Introduction
Since many organisms that live under aerobic
conditions are exposed to reactive oxygen species (ROS), they have
developed a protective or defensive system against oxidative
stress, which includes the production of a variety of antioxidant
enzymes, including superoxide dismutases, catalase and glutathione
peroxidases (1–3). These enzymes are important in
detoxifying ROS, some of which are generated during cellular
respiration. In addition to these enzymes, a distinct class of
antioxidant proteins has been identified and is referred to as
peroxiredoxins (Prxs) (4–6).
Prx is a family of proteins that act on peroxides in
a thioredoxin-dependent manner (4). In mammals, this protein family
consists of six members, Prx-1 to -6 (4,7).
Their common feature is the presence of two redox-sensitive
cysteine residues in the active site, thus referred to as a 2-Cys
type, with the exception of Prx-6, which has a single catalytic
cysteine and is thus referred to as a 1-Cys type. During the
reduction of peroxides by the 2-Cys-type Prx, the sulfhydryl groups
of these two cysteines are oxidized to a disulfide linkage, which
are then reduced by thioredoxin (4). Prxs-1–4 are highly homologous in
terms of protein structure involving the two active site cysteine
residues, while Prx-5 is classified as an atypical 2-Cys Prx due to
its low homology (4,7). It is well-known that oxidative stress
is closely associated with a number of pathological processes, and
thus, the role of the Prx family has been investigated in numerous
diseases (8–12). The expression of certain members of
the Prx family are altered in a number of human malignancies
(13–18). In addition to their role in the
detoxification of peroxides, it is also considered that this family
is involved in intracellular signal transduction involving
H2O2 (4,6,19,20).
Among the Prx family members, Prx-4 uniquely
contains an N-terminal signal peptide (21). The expression of this protein is
relatively high in the testis, pancreas and liver, as demonstrated
by immunoblotting data. While the majority of the Prx family
members are present within cells, for example, in the cytosol for
Prx-1 and Prx-2 and in the mitochondria for Prx-3, Prx-4 appears to
reside in the endoplasmic reticulum (ER) and is secretable due to
the presence of a cleavable signal peptide (21,22).
Therefore, Prx-4 has the potential to be present in extracellular
fluids, such as serum. Thus, the quantification of Prx-4 may also
be useful for the clinical examination of pathological conditions
using serum samples. Although an attempt to examine the serum
levels of Prx-4 has recently been reported for sepsis in humans, a
complete quantification was not accomplished due to the
unavailability of a standard sample and the use of an arbitrary
unit which was unique to this earlier study (23). In addition, it has not yet been
fully clarified in which pathological conditions or in which types
of disease this protein is implicated, although available evidence
suggests that Prx-4 plays a role in ER stress and the oxidative
folding of proteins in the ER (24,25).
To extend the clinical usefulness of Prx-4, a more quantitative
assay is required to determine and compare Prx-4 levels in various
mammalian tissues.
In this study, we report on the preparation of a
specific polyclonal antibody by immunization of a rabbit with a
purified recombinant Prx-4, which was previously described
(26). An enzyme-linked
immunosorbent assay (ELISA) was then developed to quantitatively
determine its levels in tissues and to investigate alterations of
its levels in association with certain pathological conditions
using animal models. The findings suggest that the serum level of
Prx-4 is associated with the pathological state of the liver and
may be of potential value for monitoring oxidative stress in the
liver.
Materials and methods
Cell culture
The rat cell lines used in this study were
maintained at 37°C in RPMI-1640 (AH66 cells), Eagle’s MEM (WB-F344
and RLN-10 cells) and DMEM (RLC-16 cells), all of which were
supplemented with 5 or 10% fetal bovine serum, streptomycin and
penicillin, under an atmosphere of 5% CO2 in humidified
air.
Animals
Male Wistar rats were purchased from Japan SLC Inc.
(Hamamatsu, Japan) at the age of 6 weeks, and Long-Evans Cinnamon
(LEC) rats and Sprague-Dawley (SD) rats were obtained at the age of
18 weeks from Charles River Japan, Inc. (Yokohama, Japan). The rats
were maintained under specific pathogen-free conditions at a
constant temperature of 20–22°C with a 12-h light/dark cycle. The
Wister rats were injected with a single dose of 50 mg/kg
streptozotocin (Sigma) to induce diabetes. In the LEC rats, serum
alanine transaminase (ALT) and aspartate transaminase (AST)
activities were determined using a transaminase assay kit (Wako
Pure Chemicals) to verify the occurrence of hepatitis. Animal
handling and care were conducted under the protocol approved by the
Institutional Animal Research Committee of Saga University.
Expression and purification of
recombinant rat Prx-4
Preparation of the recombinant Prx-4 protein was
conducted as previously described (26). The recombinant protein was obtained
from the extract of the Sf21 cells that had been infected with a
recombinant baculovirus encoding a signal peptide-truncated form of
rat Prx-4. The enzyme was purified by two successive cation
exchanges and gel filtration column chromatographies, following
polyethyleneimine precipitation. As previously described (26), the recombinant protein was
enzymatically active and homogeneous.
Preparation and biotinylation of rabbit
anti-Prx-4 IgG
A total of 500 μg of the purified rat Prx-4 was
emulsified in Freund’s complete adjuvant and injected
intracutaneously into the dorsal skin of a female rabbit. Another
500 μg of protein in incomplete adjuvant were subcutaneously
injected dorsally at two-week intervals. Antiserum was obtained
three weeks after the final booster injection. The IgG fraction was
prepared from the antiserum by ammonium sulfate precipitation and
subsequent column chromatography using Protein A Sepharose Fast
Flow (GE Healthcare). Biotinylation of the antibody was conducted
using N-hydroxysuccinimide-biotin (Pierce), according to the
manufacturer’s instructions.
Electrophoresis and immunoblot
analysis
After the protein samples were separated using
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), they were electrophoretically transferred onto a
polyvinylidene fluoride membrane (Millipore). The membrane was
blocked by treatment with 1% skimmed milk in phosphate-buffered
saline (PBS) and reacted with the polyclonal rabbit antibody. After
washing, the membrane was incubated with a horseradish
peroxidase-conjugated antibody against rabbit IgG. Following
washing, the reactive protein bands were visualized using an ECL
Advance Western Blotting Detection kit (Amersham).
ELISA
The 96-well microtiter plates (Maxisorp, Nunc) were
pre-coated with rabbit polyclonal IgG against rat Prx-4. The IgG
solution was diluted in 50 mM NaHCO3 (pH 9.6) to 50
μg/ml and added to the wells. The plates were incubated overnight
at 4°C and were then blocked by 1% bovine serum albumin (BSA) after
washing with PBS. A total of 50 μl of the appropriately diluted
samples were added in duplicate to the wells of the plates,
followed by incubation at room temperature for 2 h. Biotinylated
anti-rat Prx-4 rabbit IgG was added to the plates, and the plates
were then allowed to react at room temperature for 1 h. The plates
were then incubated at room temperature for 30 min after adding
horseradish peroxidase-conjugated streptavidin to the wells. The
plates were washed five times with PBS-Tween-20 (PBS-T) after each
step. Detection was performed using a solution of 1 mg/ml
o-phenylenediamine as the substrate with 0.015%
H2O2 in 0.1 M citrate-phosphate buffer (pH
5.4). The absorbance at 490 nm was measured using a microtiter
plate reader (Bio-Rad).
Tissue sample preparation for immunoblot
analysis and ELISA
The rats were sacrificed after appropriate periods
of time, and then the tissues were obtained and stored at −80°C
until use. The tissues were homogenized with a Dounce homogenizer
in a lysis buffer of 20 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1%
(v/v) Nonidet P-40, 10% (v/v) glycerol, 1 mM phenylmethylsulfonyl
fluoride, 2 μg/ml aprotinin and 5 μg/ml leupeptin (pH 7.4)
Protein determination
Protein contents were determined using a Bradford
protein assay kit (Pierce). BSA was used as the standard.
Statistical analysis
Data were analyzed using the t-test and the results
are expressed as the means ± standard deviation (SD). P<0.05 was
considered to indicate a statistically significant difference,
unless otherwise stated.
Results
Antibody raised against native Prx-4
protein
In earlier studies, specific antibodies have been
prepared using an inactive denatured polypeptide or a synthetic
polypeptide as the antigen (21,23,27).
The use of the native form of a protein can occassionally prove
more benificial for immunization. For example, when an antibody
that effectively recognizes a native protein is desired, the latter
is recommended. Thus, the enzymatically active recombinant Prx-4
was purified and used as the antigen to immunize rabbits in this
study. The anti-Prx-4 IgG was purified from antiserum obtained from
the immunized rabbits. The specificity of the antibody was verified
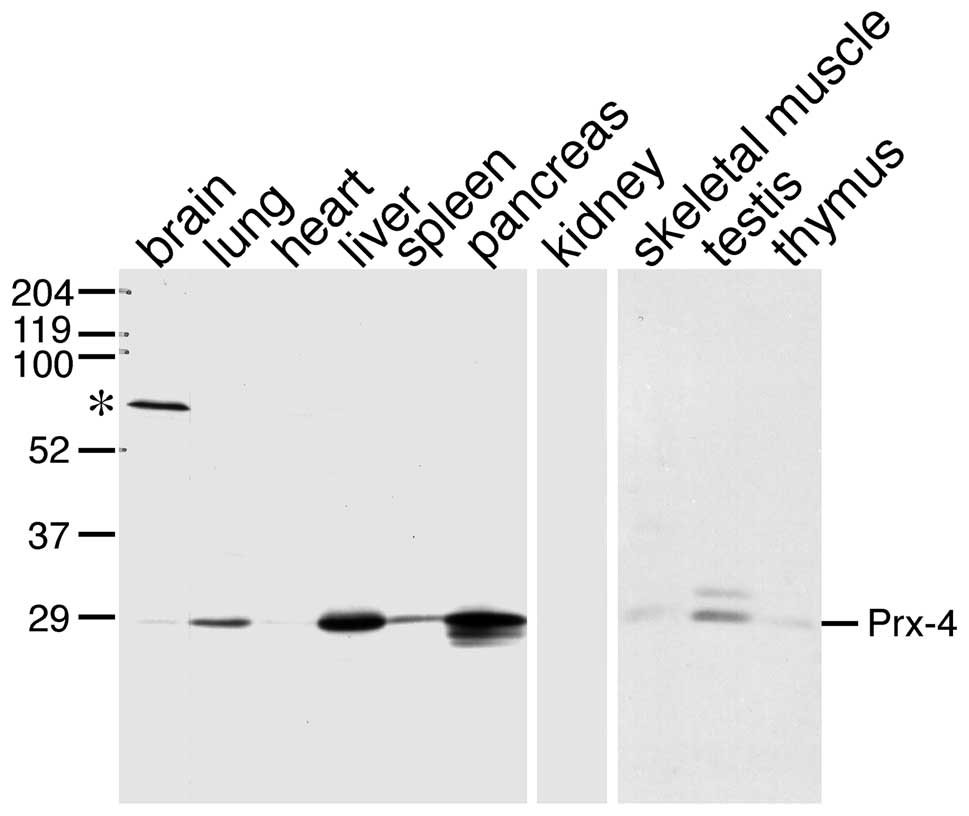
using immunoblot analysis of several rat tissues (Fig. 1). All samples examined displayed
identical protein bands corresponding to a signal peptide-processed
form, with the exception of the testis; consistent with a previous
study using a different antibody which was raised against a
bacterially produced antigen (21,27).
No other bands that could correspond to other Prx isoforms with
different molecular masses were detected, thus indicating that the
specificity of this antibody was sufficient. The results were in
accordance with data reported in a previous study, and demonstrated
that the protein levels of Prx-4 were relatively higher in the
testis, pancreas and liver.
ELISA for Prx-4 and examination of tissue
distribution
Although in a previous study, we examined tissue
distribution of Prx-4 in rats, the protein levels were roughly
estimated by immunoblot analysis (21). In order to more quantitatively
estimate the levels of Prx-4 in tissues, we developed an ELISA
using the aforementioned antibody. This ELISA involved the
immobilized antibody, the biotin-labeled antibody and
HRP-conjugated streptavidin, as described in detail in the
Materials and methods section. A typical calibration curve for the
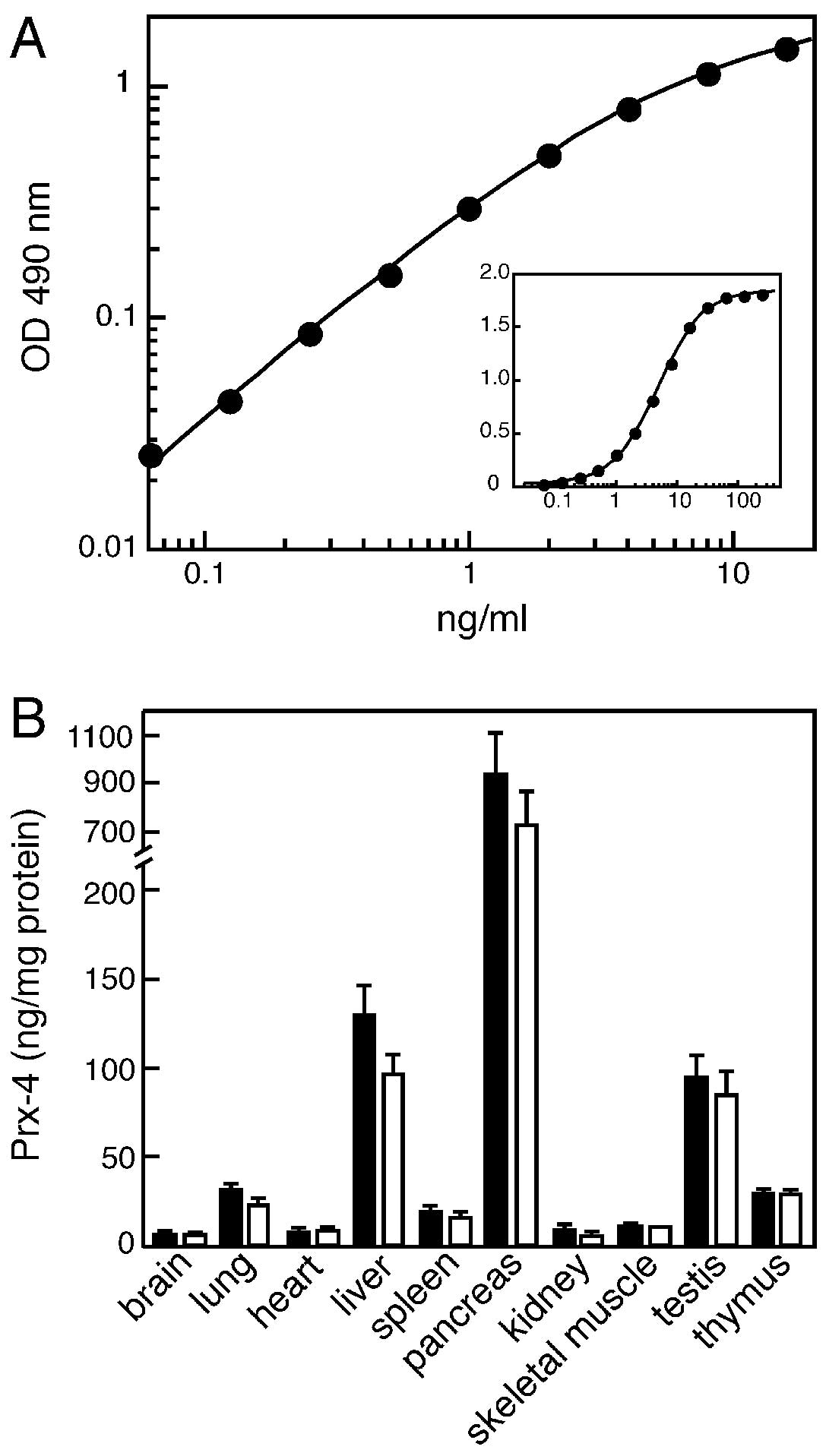
standard samples is shown in Fig.
2A. As indicated, this assay enabled us to optimally estimate
the levels in the range between 0.1 and 10 ng/ml. The re-assessment
of the tissue distribution using ELISA was essentially consistent
with the results obtained by the immunoblot analyses, as described
above, as well as in the previous study. As shown for normal
control rats in Fig. 2B, the
expression of Prx-4 was significantly higher in the pancreas, liver
and testis. The higher expression of Prx-4 in the pancreas and
liver may be associated with the possible role of this protein in
the oxidative folding of secretory proteins in the ER.
Prx-4 levels in the tissues of
streptozotocin-induced diabetic rats
The issue of whether or not Prx-4 is implicated in
diseases of the pancreas and liver has not yet been fully
investigated; thus we examined the levels of Prx-4 in certain
pathological states using animal models. Eight-week-old Wistar rats
were treated with streptozotocin to induce severe diabetes. The
occurrence of the disease was verified by high blood sugar levels
and the production of urinary sugar. Measurements of blood sugar
indicated levels of 130±35 and 440±67 mg/dl for the control rats
and streptozotocin-treated rats, respectively (P<0.01, t-test;
n=3 for each group). The protein levels of Prx-4 were determined by
ELISA in various tissues of the control and diabetic rats. As shown
in Fig. 2B, although a slight
decrease in the Prx-4 level was observed in the liver and pancreas,
there was no statistically significant difference in all tissues
examined. The mean serum level values of the proteins were
estimated to be 1.7 and 2.2 ng/ml in normal and diabetic rats,
respectively. However, a statistical analysis indicated no
significant difference despite a tendency toward an increase caused
by the streptozotocin treatment.
Alteration of Prx-4 levels in hepatic
disease model rats
Another animal model, which has been used for liver
disease, is the LEC rats (28).
LEC rats are frequently used as a Wilson’s disease animal model for
which a genetic defect was found in the copper transporter ATPase
gene, Atp7b (29,30). The rats exhibit abnormal copper
accumulation in the liver, and suffer from the development of
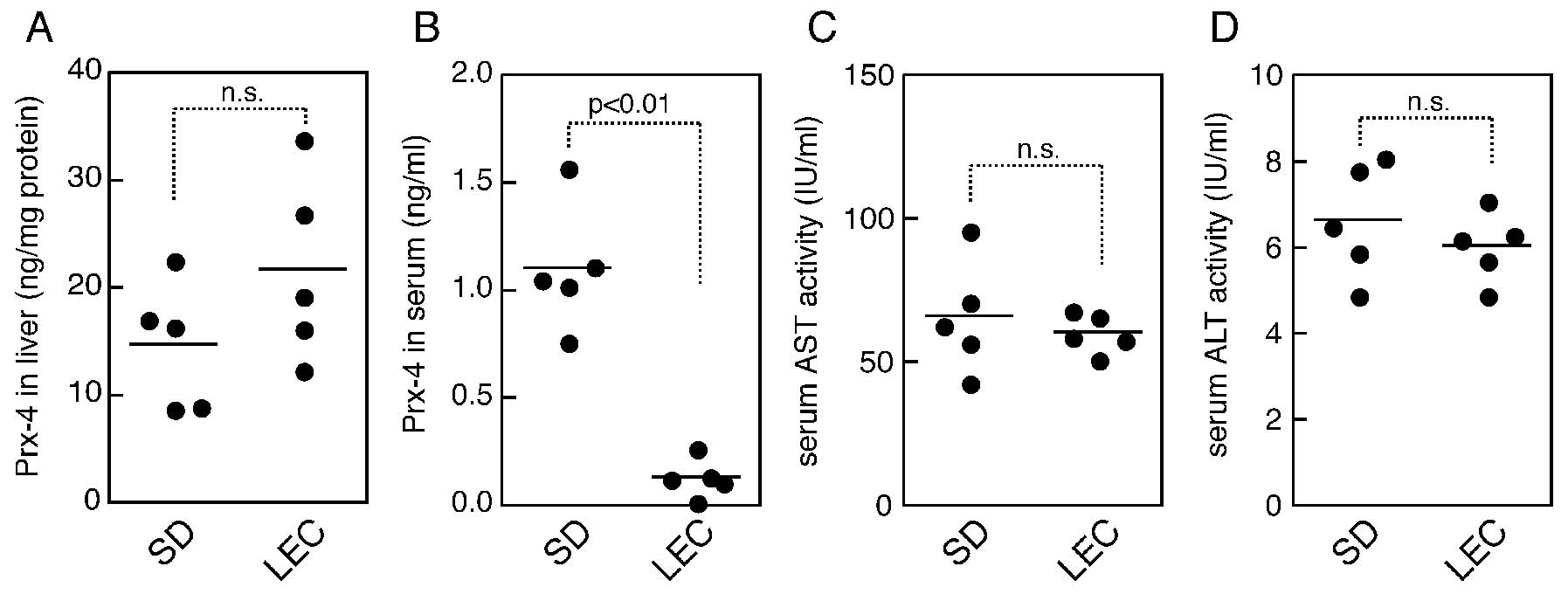
hepatitis and hepatocarcinogenesis (31). Prx-4 levels were determined in the
livers and sera of 24-week-old LEC rats. SD rats of the same age
were used as the healthy controls. The LEC rats appeared to be
healthy in this study, and did not appear to suffer from hepatitis,
as also indicated by ALT and AST assays (Fig. 3C and D). When the serum levels of
Prx-4 were determined, however, the data indicated that the levels
in the LEC rats were significantly less than those in the normal
rats (Fig. 3B). On the other hand,
in contrast to the serum levels, LEC rats demonstrated a trend
toward a higher level in the liver, compared to the controls,
although no statistically significant difference was observed for
Prx-4 levels in the liver (Fig.
3A).
Expression of Prx-4 in rat hepatic cell
lines and effects of redox stress on secretion from cells
As suggested by the lower levels of serum Prx-4 in
LEC rats, it appeared likely that the redox state affects the
release of Prx-4 from the cells since it is well-known that an
abnormal accumulation of hepatic copper causes oxidative stress in
LEC rats (32,33). Rat hepatic cell lines were used in
order to assess whether oxidative stress alters the secretion of
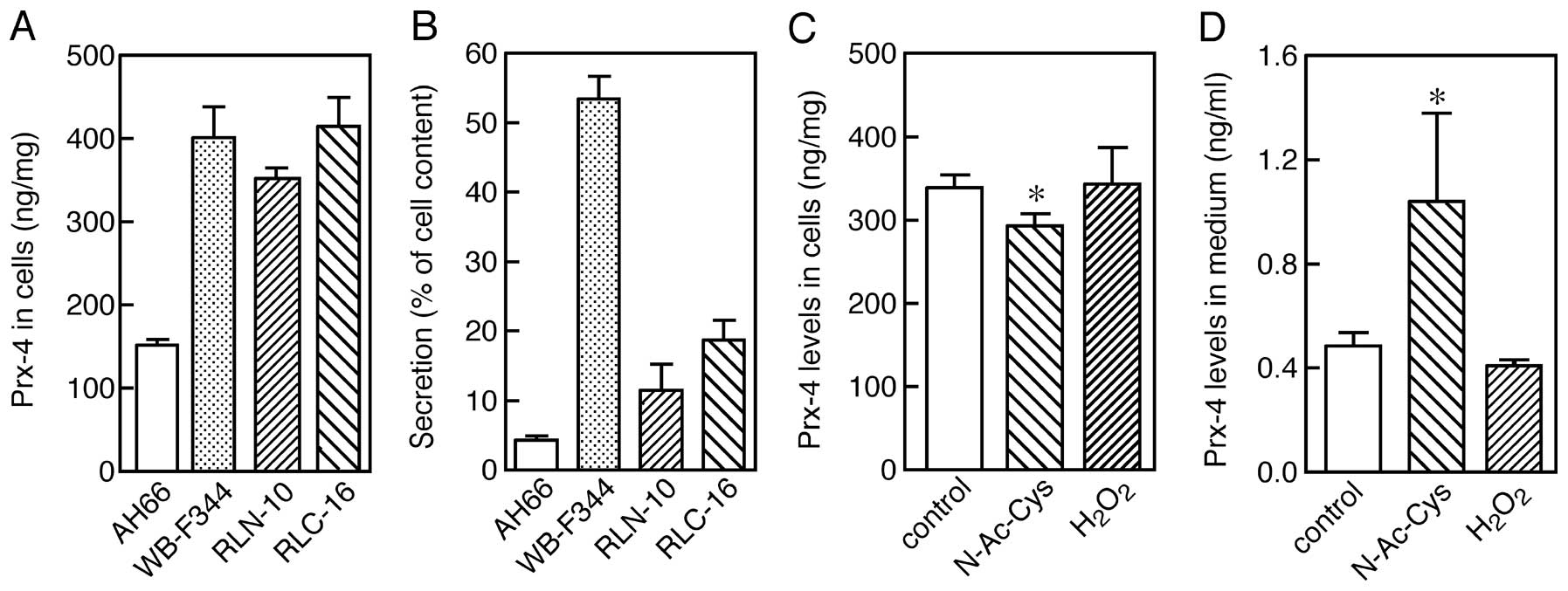
Prx-4. Four cell lines, AH66, WB-F344, RLN-10 and RLC-16, were
cultured for three days, and the Prx-4 levels in the cells and
medium were then separately determined by ELISA. The expression of
Prx-4 was observed in all cell lines examined (Fig. 4A). However, the levels of the Prx-4
protein varied in the medium, regardless of the contents associated
with the cells, suggesting that the efficiency of secretion varies
among the cell lines (Fig. 4B).
Only a single protein band, in which the signal peptide was cleaved
off, was observed for all cell lines in the immunoblot analyses
(data not shown), suggesting that variations in the extent of
secretion are not due to differences in the processing capability
of the cells. The difference in secretion appeared to be due to the
status or character of the cells rather than to a factor intrinsic
to the protein. As observed in RLN-10 cells, treatment with 5 mM
N-acetylcysteine for 24 h significantly increased the extent of
secretion into the medium, accompanied by a corresponding decrease
in the cells. However, the presence of 0.05 mM
H2O2 did not greatly suppress the secretion
of Prx-4 24 h after addition. This discrepancy between the
experiments using the reducing and the oxidizing agent may be
explained by more intensive oxidative stress in the cells cultured
under the condition of 20% O2 than in the tissues where
a partial pressure of O2 is expected to be lower
(34–36). These results suggest that the
release of Prx-4 from cells is, at least in part, affected by the
redox state of the cells.
Discussion
In this study, we prepared a polyclonal antibody
against rat Prx-4 using a purified recombinant protein as the
antigen, and subsequently developed an immunoassay for
quantitatively determining the levels of the Prx-4 protein. An
enzymatic activity assay for thioredoxin-dependent peroxidase
cannot be used to specifically evaluate the tissue levels of Prx-4
as the peroxidase activities of the mammalian Prx family are
relatively weak; much less than other typical peroxidases, such as
glutathione peroxidase. In addition, the enzymatic activity assay
does not permit the measurement of each individual member of the
Prx family. Therefore, an isoform-specific immunoassay would be
useful and desirable for such a quantification in investigations of
the role of Prx-4 in various diseases.
The development of the ELISA for Prx-4 confirmed
high levels of expression in the pancreas, liver and testis of
rats. Considering tissue mass, the total content of Prx-4 is
highest in the liver, and thus it is more likely that the supply of
the protein to the blood largely depends on the liver. As
previously described, Prx-4 appears to be highly expressed in
exocrine pancreas rather than islets (37), and another study also reported that
another Prx isoform is highly expressed in pancreatic islet cells
(38). Consistent with these
findings, it would be reasonable that no significant alteration of
Prx-4 levels in the serum would be observed in the experiments
using streptozotocin-induced diabetic rats.
LEC rats were initially identified as a mutant rat
with spontaneous hepatitis and hepatoma, and thereafter were
considered as a Wilson’s disease model. In the liver, Prx-4 levels
were slightly higher in the LEC rats compared with the control SD
rats; however, no statistically significant difference was found,
while this trend was inverse to the levels of serum Prx-4. Thus, it
appears that the release of Prx-4 from the liver is suppressed in
LEC rats. When the LEC rats were used in experiments, they did not
display any symptoms of hepatitis, i.e., no apparent jaundice and
no increase in serum transaminase activities (Fig. 3C and D), indicating that the lower
levels of serum Prx-4 were not directly associated with
inflammation. As suggested by in vitro studies using
cultured cells, however, it appears more likely that the secretion
of Prx-4 from the cells can be regulated or affected by the redox
state. Therefore, even very mild oxidative stress, which is so weak
that it does not cause hepatitis, might potentially affect the
secretion of Prx-4 into the blood.
The findings from this study suggest that the serum
levels of Prx-4 are associated with the redox state of the liver,
and therefore it is possible that this protein may be utilized as a
biomarker for oxidative stress in the liver. The application of the
anti-rat Prx-4 polyclonal antibody to human cell lines demonstrated
no apparent reactivity (data not shown). The clinical utility of
diagnosing human liver diseases will await optimizing the ELISA
procedures for use in conjunction with human samples.
References
|
1
|
Halliwell B and Gutteridge JMC: Free
Radicals in Biology and Medicine. 4th edition. Oxford University
Press; Oxford, United Kingdom: 2007
|
|
2
|
Fridovich I: Oxygen toxicity: a radical
explanation. J Exp Biol. 201:1203–1209. 1998.PubMed/NCBI
|
|
3
|
Papa S and Skulachev VP: Reactive oxygen
species, mitochondria, apoptosis and aging. Mol Cell Biochem.
174:305–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rhee SG, Kang SW, Chang TS, Jeong W and
Kim K: Peroxiredoxin, a novel family of peroxidases. IUBMB Life.
52:35–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rhee SG, Yang KS, Kang SW, Woo HA and
Chang TS: Controlled elimination of intracellular
H2O2: regulation of peroxiredoxin, catalase,
and glutathione peroxidase via post-translational modification.
Antioxid Redox Signal. 7:619–626. 2005.PubMed/NCBI
|
|
6
|
Rhee SG, Chae HZ and Kim K:
Peroxiredoxins: a historical overview and speculative preview of
novel mechanisms and emerging concepts in cell signaling. Free
Radic Biol Med. 38:1543–5152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujii J and Ikeda Y: Advances in our
understanding of peroxiredoxin, a multifunctional, mammalian redox
protein. Redox Rep. 7:123–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang XZ, Li DQ, Hou YF, Wu J, Lu JS, Di
GH, Jin W, Ou ZL, Shen ZZ and Shao ZM: Identification of the
functional role of peroxiredoxin 6 in the progression of breast
cancer. Breast Cancer Res. 9:R762007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iraqui I, Faye G, Ragu S, Masurel-Heneman
A, Kolodner RD and Huang ME: Human peroxiredoxin PrxI is an
orthologue of yeast Tsa1, capable of suppressing genome instability
in Saccharomyces cerevisiae. Cancer Res. 68:1055–1063. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song IS, Kim SU, Oh NS, Kim J, Yu DY,
Huang SM, Kim JM, Lee DS and Kim NS: Peroxiredoxin I contributes to
TRAIL resistance through suppression of redox-sensitive caspase
activation in human hepatoma cells. Carcinogenesis. 30:1106–1114.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Neumann CA, Krause DS, Carman CV, Das S,
Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH and Van
Etten RA: Essential role for the peroxiredoxin Prdx1 in erythrocyte
antioxidant defence and tumour suppression. Nature. 424:561–565.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang J, Nakamura T, Cho DH, Gu Z and
Lipton SA: S-nitrosylation of peroxiredoxin 2 promotes oxidative
stress-induced neuronal cell death in Parkinson’s disease. Proc
Natl Acad Sci USA. 104:18742–18747. 2007.PubMed/NCBI
|
|
13
|
Cha MK, Suh KH and Kim IH: Overexpression
of peroxiredoxin I and thioredoxin1 in human breast carcinoma. J
Exp Clin Cancer Res. 28:932009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karihtala P, Mantyniemi A, Kang SW,
Kinnula VL and Soini Y: Peroxiredoxins in breast carcinoma. Clin
Cancer Res. 9:3418–3424. 2003.PubMed/NCBI
|
|
15
|
Quan C, Cha EJ, Lee HL, Han KH, Lee KM and
Kim WJ: Enhanced expression of peroxiredoxin I and VI correlates
with development, recurrence and progression of human bladder
cancer. J Urol. 175:1512–1516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lehtonen ST, Svensk AM, Soini Y, Paakko P,
Hirvikoski P, Kang SW, Saily M and Kinnula VL: Peroxiredoxins, a
novel protein family in lung cancer. Int J Cancer. 111:514–521.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yanagawa T, Iwasa S, Ishii T, Tabuchi K,
Yusa H, Onizawa K, Omura K, Harada H, Suzuki H and Yoshida H:
Peroxiredoxin I expression in oral cancer: a potential new tumor
marker. Cancer Lett. 156:27–35. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang JW, Jeon HB, Lee JH, Yoo JS, Chun
JS, Kim JH and Yoo YJ: Augmented expression of peroxiredoxin I in
lung cancer. Biochem Biophys Res Commun. 289:507–512. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rhee SG, Kang SW, Jeong W, Chang TS, Yang
KS and Woo HA: Intracellular messenger function of hydrogen
peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol.
17:183–189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rhee SG: Cell signaling.
H2O2, a necessary evil for cell signaling.
Science. 312:1882–1883. 2006.PubMed/NCBI
|
|
21
|
Okado-Matsumoto A, Matsumoto A, Fujii J
and Taniguchi N: Peroxiredoxin IV is a secretable protein with
heparin-binding properties under reduced conditions. J Biochem.
127:493–501. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tavender TJ, Sheppard AM and Bulleid NJ:
Peroxiredoxin IV is an endoplasmic reticulum-localized enzyme
forming oligomeric complexes in human cells. Biochem J.
411:191–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schulte J, Struck J, Bergmann A and Kohrle
J: Immunoluminometric assay for quantification of peroxiredoxin 4
in human serum. Clin Chim Acta. 411:1258–1263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tavender TJ and Bulleid NJ: Peroxiredoxin
IV protects cells from oxidative stress by removing
H2O2 produced during disulphide formation. J
Cell Sci. 123:2672–2679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bertolotti M, Yim SH, Garcia-Manteiga JM,
Masciarelli S, Kim YJ, Kang MH, Iuchi Y, Fujii J, Vene R,
Rubartelli A, Rhee SG and Sitia R: B- to plasma-cell terminal
differentiation entails oxidative stress and profound reshaping of
the antioxidant responses. Antioxid Redox Signal. 13:1133–1144.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ikeda Y, Ito R, Ihara H, Okada T and Fujii
J: Expression of N-terminally truncated forms of rat
peroxiredoxin-4 in insect cells. Protein Expr Purif. 72:1–7. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsumoto A, Okado A, Fujii T, Fujii J,
Egashira M, Niikawa N and Taniguchi N: Cloning of the peroxiredoxin
gene family in rats and characterization of the fourth member. FEBS
Lett. 443:246–250. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoshida MC, Masuda R, Sasaki M, Takeichi
N, Kobayashi H, Dempo K and Mori M: New mutation causing hereditary
hepatitis in the laboratory rat. J Hered. 78:361–365.
1987.PubMed/NCBI
|
|
29
|
Li Y, Togashi Y, Sato S, Emoto T, Kang JH,
Takeichi N, Kobayashi H, Kojima Y, Une Y and Uchino J: Spontaneous
hepatic copper accumulation in Long-Evans Cinnamon rats with
hereditary hepatitis. A model of Wilson’s disease. J Clin Invest.
87:1858–1861. 1991.PubMed/NCBI
|
|
30
|
Wu J, Forbes JR, Chen HS and Cox DW: The
LEC rat has a deletion in the copper transporting ATPase gene
homologous to the Wilson disease gene. Nat Genet. 7:541–545. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Masuda R, Yoshida MC, Sasaki M, Dempo K
and Mori M: High susceptibility to hepatocellular carcinoma
development in LEC rats with hereditary hepatitis. Jpn J Cancer
Res. 79:828–835. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marquez-Quinones A, Cipak A, Zarkovic K,
Fattel-Fazenda S, Villa-Trevino S, Waeg G, Zarkovic N and Gueraud
F: HNE-protein adducts formation in different pre-carcinogenic
stages of hepatitis in LEC rats. Free Radic Res. 44:119–127. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samuele A, Mangiagalli A, Armentero MT,
Fancellu R, Bazzini E, Vairetti M, Ferrigno A, Richelmi P, Nappi G
and Blandini F: Oxidative stress and pro-apoptotic conditions in a
rodent model of Wilson’s disease. Biochim Biophys Acta.
1741:325–330. 2005.PubMed/NCBI
|
|
34
|
Madhani M, Barchowsky A, Klei L, Ross CR,
Jackson SK, Swartz HM and James PE: Antibacterial peptide PR-39
affects local nitric oxide and preserves tissue oxygenation in the
liver during septic shock. Biochim Biophys Acta. 1588:232–240.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sato H, Shiiya A, Kimata M, Maebara K,
Tamba M, Sakakura Y, Makino N, Sugiyama F, Yagami K, Moriguchi T,
Takahashi S and Bannai S: Redox imbalance in cystine/glutamate
transporter-deficient mice. J Biol Chem. 280:37423–37429. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khan N, Williams BB, Hou H, Li H and
Swartz HM: Repetitive tissue pO2 measurements by
electron paramagnetic resonance oximetry: current status and future
potential for experimental and clinical studies. Antioxid Redox
Signal. 9:1169–1182. 2007.
|
|
37
|
Nagaoka Y, Iuchi Y, Ikeda Y and Fujii J:
Glutathione reductase is expressed at high levels in pancreatic
islet cells. Redox Rep. 9:321–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fujii T, Fujii J and Taniguchi N:
Augmented expression of peroxiredoxin VI in rat lung and kidney
after birth implies an antioxidative role. Eur J Biochem.
268:218–225. 2001. View Article : Google Scholar : PubMed/NCBI
|