Introduction
Recent studies have identified autophagy, originally
observed in yeast, as a highly evolutionarily conserved mechanism
universally present in all eukaryotic cells (1). Certain overaggregations of
intracellular proteins, organelles and pathogens are too large to
be effectively degraded by the proteasome. At present, autophagy is
the only known cell biological pathway able to eliminate these
aggregates in order to maintain normal cell function. Functional
deficit of autophagy contributes to a variety of diseases,
including cancer, neurodegeneration and infectious diseases
(2). The LC3 protein is an
autophagosomal membrane marker that is usually dispersed throughout
the cytoplasm. The cytoplasmic form of LC3 (LC3-I) is conjugated to
phosphatidylethanolamine to produce the
LC3-phosphatidylethanolamine conjugate (LC3-II), which is recruited
to autophagosomal membranes under stimulation of starvation, lack
of growth factors or immune factors, and certain intracellular
pathogens, and is involved in the formation of autophagosomes.
Currently, as LC3-II is involved in the entire
formation process of autophagosomal membranes, aggregation of
GFP-LC3 fusion proteins in the autophagosome observed under
fluorescence microscopy has become the most widely used method to
evaluate the occurrence of autophagy (3). One of the effective mechanisms that
macrophages, important immune cells, use to kill intracellular
pathogens, including Mycobacterium tuberculosis, depends on
the autophagy pathway, which performs an immune defense role
(4). Owing to the insufficient
transfection rate of liposome-transfected cells, in this study, we
screened macrophage cell lines with stable expression of the
GFP-LC3 protein, which may aid in basic investigations examining
the autophagic function of macrophages or act as the cellular
platform for drug screening.
Materials and methods
Plasmids
The pEGFP-C1 plasmids and DH5α bacterial strains
were gifts from Professor Xinbing Yu (Zhongshan School of Medicine,
Sun Yat-Sen University, China). As in previous studies (5–7),
cDNA encoding the LC3 protein was obtained by RT-PCR amplification
using the specific primers (sense, ttactcgagatatgccctccgaccggcctttc
and anti-sense, accg gatcctcagaagccgaaggtttcttg). The PCR products
encoding the LC3 protein were digested using XhoI and
BamHI restriction endonucleases (Fermentas, Canada). The
products were then inserted into the corresponding sites of the
pEGFP-C1 plasmid digested with the same restriction enzymes in
order to construct the pEGFP-LC3 plasmid. The plasmids were then
transformed into DH5α bacterial strains to screen for the
recombinant plasmid. These recombinants were identified by DNA
sequencing. Plasmid DNA was prepared and purified using a plasmid
maxi kit (Tiangen Biochemical Technology Ltd., Co., Beijing,
China), according to the manufacturer’s instructions, suspended in
endotoxin-free physiologic saline, and stored at −20°C until
use.
Cell transfection and stable cell line
screening
Transient transfections were performed using
Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA).
RAW264.7 cells were cultured in DMEM medium (Gibco) containing 10%
fetal bovine serum (FBS) and antibiotics in 5% CO2 at
37°C. The growth rate of RAW264.7 cells was then observed under an
inverted microscope. When the confluence reached 85%, the cells
were plated in 6-well plates and supplemented with 2 μg
plasmid per well [plasmid (μg): Lipofectamine 2000
(μl) = 1:3]. Twenty-four hours after transfection, cells
were digested with 0.25% trypsin and the cultures were transferred
to the plates for further culture with DMEM medium containing 600
mg/l G418 and 10% FBS for 10 days. When the amount of resistant
cell clones was observed, they were digested with 0.25% trypsin and
then transferred to a new culture flask using an aseptic pipette
for further culture. Subsequently, the pEGFP-LC3 recombinant
plasmids or pEGFP empty plasmids and pEGFP-LC3 recombinant
bacterium liquid PCR products were cut using specific enzymes. The
fragments were then subjected to agarose gel electrophoresis.
Autophagy induction and suppression
When stable cell growth was observed, DMEM medium
was blotted and cells were washed three times with PBS at 37°C. The
cells were then incubated in Earle’s balanced salts solution
(starve group) for 1 h at 37°C, or were simultaneously added to
wortmannin at a final concentration of 50 nM (starve+wort group).
After 1 h, at least 200 GFP-positive cells were selected for
observation under an inverted fluorescence microscope (x400) in
order to count the number of GFP-LC3 punctas. Under fluorescence
microscopy, several bright green fluorescent punctas were observed
in the cells. One puncta was regarded as equal to one
autophagosome. The results are presented as the average number of
punctas per cell.
Statistical analysis
Data were presented as the mean ± SD. ANOVA was used
to compare the means of more than two samples. P<0.05 was
considered statistically significant.
Results
Successful construction of pEGFP-LC3
vector
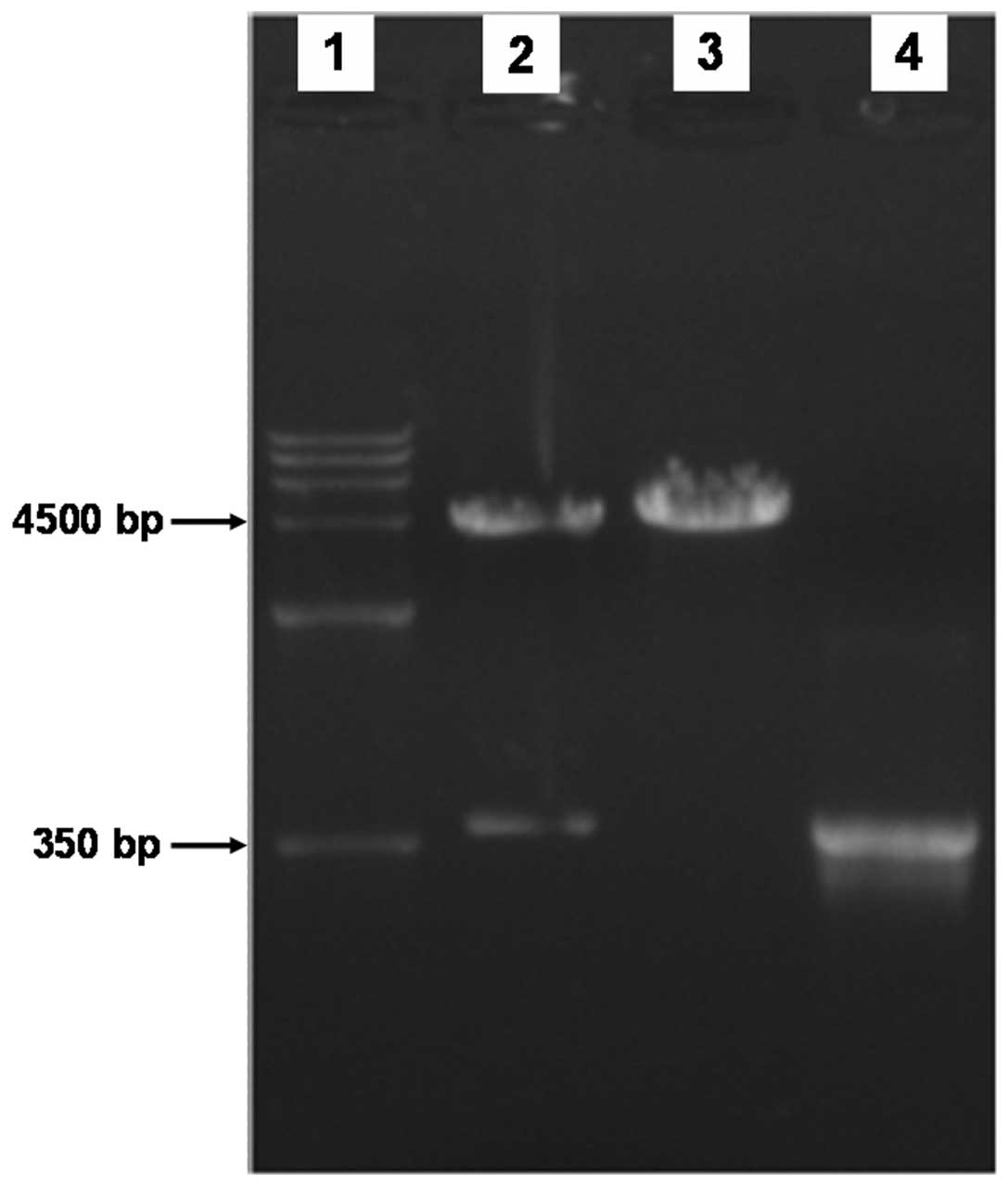
The open reading frame of the cDNA coding for the
mouse LC3 protein was 366 bp. The 366-bp cDNA products were
obtained by RT-PCR amplification using mouse cDNA from
transcriptase as templates with specific primers, and then the
recombinant plasmid was constructed. The 366-bp product was also
amplified by PCR using positive bacterium liquid as a template. To
determine whether the pEGFP-LC3 vectors were constructed
successfully, the recombinant plasimds were cut into fragments by
restriction enzymes, and agarose gel electrophoresis was performed.
We identified bands of the target gene at 366 bp (Fig. 1). Additionally, the sequencing
results revealed that the recombinant gene sequences were
completely matched.
Successful screening of RAW264.7 cell
lines with stable expression of GFP-LC3
After the RAW264.7 cell lines screened by G418 were
amplified and cultured, we found that the cell clones were able to
stably express the GFP-LC3 fusion protein. Upon stimulation of
starvation or lack of growth factor, the LC3 originally distributed
in the cytoplasm was conjugated to the lipid
phosphatidylethanolamine, forming LC3-II (the lipidated form).
LC3-II was then recruited to the outer and inner membranes at each
stage of autophagosome formation, and punctate aggregation
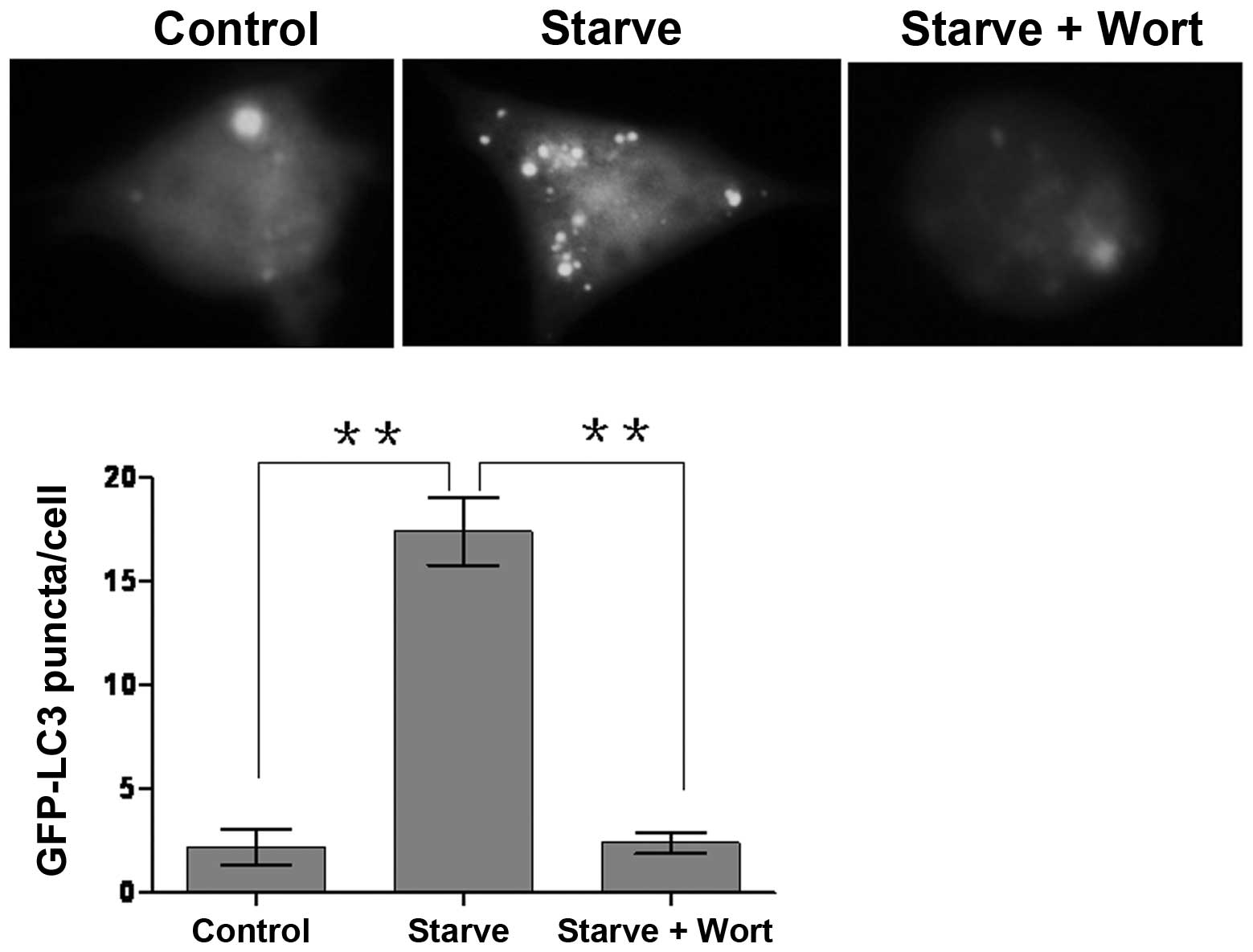
occurred. In this study, we observed GFP-LC3 punctate structures in
stably transfected cell lines by fluorescence microscopy. GFP-LC3
punctas of the majority of cells were homogeneously distributed in
the cytoplasm in complete medium supplemented with FBS. Autophagic
punctas were significantly increased in Earle’s balanced salts
solution, suggesting that treatment with starvation is able to
effectively induce the formation of autophagosomes (Fig. 2). However, upon wortmannin
treatment, the number of autophagosomes was evidently reduced,
indicating that wortmannin is capable of suppressing autophagosome
formation.
Discussion
In the recent years, the number of studies
investigating autophagy in several areas, including cancer, immune
factors, infection, inflammation and neurodegeneration, has been on
the increase (2). Autophagy is a
complex, dynamic process involved in the initation of autophagy,
elongation and formation of the autophagosome, and
autophagosome-lysosomal fusion (8,9).
Accurate detection of autophagy is important for the study of the
biological functions of autophagy. Currently, although over 30
autophagy-related proteins (Atgs) have been identified, only LC3,
the mammalian counterpart of yeast Atg8, is able to act as a marker
of an autophagosome (3). LC3
occurs at all phases of autophagosome membrane formation, and
therefore may be able to demonstrate the dynamic process of
autophagosome formation.
LC3 undergoes two important changes in the process
of autophagosome formation. The first is lipidation of the
originally free LC3, which forms LC3-II bound to
phosphatidylethanolamine (PE). The other is the translocation of
cytoplasmic LC3 distribution, which is localized in the
autophagosome membrane. According to the characteristics of the
conversion of LC3 to the lipidated LC3-II and the differences in
mobility between LC3 and LC3-II in polyacrylamide gel
electrophoresis (PAGE), we were able to detect the ratio of
LC3/LC3-II by western blot analysis in order to indirectly reflect
the formation of autophagosomes. When the formation of
autophagosomes increased, the ratio of LC3/LC3-II was reduced;
inversely, the ratio of LC3/LC-II increased (3,10).
When the LC3 protein was combined with fluorescent
tags, we were able to observe the formation of autophagosomes using
autophagy puncta formation experiments based on the characteristics
of LC3 attached to the autophagosome membrane. We then counted the
number of fluorescent punctas per cell in the RAW264.7 cells and
performed statistical quantitative analysis. In this study, LC3
proteins were combined with GFP tags. After the recombinant
plasmids were successfully transfected into RAW264.7 cells, which
had increased the expression of recombinant plasmids in 24–48 h, we
counted the number of autophagosome punctas in the cells. However,
cells instantaneously transfected with liposomes had two
shortcomings; unstable efficiency of transfection leading to a
reduction in cells effectively expressing GFP-LC3, and degradation
of the recombinant plasmid resulting in a reduction in GFP-LC3
autophagy punctas. Therefore, we screened the RAW264.7 cell lines
stably expressing GFP-LC3 using G418. The screened cell lines
expressed the GFP-LC3 protein for an extended period of time.
Following the starvation stimuli, the GFP-LC3 proteins, which
originally homogeneously existed in the cytoplasm, aggregated to
form bright autophagy puncta, suggesting that the cell line is
useful in studies regarding the formation of autophagosomes.
Of note is that due to the dynamic process of
autophagy, the number of autophagosomes at certain times should be
the balanced result of autophagosome formation and conversion.
Aggregation of the autophagosome is likely to reflect the induction
of autophagy and the consequence of blockage of the downstream
autophagy pathway. Therefore, it is insufficient to adequately
represent the activity of autophagy using one or two methods
(3). According to the complex
characteristics of dynamic autophagy, it may be more accurate to
evaluate autophagy function using autophagy flux; the increase in
autophagy flux represents the enhancement of autophagy activity.
Conversely, autophagy activity is reduced (3,10,11).
In conclusion, in this study, the stable cell lines provided a more
reliable cell platform for detecting marked autophagy flux.
Acknowledgements
This study was supported by the Anhui Provincial
Natural Science Foundation (No. 1208085QH162), AUST Grants (Dong Hu
and Jing Wu), the Colleges and Universities Education Grant of
Anhui Province (no. 2008jp1042) and the National Natural Science
Foundation Grants of China (no. 81041083 and no. 81172778).
References
|
1
|
Levine B, Mizushima N and Virgin HW:
Autophagy in immunity and inflammation. Nature. 469:323–335. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deretic V, Delgado M, Vergne I, Master S,
De Haro S, Ponpuak M and Singh S: Autophagy in immunity against
Mycobacterium tuberculosis: a model system to dissect
immunological roles of autophagy. Curr Top Microbiol Immunol.
335:169–188. 2009.
|
|
5
|
Hu D, Wu J, Hu F, Yang Y, Liang C, Chen J,
Wang L, Wang P, Wang X, Xu J, Hu X and Yu X: Stage and tissue
specific differences in SjBMI1, a Polycomb protein in
Schistosoma japonicum. Parasitol Res. 106:677–682. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu J, Hu D, Yang G, Zhou J, Yang C, Gao Y
and Zhu Z: Down-regulation of BMI-1 cooperates with artemisinin on
growth inhibition of nasopharyngeal carcinoma cells. J Cell
Biochem. 112:1938–1948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu D, Wu J, Tang X, Hu F, Yang Y, Du J, Ye
S and Zhang R: Molecular cloning and tissue distribution of a
Schistosoma japonicum gene encoding AMY-1. Mol Med Rep.
4:1267–1271. 2011.PubMed/NCBI
|
|
8
|
Tang H, Da L, Mao Y, Li Y, Li D, Xu Z, Li
F, Wang Y, Tiollais P, Li T and Zhao M: Hepatitis B virus X protein
sensitizes cells to starvation-induced autophagy via up-regulation
of beclin 1 expression. Hepatology. 49:60–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Virgin HW and Levine B: Autophagy genes in
immunity. Nat Immunol. 10:461–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rubinsztein DC, Cuervo AM, Ravikumar B,
Sarkar S, Korolchuk V, Kaushik S and Klionsky DJ: In search of an
‘autophagomometer’. Autophagy. 5:585–589. 2009.
|
|
11
|
Ganley IG, Wong PM, Gammoh N and Jiang X:
Distinct autophagosomal-lysosomal fusion mechanism revealed by
thapsigargin-induced autophagy arrest. Mol Cell. 42:731–743. 2011.
View Article : Google Scholar : PubMed/NCBI
|