Introduction
TRESK, the most recently reported type of two-pore
potassium ion channel, is expressed in the center and peripheral
nervous system, including the cerebellum, the cerebral cortex and
the dorsal root ganglia (DRG) (1).
TRESK is an outwardly rectifying K+ current channel that
contributes to the resting potential and is the most important
background potassium channel with a relatively small conductance.
Neuropathic pain (NP) is closely related to the regulation of
certain potassium channels in DRG neurons. It was previously shown
that the change of TRESK is likely to be involved in the
development of NP (2–4).
Capsaicin is a selective sensory nerve stimulator,
and small doses of it excite sensory nerve endings, releasing nerve
transmitters (5,6). Substance P (SP), as a nerve
transmitter, is a member of the tachykinin family, which is widely
distributed in the peripheral nervous system. SP functions mainly
as a nociceptive stimulus receptor, and is also involved in
inflammatory response (7,8). Numerous studies have demonstrated
that DRG-released SP is important in the peripheral mechanism of
NP.
We recently examined the expression level of TRESK
mRNA in the DRG of NP of rats. Results of that study demonstrated
that downregulation of the TRESK background current in DRG may be
related to NP (9). In addition,
rat TRESK full-length cDNA was cloned and recombinant TRESK
adenovirus vector was constructed successfully in our previous
study (10).
In the present study, therefore, we examined whether
the activation of TRESK detected by TRESK mRNA and protein by the
TRESK gene recombinant adenovirus vector inhibits the
capsaicin-evoked SP release in DRG neurons.
Materials and methods
Materials
TRESK adenovirus vector (pAdTrack-CMV-TRESK),
negative adenovirus, PCR primer (Shanghai R&S Biotechnology
Co., Ltd., Shanghai, China); the rabbit polyclonal anti-TRESK
antibody, TRESK-conjugated anti-rabbit antibody (Santa Cruz
Biotechnology, Santa Cruz, CA, USA); the SP radio-immunity kit (the
Second Army Medical College, Shanghai, China); the Superscript kit
(Life Technologies, Gaithersburg, MD, USA); and the DNA engine
Opticon 2 real-time PCR detection system (Bio-Rad, Hercules, CA,
USA) were used in this study.
Isolation and culture of DRG neurons
DRGs were removed from young adult SD rats (6–9
weeks) and were dissociated into single isolated neurons by enzyme
treatment of 0.125% collagenase for 90 min (twice), followed by
0.25% trypsin for 30 min at 37˚C and by trituration with
fire-polished Pasteur pipettes of decreasing tipdiameter (11). The cells (2–3 DRG/well) were
subsequently plated on polyethyleneimine and laminin-coated 96-well
tissue-culture plates and incubated in DMEM containing 10%
heat-inactivated horse serum, 1% penicillin/streptomycin and 200 mM
glutamine. The cultures were maintained at 37˚C in a
water-saturated atmosphere with 5% CO2 for 5 days prior
to the experiment. On the fifth day of culture, neurons exhibited
globular cell bodies and extended axonal processes. Various
non-neuronal cells, such as Schwann cells, fibroblasts and
satellite cells, were also present as an oxford gray cellular
background. Approval was received by the First People's Hospital of
Foshan ethics committee.
Experimental groups
DRG neurons were cultured in 54 wells and divided
randomly into six groups, 9 holes per group, per well. Group C
received no treatment. Each well in the S group was incubated for
10 min with fresh 300 nmol/l capsaicin culture medium. Each well in
the NC group was administered 3×107 negative adenovirus
(Shanghai R&S Biotechnology Co., Ltd.). Each well in the NCS
group was given 3×107 negative adenovirus and then
incubated for 10 min with fresh 300 nmol/l capsaicin culture medium
72 h later. Each well in the R group was given 3×107
pAdTrack-CMV-TRESK (Shanghai R&S Biotechnology Co., Ltd.). Each
well in the RS group was given 3×107 pAdTrack-CMV-TRESK
and then incubated for 10 min with fresh 300 nmol/l capsaicin
culture medium 72 h later.
Measurement of TRESK mRNA
Total RNA harvested from 3 wells of each group by
the acid guanidinium thiocyanate-phenol-chloroform extraction
method was separately subjected to reverse transcription into cDNA
using a Superscript kit (Life Technologies), according to the
manufacturer's protocol. cDNA sample (2 μg) was used immediately in
a real-time PCR assessment of TRESK mRNA levels with iQ SYBR-Green
Supermix, and forward primer (5′-GGTGCCAACGATGATCT-3′) and reverse
primer (5′-CTGCTGGGCTGTGGGTCTAG-3′) on a DNA engine Opticon 2
real-time PCR detection system (Bio-Rad). The thermal cycler
parameters used were: denaturation of 1 cycle of 2 min at 95˚C,
followed by 40 cycles of 20 sec at 95˚C, annealing for 15 sec at
57˚C and extension 20 sec at 72˚C. A glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) control was run simultaneously with the same
reaction recipe listed in the instructions manual for the iQ
SYBR-Green Supermix. The 2−ΔΔCt method was used to
calculate the expression level of TRESK mRNA (12).
Measurement of TRESK protein
Three wells were removed from each group, and the
cells were processed using western blotting. Primary antibodies
were raised against TRESK (1:1,000 dilution; rabbit polyclonal
TRESK antibodies; Santa Cruz) or β-actin. After washing, the
membranes were further incubated with horseradish
peroxidase-conjugated anti-rabbit secondary antibody (1:2,000
dilution; Santa Cruz Biotechnology) for 1 h at room temperature
(22˚C). The proteins were then used for the detection of
chemiluminescence, according to the manufacturer's
instructions.
Measurement of SP content
We measured the SP content of the culture medium
(serum-free DMEM) in the last 3 wells from each group at 72 h
following grouping. Cells were removed from the medium and were
maintained for 10 min with 300 nmol/l capsaicin in groups S, NCS
and RS. Subsequently, the medium from the cells in each group was
collected and centrifuged at 1,200 × g for 5 min, followed by a
radioimmunoassay with the SP radioimmunity kit (the Second Army
Medical College, Shanghai, China) of the medium (13).
Statistical analysis
The data are presented as the means ± SD.
Statistical analyses were performed by the multiple t-test with the
Bonferroni correction following ANOVA. P<0.05 was considered to
indicate a statistically significant difference.
Results
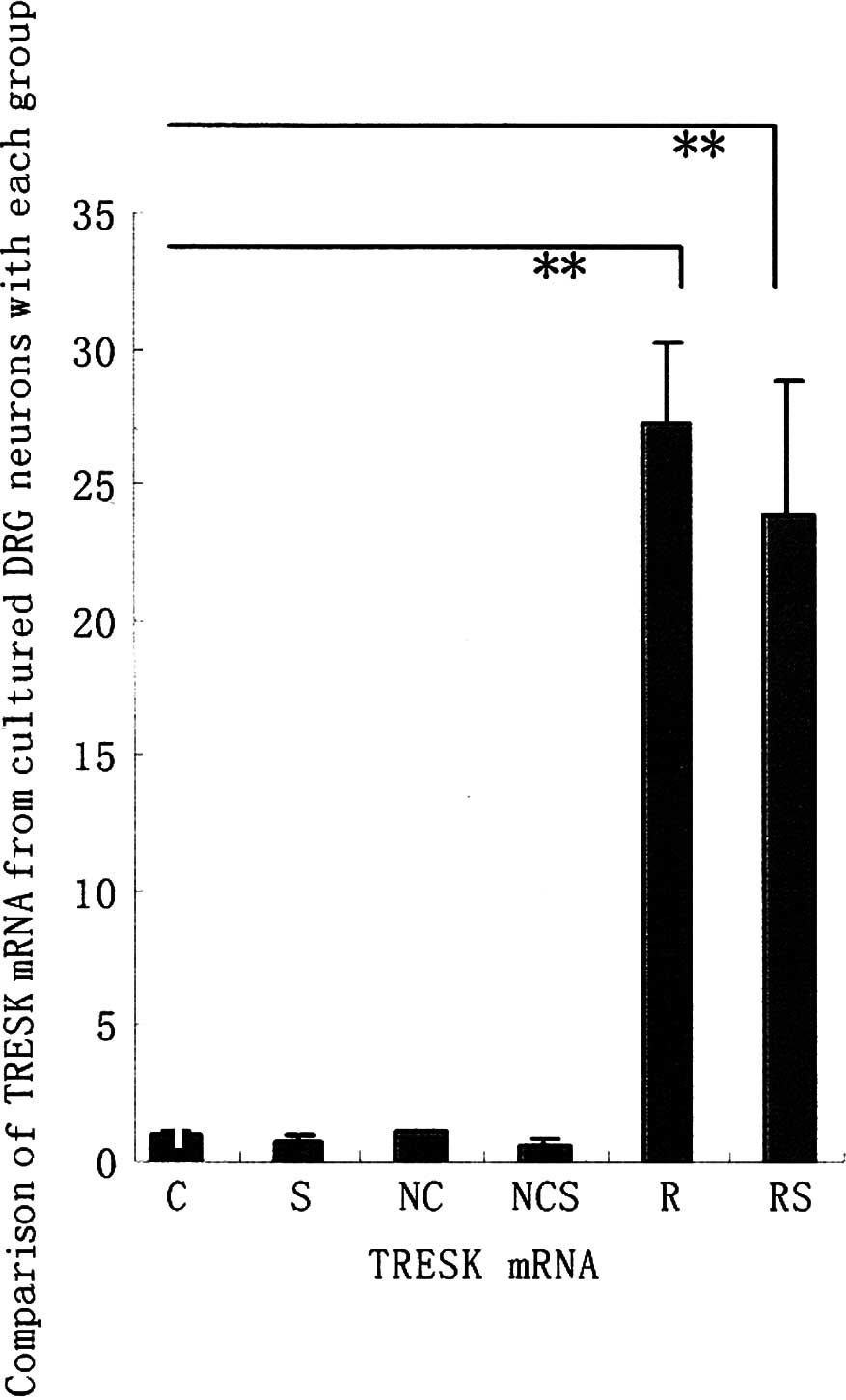
Comparison of TRESK mRNA from cultured
DRG neurons of each group
In our previous studies, we successfully constructed
the recombinant TRESK adenovirus vector (pAdTrack-CMV-TRESK). This
vector was found to upregulate TRESK mRNA expression levels in the
DRG neurons of rats (10).
Therefore, we investigated whether these mass
duplicate pAdTrack-CMV-TRESK are affected in the enhancement of
TRESK mRNA from cultured DRG neurons exposed to long-term (72 h)
treatment with adenovirus vector. Fig.
1A shows that the TRESK mRNA from cultured DRG neurons was
significantly enhanced when exposed to long-term (72 h) treatment
with pAdTrack-CMV-TRESK in groups R and RS. However, no increase in
the TRESK mRNA levels of the rat DRG neurons was observed following
a 72-h exposure of negative adenovirus in groups NC and NCS, as was
the case with groups C and S as controls (data not shown).
Comparison of TRESK protein from cultured
DRG neurons of each group
The protein levels of TRESK exposed to long-term (72
h) treatment with pAdTrack-CMV-TRESK or negative adenovirus vector
were examined using western blotting. The increase in the protein
levels of TRESK from cultured DRG induced by pAdTrack-CMV-TRESK was
significantly enhanced when exposed to long-term (72 h) treatment
in groups R and RS. However, no increase in the TRESK protein
levels of the rat DRG neurons was observed following a 72-h
exposure of negative adenovirus in groups NC, NCS, C and S as
controls (Fig. 1B) (data not
shown).
Comparison of capsaicin-induced SP
release from cultured DRG neurons of each group
To investigate whether pre-treatment with
upregulation of TRESK mRNA causes the attenuation of SP release
evoked by capsaicin, the levels of capsaicin-induced SP release
from cultured DRG neurons pre-treated with pAdTrack-CMV-TRESK or
negative adenovirus vector were examined. Fig. 1C shows that the release of SP from
cultured DRG neurons was significantly enhanced when incubated for
10 min with capsaicin in groups S, NCS and RS. Although the
capsaicin-evoked SP release was significantly enhanced by
pre-treatment with pAdTrack-CMV-TRESK in comparison to the control
group which was not incubated with capsaicin, the capsaicin-evoked
SP release from cultured DRG was significantly attenuated by
exposure to long-term (72 h) treatment with pAdTrack-CMV-TRESK in
the RS group compared to the NCS and S groups. There was no
significant difference in the release of SP from cultured DRG
neurons in the R, NC and C groups (data not shown).
Discussion
Results of the present study show that long-term (72
h) exposure of cultured rat DRG neurons to the pAdTrack-CMV-TRESK
resulted in the enhancement of TRESK mRNA, causing the protein
levels of TRESK to increase, leading to the attenuation of SP
release triggered by capsaicin.
Two-pore potassium is now considered an important
ion channel involved in the pain mechanism (14,15).
As a subtype of two-pore potassium channel, TRESK is similar in
distribution, physiology and pharmacology to other subtypes, which
are related to pain (1,3,4).
TRESK was found to be greatly expressed in the DRG neurons of rats,
and it was the main background current in DRG at 37 or 24˚C
(2,16). In particular, many studies have
demonstrated that DRG is important in the peripheral mechanism of
NP (17,18). These findings show that TRESK may
be involved in the development of NP. Furthermore, gliocyte is
involved in the occurrence and maintainance of NP, while it
exhibits permeability for potassium channel at resting potential.
As a background potassium current for resting potential, TRESK is
likely to have a key role in the excitation mechanism of gliocyte
(19,20).
SP is an 11-amino acid peptide sensory
neurotransmitter, which is synthesized in the DRG and released from
primary afferent neurons to convey information regarding various
noxious stimuli.
SP release from primary afferent neurons is a highly
complex process that often involves critical intracellular
effectors, such as ion channel (21,22).
Further investigations are required to ascertain whether one or
more substances are involved in the regulation of SP release by the
application of capsaicin.
Previously, we cloned the rat TRESK full-length cDNA
and successfully constructed the recombinant TRESK adenovirus
vector. This vector effectively upregulated TRESK mRNA and protein
levels in the DRG neurons of rat (10). Therefore, the present findings
indicate that the mass duplicate pAdTrack-CMV-TRESK may be a means
of clarifying whether any change in TRESK in DRG neurons is
involved in the progression of NP. Our results demonstrated that
the upregulation of TRESK in DRG neurons is involved in the
regulation of capsaicin-evoked SP release. It is thus possible that
TRESK is involved in the mechanism of NP.
Following our previous study (9), the present findings demonstrate that
the modulation of TRESK of DRG neurons is involved in NP;
consequently, another mechanism of NP is possible: TRESK has the
function which maintains the balance of resting potential in the
DRG neurons. When the DRG neurons accepted the signal from
peripheroceptor, such as acid or other hurt stimulation, it
immediatedly downregulates TRESK and then induces the polarization
of neurons. Additionally, the increase of the introvertive
potassium current evokes the release of SP and then delivers more
signal transmitters to the central receptor (23,24).
If the hurt stimulation cannot be relieved, acute pain changes into
NP.
By contrast, the release of SP is regulated with
calcineurin. Moreover, a strong and persistent stimulation, such as
peripheral nervous injury, clearly decreases the release of
calcineurin (25–27). Therefore, the fact that calcineurin
agonist cures chronic pain indicates that it maintains the TRESK
current to regulate the release of SP to inhibit the development of
NP.
In conclusion, we have demonstrated that the
activation of TRESK by its gene recombinant adenovirus vector
induces the enhancement of TRESK mRNA in the DRG neurons of rats,
thereby raising the protein levels of TRSK and attenuating the
capsaicin-evoked SP release from cultured DRG neurons. It is
possible that TRESK in DRG has an accommodating effect on the SP
release process. These observations provide evidence that TRESK in
DRG may be involved in the progression of NP through the
neuromodulatory actions of SP.
References
|
1
|
D KangE MariashD KimFunctional expression
of TRESK-2, a new member of the tandem-pore K+ channel
familyJ Biol
Chem2792806328070200410.1074/jbc.M40294020015123670
|
|
2
|
D KangD KimTREK-2(K2P 10.1) and TRESK(K2P
18.1) are major background K+ channels in dorsal root
ganglion neuronsAm J Physiol Cell Physiol2911381462006
|
|
3
|
C LiuJD AuHL ZouJF CottenCS YostPotent
activation of the human tandem pore domain K channel TRESK with
clinical concentrations of volatile anestheticsAnesth
Analg9917151722200410.1213/01.ANE.0000136849.07384.4415562060
|
|
4
|
B KeshavaprasadC LiuJD AuCH KindlerJF
CottenCS YostSpecies-specific differences in response to
anesthetics and other modulators by the K2P channel TRESKAnesth
Analg10110421049200510.1213/01.ane.0000168447.87557.5a16192517
|
|
5
|
M RigoniM TrevisaniD GazzieriNeurogenic
responses mediated by vanilloid receptor-1 (TRPV1) are blocked by
the high affinity antagonist, iodo-resiniferatoxinBr J
Pharmacol138977985200310.1038/sj.bjp.070511012642400
|
|
6
|
J NemethD ReglodiG PozsgaiEffect of
pituitary adenylate cyclase activating polypeptide-38 on sensory
neuropeptide release and neurogenic inflammation in rats and
miceNeuroscience143223230200610.1016/j.neuroscience.2006.07.02816938409
|
|
7
|
MS CarterJE KrauseStructure, expression
and some regulatory mechanisms of the rat preprotachykinin gene
encoding substance P, neurokinin A, neuropeptide K and neuropeptide
gammaJ Neurosci10220322141990
|
|
8
|
A Ribeiro-da-SilvaT HökfeltNeuroanatomical
localisation of substance P in the CNS and sensory
neuronsNeuropeptides34256271200010.1054/npep.2000.083411049730
|
|
9
|
J ZhouSL YaoCX YangChanges in expression
of TRESK mRNA in dorsal root ganglion in a rat model of neuropathic
painChin J Anesthesiol311831852011
|
|
10
|
J ZhouSL YaoCX YangConstruction of the
recombinant adenovirus vector of rat TRESK geneChin J
Anesthesiol312962982011
|
|
11
|
HB TangA InoueK OshitaY
NakataSensitization of vanilloid receptor 1 induced by bradykinin
via the activation of second messenger signaling cascades in rat
primary afferent neuronsEur J
Pharmacol4983743200410.1016/j.ejphar.2004.07.07615363973
|
|
12
|
P HalfonM BourlièreG PénarandaH KhiriD
OuzanReal-time PCR assays for hepatitis C virus (HCV) RNA
quantitation are adequate for clinical management of patients with
chronic HCV infectionJ Clin
Microbiol4425072511200610.1128/JCM.00163-0616825372
|
|
13
|
A InoueT HashimotoI
Hide5-Hydroxytrytamine-facilitated release of substance P from rat
spinal cord slices is mediated by nitric oxide and cyclic GMPJ
Neurochem68128133199710.1046/j.1471-4159.1997.68010128.x8978718
|
|
14
|
F VianaE de la PeñaC BelmonteSpecificity
of cold thermotransduction is determined by differential ionic
channel expressionNat Neurosci5254260200210.1038/nn80911836533
|
|
15
|
A AllouiK ZimmermannJ MametTREK-1, a
K+ channel involved in polymodal pain perceptionEMBO
J25236823762006
|
|
16
|
T DoblerA SpringaufS TovornikTRESK
two-pore-domain K+ channels constitute a significant
component of background potassium currents in murine dorsal root
ganglion neuronesJ Physiol5858678792007
|
|
17
|
TK BaumannKJ BurchielSL IngramME
MartensonResponses of adult human dorsal root ganglion neurons in
culture to capsaicin and low
pHPain653138199610.1016/0304-3959(95)00145-X8826487
|
|
18
|
TK BaumannP ChaudharyME
MartensonBackground potassium channel block and TRPV1 activation
contribute to proton depolarization of sensory neurons from humans
with neuropathic painEur J
Neurosci1913431351200410.1111/j.1460-9568.2004.03097.x15016092
|
|
19
|
YR WenMR SuterY KawasakiNerve conduction
blockade in the sciatic nerve prevents but does not reverse the
activation of p38 mitogen-activated protein kinase in spinal
microglia in the rat spared nerve injury
modelAnesthesiology107312321200710.1097/01.anes.0000270759.11086.e717667577
|
|
20
|
W GuoH WangM
WatanabeGlial-cytokine-neuronal interactions underlying the
mechanisms of persistent painJ
Neurosci2760066018200710.1523/JNEUROSCI.0176-07.200717537972
|
|
21
|
M TominagaM NumazakiT IidaRegulation
mechanisms of vanilloid receptorsNovartis Found
Symp261412200410.1002/0470869127.ch2
|
|
22
|
H NakagawaA HiuraCapsaicin, transient
receptor potential (TRP) protein subfamilies and the particular
relationship between capsaicin receptors and small primary sensory
neuronsAnat Sci Int81135155200610.1111/j.1447-073X.2006.00141.x
|
|
23
|
HS SmithArachidonic acid pathways in
nociceptionJ Support Oncol42772872006
|
|
24
|
YC WooSS ParkAR SubietaTJ BrennanChanges
in tissue, Ph and temperature after incision indicate acidosis may
contribute to postoperative
painAnesthesiology101468475200410.1097/00000542-200408000-0002915277931
|
|
25
|
G CzirjákP EnyediTargeting of calcineurin
to an NFAT-like docking site is required for the calcium-dependent
activation of the background K+ channel, TRESKJ Biol
Chem2811467714682200616569637
|
|
26
|
G CzirjákP EnyediZinc and mercury ions
distinguish TRESK from the other two-pore-domain K+
channelsMol Pharmacol6910241032200616354767
|
|
27
|
G CzirjákZE TóthP EnyediThe two-pore
domain K+ channel, TRESK, is activated by the
cytoplasmic calcium signal through calcineurinJ Biol
Chem27918550185582004
|