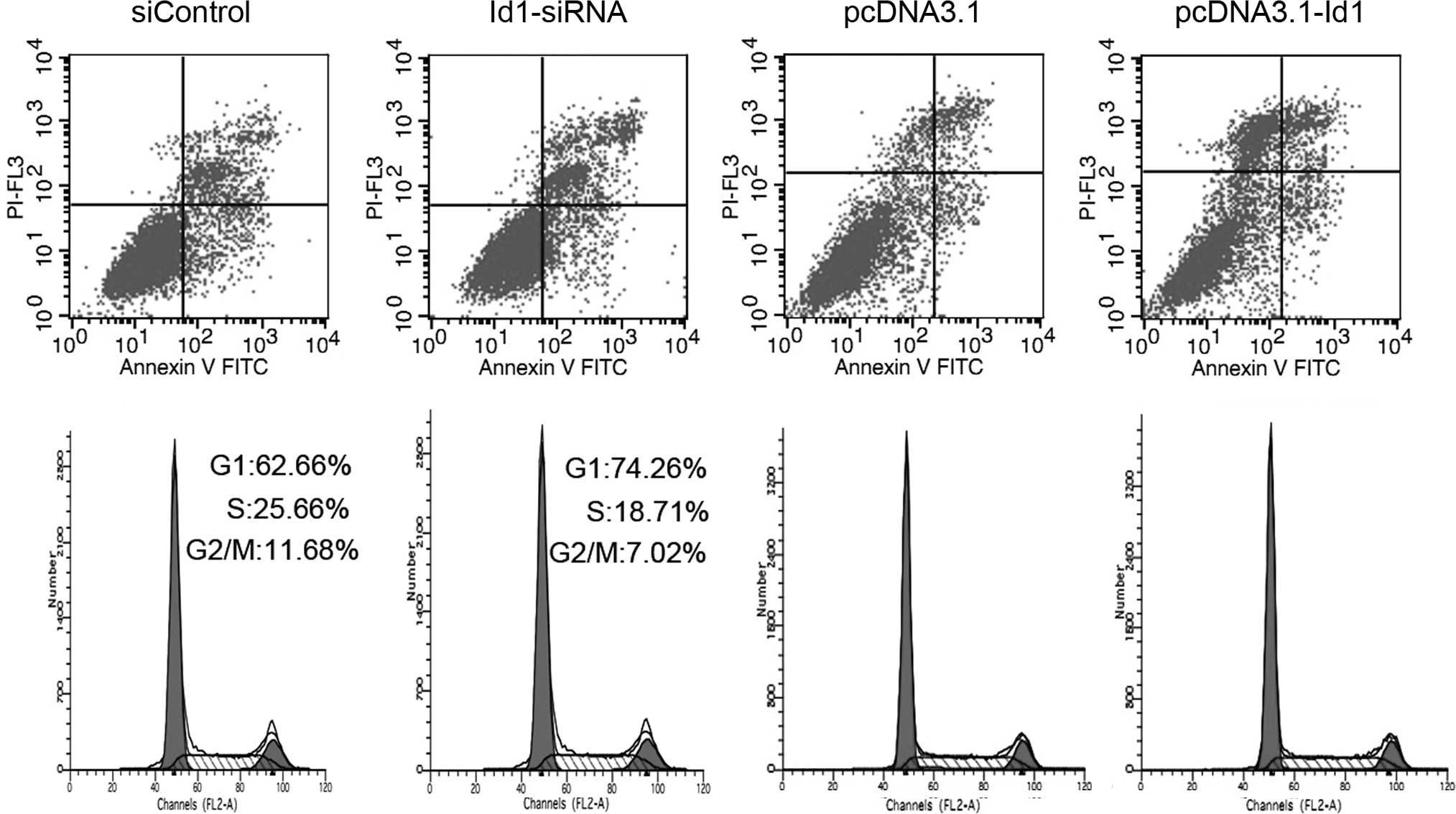
Regulation of Id1 did not affect the
apoptosis of SGC-7901 cells
To examine the effect of altered Id1 expression on
cell apoptosis, transfected cells were analyzed by Annexin
V-FITC/PI double staining. As shown in Fig. 3A, compared to the control group, no
significant change was found in the annexin V-positive or annexin V
and PI-double positive cell fractions in the Id1 siRNA-transfected
group. Moreover, Id1 overexpression in SGC-7901 cells transfected
with pcDNA3.1-Id1 showed no impact on cell apoptosis; it suggested
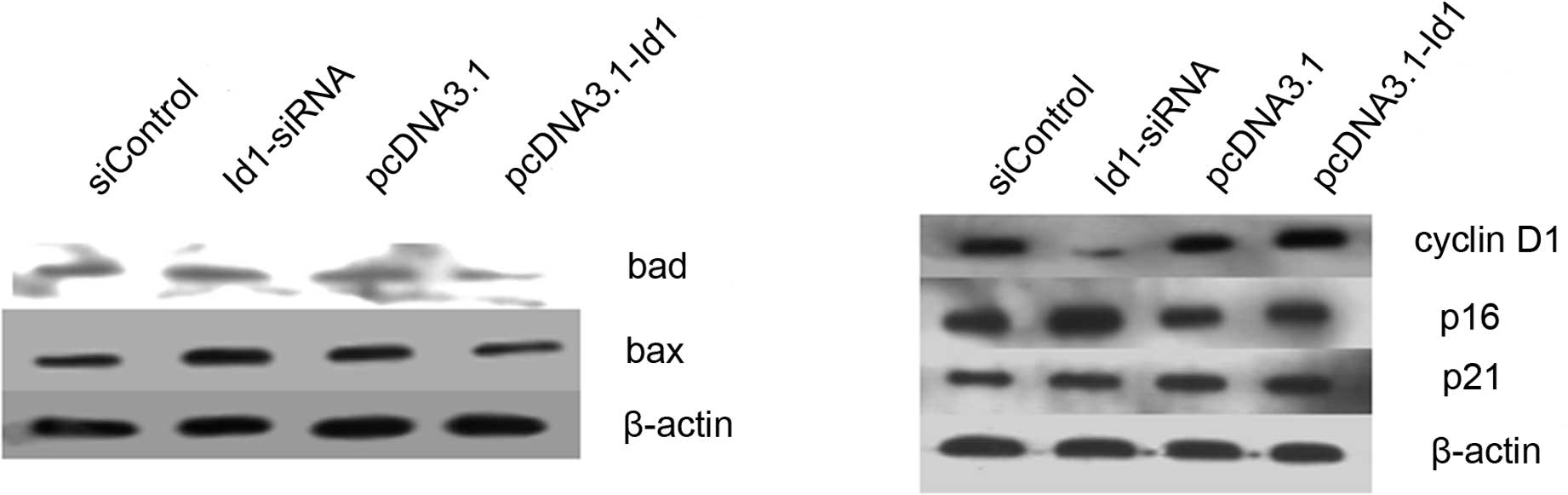
that Id1 is not involved in the apoptosis of SGC-7901 cells. Bax
and Bad are important in the apoptotic pathway. In this study,
although the changes of Id1 levels did not affect the apoptosis of
SGC-7901 cells, we detected the protein levels of Bax and Bad in
SGC-7901 cells. As shown in Fig.
4A, the expression of Bax and Bad increased when cells were
transfected with Id1 siRNA, as expected, whereas their expression
decreased when cells were transfected with pcDNA3.1-Id1.
Effects of Id1 expression on cell cycle
distribution
Cell cycle distribution was analyzed by flow
cytometry after the cells were transfected for 72 h. As shown in
Fig. 3B, the proportion of the
G2/M phase was decreased in Id1 siRNA-transfected cells compared to
the control group. The cell cycle did not change when Id1 was
overexpressed in SGC-7901 cells. We further analyzed the changes of
certain cell cycle regulators. Cyclin D1 was decreased in the Id1
knockdown group, while p16 and p21 were increased; cyclin D1 was
elevated in the Id1 overexpression group, while p16 and p21 were
decreased (Fig. 4B).
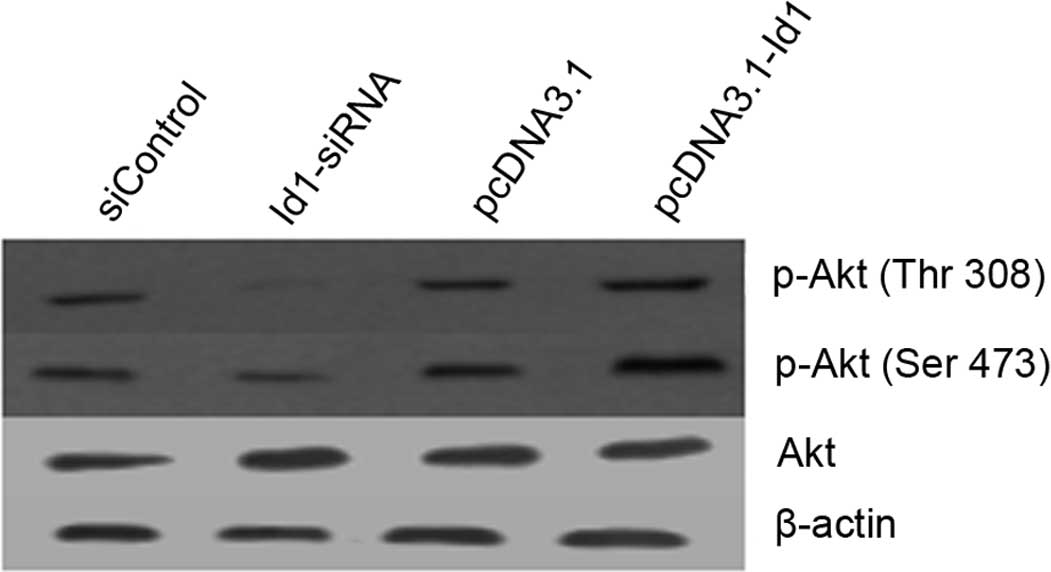
Akt pathway is involved in the growth
inhibition of SGC-7901 cells following transfection with Id1
siRNA
The correlation between Id1 and p-Akt expression in
SGC-7901 cells was examined by western blotting. As shown in
Fig. 5, Akt phosphorylation at
Thr308 and Ser473 was reduced in Id1 siRNA-treated SGC-7901 cells,
but enhanced in pcDNA3.1-Id1-treated SGC-7901 cells. These results
suggest that the expression of p-Akt is associated with Id1
expression in SGC-7901 cells.
Discussion
Id proteins are involved in cell differentiation,
proliferation, migration and angiogenesis. Recent studies have
investigated the role of Id1 in cancer development in several tumor
models (22,23). Data on its role in gastric
carcinoma remain scarce and are derived mainly from tissue
resources (17,18). A previous report has shown that
Id1, 3 double-knockdown impaired the ability of gastric cancer
cells to form peritoneal metastasis. Findings of that study also
suggested that proliferation and motility may be inhibited in Id1,
3 double-knockdown gastric cancer cells (19). However, the single role of Id1 in
gastric cancer cells was not investigated.
We investigated the role of Id1 in the proliferation
of gastric carcinoma by changing the Id1 levels in SGC-7901 cells.
Id1-siRNA and the vector expressing Id1 were utilized to regulate
the levels of Id1 in SGC-7901 cells. RT-PCR and western blotting
showed that the levels of Id1 were successfully regulated by
Id1-siRNA and pcDNA3.1-Id1. In this study, the downregulation of
Id1 in SGC-7901 cells inhibited cell proliferation and decreased
the proportion of G2/M phase of the cell cycle, while the
upregulation of Id1 did not show these effects; the reason has yet
to be determined. However, both the downregulation and upregulation
of Id1 levels changed the levels of several cell cycle-related
genes in this study, which is consistent with other recent reports
(14,23–25).
Our data suggest that Id1 is not involved in the apoptosis of
SGC-7901 cells, but that the apoptosis-associated genes Bax and Bad
are affected by Id1 levels. Therefore, the downregulation of Id1
did not elevate the levels of Bax and Bad to the extent that cell
apoptosis occurred.
p-Akt is known to be involved in cell proliferation
in several tumor models. We investigated whether p-Akt (Thr308,
Ser473) was involved in Id1-associated proliferation of SGC-7901
cells. Our findings regarding p-Akt in SGC-7901 cells are in
agreement with those of other studies in that the downregulation of
Id1 was capable of decreasing the levels of p-Akt (Thr308,
Ser473).
In conclusion, our findings have shown for the first
time that the Akt pathway is involved in Id1 in the proliferation
of gastric cancer cells. Therefore, targeting Id1 may be a novel
strategy for the treatment of gastric cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (Grant no. 30672358 to Zhenyu Zhu).
References
|
1
|
A JemalF BrayMM CenterJ FerlayE WardD
FormanGlobal Cancer StatisticsCA Cancer J
Clin616990201110.3322/caac.20107
|
|
2
|
AI NeugutM HayekG HoweEpidemiology of
gastric cancerSemin Oncol232812911996
|
|
3
|
C PrinzS SchwendyP VolandH. pylori
and gastric cancer: shifting the global burdenWorld J
Gastroenterol1254582006
|
|
4
|
DM RoderThe epidemiology of gastric
cancerGastric Cancer5Suppl 1511200210.1007/s10120-002-0203-6
|
|
5
|
HA SikderMK DevlinS DunlapB RyuRM AlaniId
proteins in cell growth and tumorigenesisCancer
Cell3525530200310.1016/S1535-6108(03)00141-712842081
|
|
6
|
MB RuzinovaR BenezraId proteins in
development, cell cycle and cancerTrends Cell
Biol13410418200310.1016/S0962-8924(03)00147-812888293
|
|
7
|
J PerkI Gil-BazoY ChinReassessment of Id1
protein expression in human mammary, prostate, and bladder cancers
using a monospecific rabbit monoclonal anti-Id1 antibodyCancer
Res661087010877200610.1158/0008-5472.CAN-06-264317108123
|
|
8
|
XH SunNG CopelandNA JenkinsD BaltimoreId
proteins Id1 and Id2 selectively inhibit DNA binding by one class
of helix-loop-helix proteinsMol Cell Biol115603561119911922066
|
|
9
|
D LydenAZ YoungD ZagzagId1 and Id3 are
required for neurogenesis, angiogenesis and vascularization of
tumour xenograftsNature401670677199910.1038/4433410537105
|
|
10
|
PY DesprezE HaraMJ BissellJ
CampisiSuppression of mammary epithelial cell differentiation by
the helix-loop-helix protein Id-1Mol Cell
Biol153398340419957760836
|
|
11
|
R BenezraS RafiiD LydenThe Id proteins and
angiogenesisOncogene2083348341200110.1038/sj.onc.120516011840326
|
|
12
|
RM AlaniAZ YoungCB ShifflettId1 regulation
of cellular senescence through transcriptional repression of
p16/Ink4aProc Natl Acad Sci
USA9878127816200110.1073/pnas.14123539811427735
|
|
13
|
A CiarrocchiV JankovicY ShakedId1
restrains p21 expression to control endothelial progenitor cell
formationPLoS ONE2e1338200710.1371/journal.pone.000133818092003
|
|
14
|
YJ ChengJW TsaiKC HsiehYC YangYJ ChenMS
HuangSS YuanId1 promotes lung cancer cell proliferation and tumor
growth through Akt-related pathwayCancer
Lett307191199201110.1016/j.canlet.2011.04.00321536374
|
|
15
|
S ParrinelloCQ LinK MurataId-1, ITF-2, and
Id-2 comprise a network of helix-loop-helix proteins that regulate
mammary epithelial cell proliferation, differentiation, and
apoptosisJ Biol Chem2763921339219200110.1074/jbc.M104473200
|
|
16
|
K TanakaJB PracykK TakedaExpression of Id1
results in apoptosis of cardiac myocytes through a redox-dependent
mechanismJ Biol
Chem2732592225928199810.1074/jbc.273.40.259229748268
|
|
17
|
HY YangHL LiuHC JiangExpression and
prognostic values of Id-1 and Id-3 in gastric adenocarcinomaJ Surg
Res167258266201110.1016/j.jss.2009.08.00620080245
|
|
18
|
Q WangSW TsaoX WangOverexpression of Id-1
in gastric adenocarcinoma: implication for a novel diagnostic
markerAnticancer Res24881886200415161041
|
|
19
|
T TsuchiyaY OkajiNH TsunoD
SakuraiTargeting Id1 and Id3 inhibits peritoneal metastasis of
gastric cancerCancer
Sci96784790200510.1111/j.1349-7006.2005.00113.x16271072
|
|
20
|
Y ZhangJ HanX YangC ShaoZ XuR ChengW CaiJ
MaZ YangG GaoPigment epithelium-derived factor inhibits
angiogenesis and growth of gastric carcinoma by down-regulation of
VEGFOncol Rep26681686201121617872
|
|
21
|
YX LingJ TaoSF FangcZ HuiQR
FangDownregulation of Id1 by small interfering RNA in prostate
cancer PC3 cells in vivo and in vitroEur J Cancer
Prev20917201110.1097/CEJ.0b013e32833ebaa020881502
|
|
22
|
O GautschiCG TepperPR PurnellRegulation of
Id1 expression by Src: implications for targeting of the bone
morphogenetic protein pathway in cancerCancer
Res6822502258200810.1158/0008-5472.CAN-07-640318381431
|
|
23
|
H GengBL RademacherJ PittsenbargerID1
enhances docetaxel cytotoxicity in prostate cancer cellsCancer
Res7032403248201010.1158/0008-5472.CAN-09-318620388787
|
|
24
|
B LiSW TsaoYY LiX WangALM CheungId-1
promotes tumorigenicity and metastasis of human esophageal cancer
cells through activation of PI3K/AKT signaling pathwayInt J
Cancer12525762585200910.1002/ijc.2467519551863
|
|
25
|
A SwarbrickMC AkerfeldtCSL LeeEA
MusgroveRegulation of cyclin expression and cell cycle progression
in breast epithelial cells by the helix-loop-helix protein
Id1Oncogene24381389200510.1038/sj.onc.120818815489884
|