Introduction
Transplantation of autogenous or allogenous
chondrocytes has been used to repair defective cartilage. However,
the limited sources for a sufficient number of chondrocytes
restricts the development and clinical application of
chondrocyte-based therapies (1).
Therefore, finding an effective method to collect ‘target cells’ is
crucial.
Increasing evidence has shown that an extract
harvested from one type of differentiated somatic cell is capable
of altering another cell phenotype in vitro, a method of
reprogramming that has developed in recent years (2–5).
This method was first introduced by Hakelien et al (2). Those authors found that 293T cells
permeabilized with streptolysin O (SLO) exposed to a T-cell extract
were capable of adopting T cell-specific properties, such as
expressing T cell-specific receptors and assembling the
interleukin-2 receptor. Supported by a number of studies, use of
this method suggests that altering cell fate using cell extract is
feasible and effective, and may be applied to multiple cell
types.
The most common cells used as ‘donor cells’, which
receive another cell extract and differentiate into ‘target cells’,
are fibroblasts and adult stem cells, as they can be harvested
easily and exhibit marked proliferative capacity. Compared with
fibroblasts, adult stem cells have a broad prospect of application
as a result of their potential of multi-directional differentiation
into cells including adipocytes, osteoblasts and chondrocytes in
response to lineage-specific induction factors (6,7).
Bone marrow-derived mesenchymal stem cells (BM-MSCs), widely used
in regenerative medicine, have also been successfully induced into
chondrocytes by the induction of growth factors (8,9),
co-culturing with chondrocytes (10,11)
or low-intensity ultrasound (12).
The aim of this study was to observe whether
chondrocyte extract induced the differentiation of BM-MSCs into
chondrocytes in a monolayer culture. Moreover, we investigated
whether the maintenance time of phenotypic alteration could be
prolonged and whether cells were able to produce cartilage-specific
extracellular matrix (ECM) components when cultured at a high
density.
Materials and methods
Animals
All animal procedures in this study were approved by
Shanghai Jiaotong University Medical Center, Institutional Animal
Care and Use Committee. The animals were maintained in a specific
pathogen-free environment. The surgical procedures were performed
under aseptic conditions.
BM-MSC preparation
Swine bone marrow was obtained from the posterior
superior iliac crest of newborn pigs. Briefly, after washing twice
in phosphate-buffered saline (PBS; Gibco-BRL, Grand Island, NY,
USA) to remove red cell fragments, cells were resuspended in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco) with low glucose
plus 10% fetal bovine serum (FBS; Gibco) and plated in 100
mm2 culture dishes at a density of 2.0×105
cells/cm2 at 37°C with 5% CO2. Non-adherent
cells were removed by medium change after 5 days. The adherent
cells were collected when >80% confluence was achieved and then
subcultured. BM-MSCs at passage 2 were used for subsequent
experiments.
Chondrocyte isolation and culture
The swine articular cartilage was cut into 2×2 mm
slices and washed twice with PBS. After being digested with 0.25%
trypsin plus 0.02% EDTA at 37°C for 30 min, the cartilage slices
were further digested with 0.1% collagenase II in serum-free DMEM
at 37°C for 12–16 h. Chondrocytes were then harvested, counted and
seeded into culture dishes at a cell density of
2.5×104/cm2 for the culture and subculture in
DMEM with high glucose and 10% FBS. Chondrocytes at passage 1 were
used in this study.
Chondrocyte extract
After being harvested, chondrocytes at passage 1
were washed in Ca2+- and Mg2+-free Hank’s
balanced salt solution (HBSS, Sigma, St. Louis, MO, USA) twice and
cell lysis buffer (100 mM HEPES, pH 8.2, 50 mM NaCl, 5 mM
MgCl2, 1 mM dithiothreitol and protease inhibitors),
sedimented at 1,000 rpm, resuspended in 0.8 ml cold cell lysis
buffer and incubated for 50 min on ice. Cells were sonicated on ice
in 200 μl aliquots using a sonicator fitted with a 3-mm diameter
probe until the cells and nuclei were lysed, as judged by
microscopy. The lysate was sedimented at 15,000 rpm for 15 min at
4°C to pellet the coarse material. The supernatant was aliquoted,
frozen in liquid nitrogen and stored at −80°C. The protein
concentration of the extract was determined by the Bradford assay.
In this study, the chondrocyte extract contained 10–15 mg/ml
protein.
Streptolysin O-mediated permeabilization
and cell extract treatment
BM-MSCs were washed in cold PBS and in cold HBSS.
Cells were resuspended in SLO solution at a concentration of 100
cells/μl with a SLO concentration of 230 ng/ml. Samples were
incubated in an H2O bath for 50 min at 37°C with
occasional agitation. Cells were then collected by sedimentation at
1,800 rpm for 5 min at 37°C. A permeabilization efficiency of
>60% was obtained, as assessed by monitoring the uptake of
propidium iodide (PI).
Following permeabilization, cells were resuspended
at 1000 cells/μl in 100 μl cell extract containing an ATP-
regenerating system and 1 mmol/l of each NTP. Cells were cultured
for 1 h at 37°C with occasional agitation. To reseal the cells,
cell suspension with cell extract was diluted with DMEM containing
2 mM CaCl2 and cultured for 2 h at 37°C. The supernatant
was discarded, fresh DMEM containing no CaCl2 was added
and cells were cultured until use. According to the different
treatments, there were 4 groups: SLO treatment(+) cell extract(+)
group; SLO treatment(−) cell extract(+) group; SLO treatment(−)
cell extract(−) group; and chondrocyte group, which was regarded as
a positive control.
Immunofluorescence
Immunofluorescence analysis was performed on the
seventh day. The cells were fixed with 4% paraformaldehyde for 15
min. The fixed specimens were washed and incubated with primary
anti-collagen type II (COL II) monoclonal antibody (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) diluted in 0.5% bovine serum
albumin (BSA) at 4°C overnight and then washed and incubated with
secondary antibody for 30 min at 37°C. DAPI was used as a nuclear
counterstain. Images were captured on a NIKON microscope using IPP
software.
Transcription analysis
Reverse transcription-polymerase chain reaction
(RT-PCR) was performed on the seventh day using the primers: COL
II: forward (5′-3′): GATGGGCAGAGGTATAATGAT and reverse (5′-3′):
CTTTTTCACCTTTGTCACCAC (product size, 322 bp); aggrecan: forward
(5′-3′): ATGACTCTGGGATCTATCGCT and reverse (5′-3′):
CGTAGGTCTCATTGGTGTCA-3 (product size, 352 bp); GADPH: forward
(5′-3′): ACATCAAGGAGAAGCTCTGCTACG and reverse (5′-3′):
GAGGGGCGATGATCTTGATCTTCA (product size, 366 bp). The reactions were
performed in a T3 thermocycler (Biometra, Goettingen, Germany). The
amplified products were separated on 1.5% agarose gels and
visualized by ethidium bromide (Sigma-Aldrich).
Chondrogenic differentiation of
treated-BM-MSCs in micromass culture
Based on the results obtained above, micromass
culture was performed. Briefly, reversibly permeabilized BM-MSCs
with added cell extract were collected by resuspension at a density
of 2.0×107 cells/ml in the growth medium. The cell
suspension was then spotted in 15 μl drops into dishes. The cells
were incubated for 2 h at 37°C to allow attachment and then
maintained in the complete medium until day 14. The specimens were
then embedded in paraffin and cut into 10 μm sections. The sections
were stained with hematoxylin and eosin (H&E) and toluidine
blue to reveal the histological structure and chondroitin sulfate
deposition, which is a substance in cartilage matrix, respectively.
The expression of COL II was detected using mouse anti-pig
monoclonal antibody (1:100 in 0.5% BSA, Santa Cruz Biotechnology),
followed by horseradish peroxidase (HRP)-conjugated anti-mouse
antibody (1:500 in PBS, Santa Cruz Biotechnology) and color
development with diaminobenzidine tetrahydrochloride (DAB, Santa
Cruz Biotechnology).
Results
Reversible permeabilization of
BM-MSCs
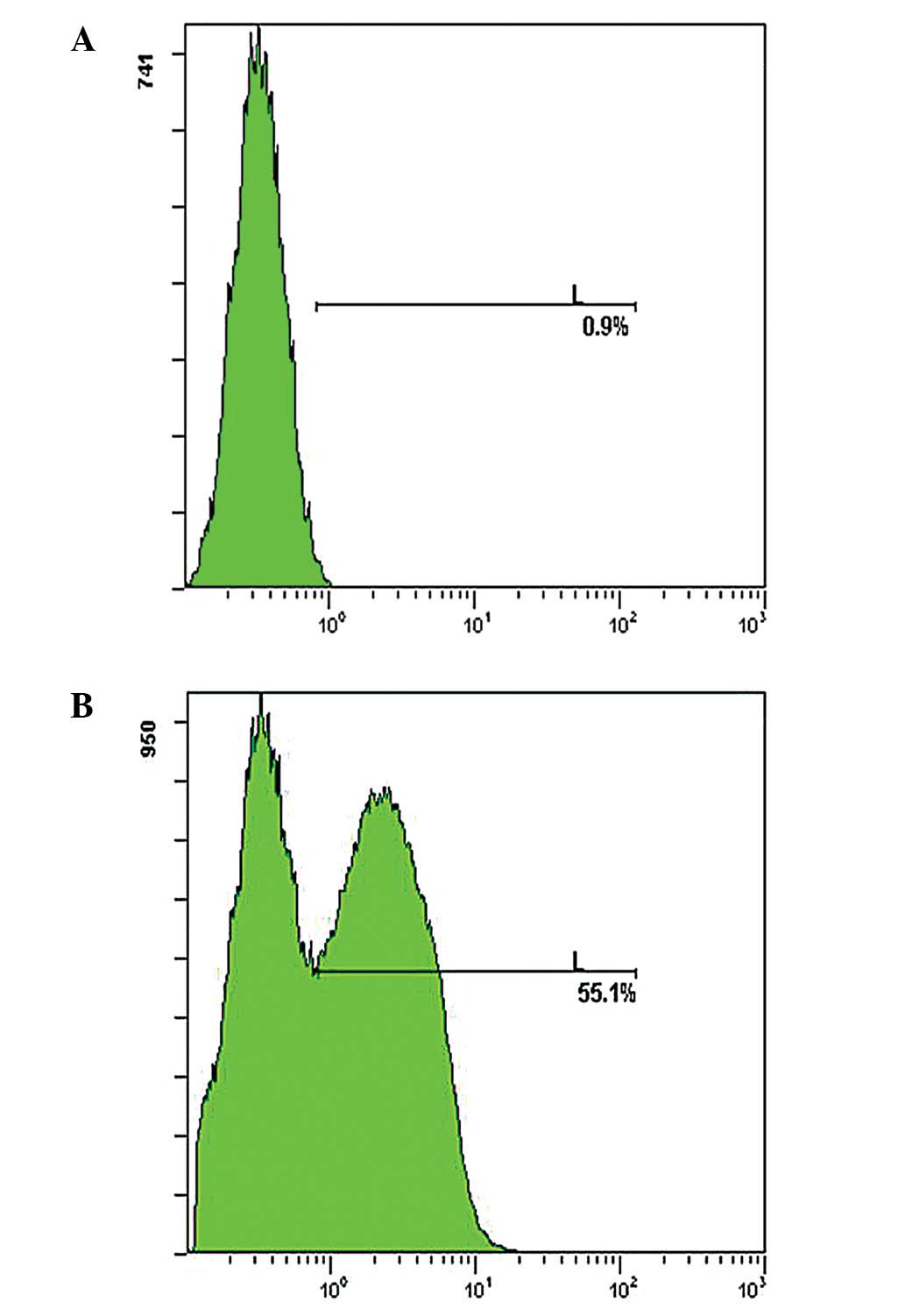
In this study, BM-MSCs were treated with various
concentrations of SLO (230, 300 and 400 ng/ml, respectively) and
the proliferative ability of cells was detected. Results showed
that an enhanced SLO concentration slightly increased
permeabilization, however, a marked decrease was observed in the
proliferative ability of cells (data not shown). SLO (230 ng/ml)
was used, as a permeabilization efficiency of >55% was obtained
and it was proven to be least detrimental (Fig. 1).
Exposure of BM-MSCs to the chondrocyte
extract leads to the induction of chondrocyte-specific genes and
protein
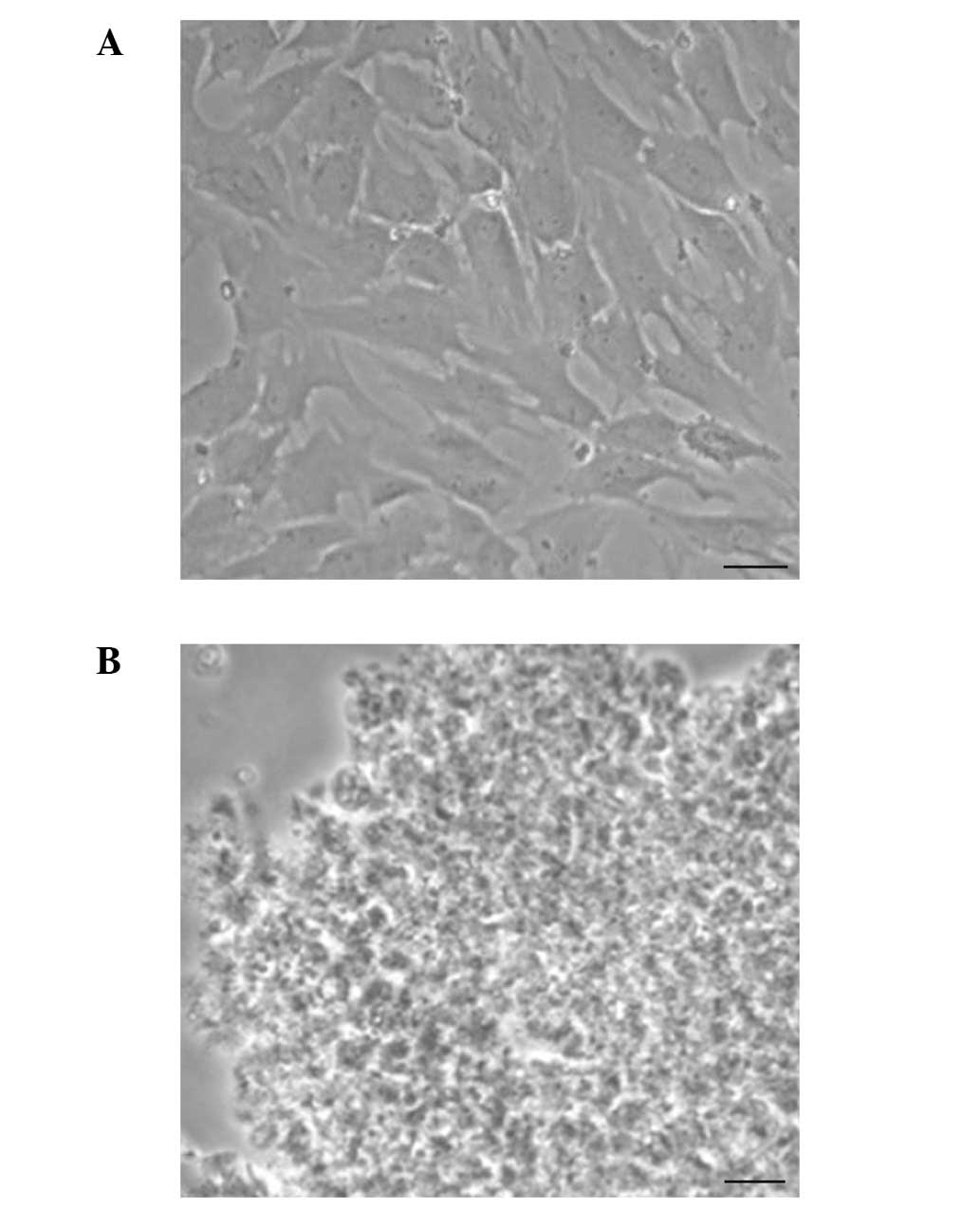
Chondrocytes were sonicated and observed under a
microscope, which revealed that there was no cell and nucleus
structure (Fig. 2). Consistent
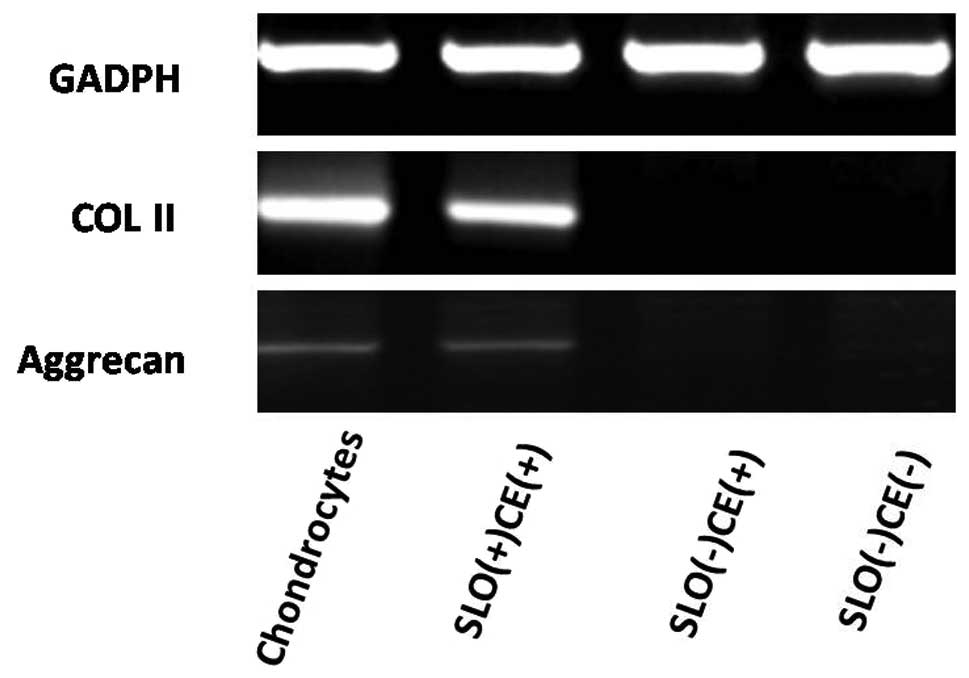
with the uptake of PI, following exposure of BM-MSCs to the
chondrocyte extract in an ATP-regeneration 7 days later, cells
treated with SLO-expressed chondrocyte-specific genes such as COL
II and aggrecan on the seventh day (Fig. 3). The immunofluorescent staining
revealed that reprogrammed BM-MSCs were positive for COL II
(Fig. 4); whereas, no changes were
observed in intact (non-permeabilized) BM-MSCs. However, at 14
days, there was no relevant expression.
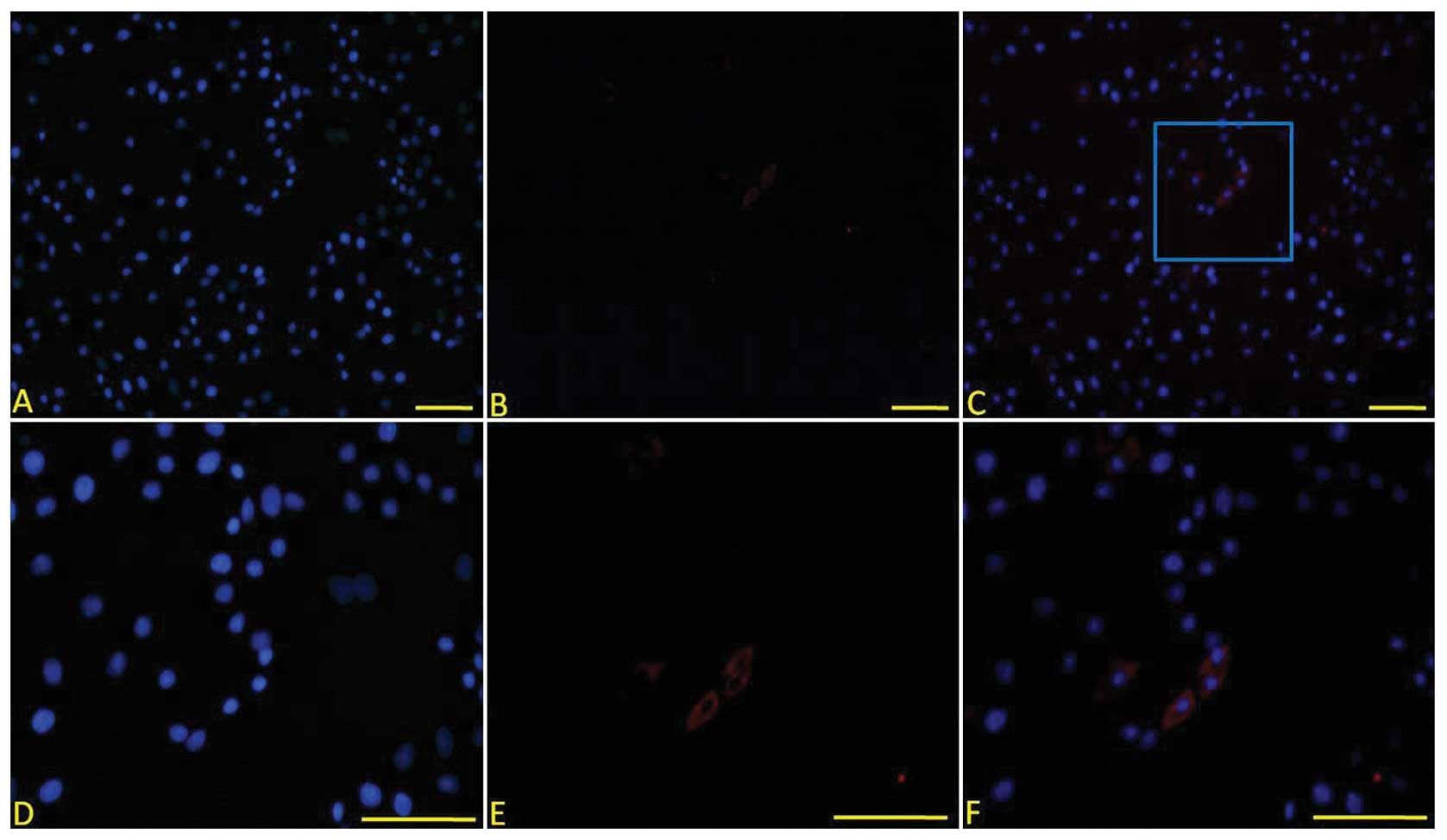 | Figure 4COL II expression in the cells 7 days
after cell treatment. COL II was positive in the cells treated with
SLO and the cell extract. (A) DAPI staining, (B) anti-COL II
immunofluorescent staining, (C) merged image of A and B revealed
that some cells expressed COL II, (D) high magnification of DAPI
staining of positive cells in the blue frame in (C), (E) high
magnification of anti-COL II immunofluorescent staining of positive
cells in the blue frame in (C) and (F) merged image of (D) and (E)
(original magnification: A–C, ×100, D–E, ×200). COL II, type II
collagen, SLO, streptolysin O. |
Maintenance of the chondrogenic phenotype
of reprogrammed BM-MSCs in micromass culture
Previous investigations have demonstrated that a
high density culture manner, such as micromass or pellet culture,
is essential for the chondrogenic differentiation of BM-MSCs. Using
monolayer culture, examinations could not detect relevant
chondrocyte-specific expression 14 days after cell treatment. Thus,
to maintain the chondrogenic differentiation of reprogrammed
BM-MSCs, micromass culture was used in this study. When the
induction time was extended to 14 days, the cells in the cell mass
formed a lacunae structure with chondrocyte-like cells located
inside secreting chondroitin sulfate. The expression of COL II was
also observed in the cell mass (Fig.
5).
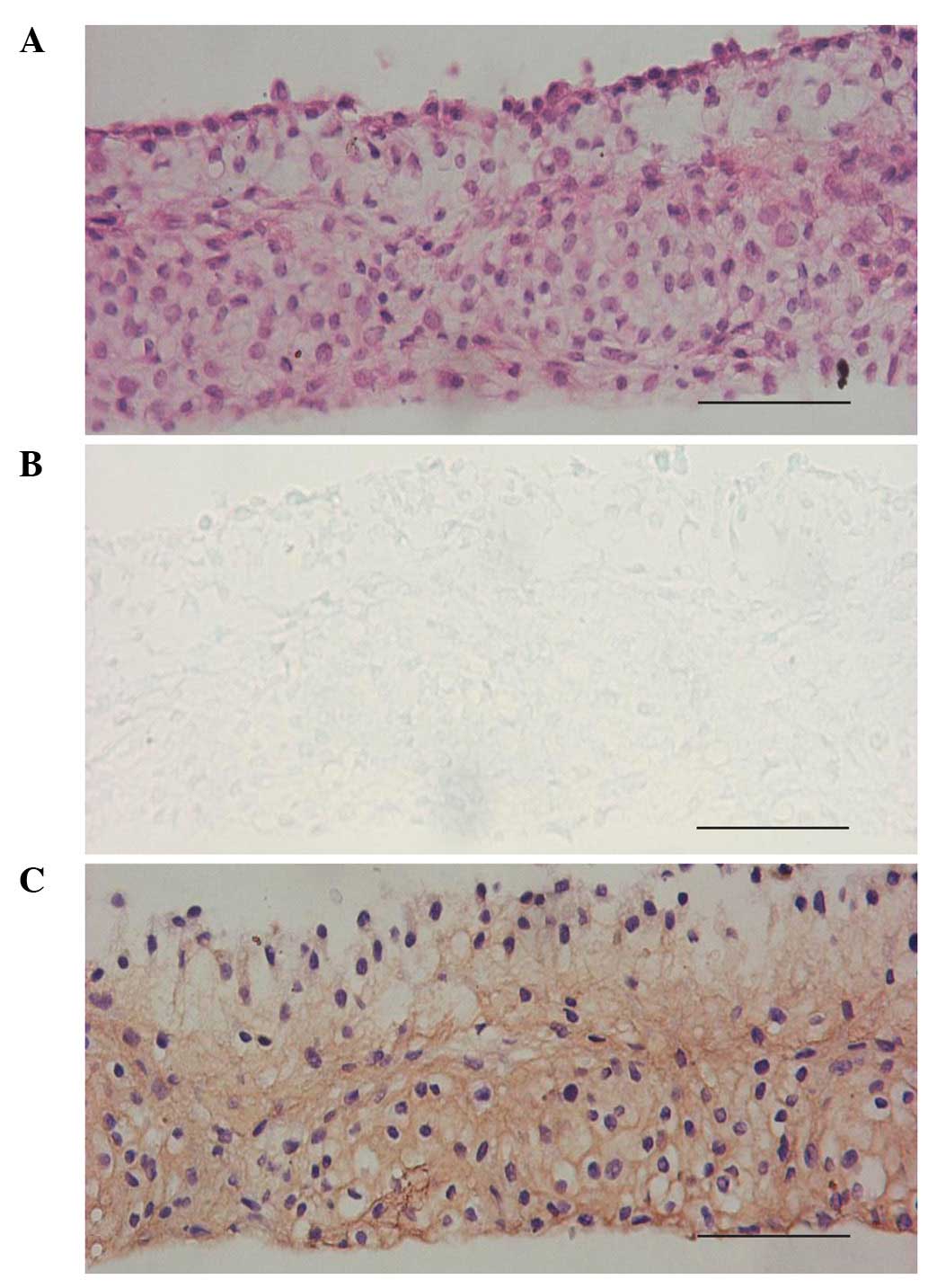 | Figure 5Histological morphology of
chondrogenically transdifferentiated BM-MSCs cultured in micromass
in the presence of SLO and chondrocyte extract simultaneously on
the 14th day. (A) H&E staining showed that certain cells in the
micromass formed lacunae structure, (B) toluidine blue staining
indicated that certain cells secreted chondroitin sulfate, (C)
anti-COL II immunohistochemical staining was positive in the cell
mass (original magnification, ×200). BM-MSCs, bone marrow-derived
mesenchymal stem cells, SLO, streptolysin O, COL II, type II
collagen, H&E, hematoxylin and eosin. |
Discussion
Cell extract-based systems for reprogramming cell
fate have been developed with the aim of eliciting somatic cell
transdifferentiation or dedifferentiation. Previous studies have
demonstrated that cell extracts harvested from embryonic stem cells
(ESCs) (13) or somatic cells
(3) are capable of inducing
nuclear de-/transdifferentiation of ‘donor cells’. The current
study provided permeabilized BM-MSCs with the chondrocyte extract
and observed changes in the gene and protein expression following
cell culture. A high-density culture method was also found to
prolong the maintenance time of the phenotype change of
reprogrammed cells.
The main component of the cell extract was thought
to be protein, as heat-treated (95°C, 5 min) extracts were not able
to induce any changes (3,5). In this study, the cell extract was
obtained using cell lysis and ultrasonic homogenization. By this
method, the protein molecular weight varies within a wide range. To
enable the cell extract to enter ‘donor cells’ effectively, BM-MSCs
were treated with SLO as described previously (2,3).
Following the binding of SLO to membranes, toxin monomers diffuse
laterally in the bilayer and oligomerize to form homotypic
aggregates that represent large transmembrane pores whose diameters
may reach 35 nm, allowing molecules <100 kDa to enter the cell
cytosol (14–16). In the presence of Ca2+,
the permeabilized cells were resealed for subsequent culture. This
finding was consistent with other authors who reported that
resealing was temperature-independent and even occurred eat 4°C
(14).
Findings of previous studies have shown that a rapid
gene change may be induced over a 1–8 h culture (17) and that these genes would
consistently persist up to days 7–10 (3,18).
In our study, no gene alteration was evident on day 3. However, 7
days later, a clear expression of COL II and aggrecan, the two main
chondrogenic gene markers, was demonstrated by RT-PCR analysis in
the group of permeabilized BM-MSCs in the presence of the cell
extract. Consistent with gene change, COL II protein expression was
also positive in this group. These results suggested that exposure
to the chondrocyte extract initiated a series of changes driving
BM-MSCs cultured in a monolayer towards chondrocyte-like cells 7
days after treatment. No changes were observed in intact
(non-permeabilized) BM-MSCs incubated in chondrocyte extract. These
results are consistent with those of Taranger et al
(19) and Bru et al
(17), but not with those by
Hansis et al (20).
However, no changes were detected on day 14. In addition to this
finding, the percentage of permeabilized cells in this study was
over 55% and was discrepant with COL II-positive cells. There are
various reasons for this finding: i) A low concentration of cell
extract leads to incomplete transdifferentiation; ii) preferential
growth of BM-MSCs inhibits the proliferation of successfully
reprogrammed BM-MSCs; iii) the culturing method makes the
chondrogenic microenvironment deficient and iv) the changes were
transient and could not be detected at the 14th day.
Considering the fact that chondroadherin interaction
with cells may be crucial for maintaining the adult chondrocyte
phenotype and cartilage homeostasis, cell-cell contact may be
responsible for the reprogramming (21). Fourteen days after micromass
culture, the cells presented a crumb structure. H&E staining
showed cells in the crumb presenting lacunae structure with
chondroitin sulfate and were COL II-positive. This suggests that
cytokines secreted by BM-MSC-converted chondrocytes are likely to
constantly induce BM-MSCs towards chondrocytes.
The main regulatory component involved in the cell
extract was believed to initiate a transcriptional program specific
for the alteration (3). Hakelien
et al (2) observed the
import of transcription factors into 293T nuclei in their study. At
the same time, demethylation of the target cell-specific gene
promoter and histone acetylation were also observed (3,5,13,17).
Cell reprogramming is of interest for the
development of novel cellular therapeutics and is potentially
useful for producing isogenic replacement cells for the treatment
of a wide variety of diseases. The incomplete transcriptional and
epigenetic reprogramming may not lead the cells harvested in this
manner to be totally the same as the ‘target’ cells, but own some
of the desired functions of the ‘target’ cells. To a certain
degree, it is sufficient for cell programming to exhibit the
necessary therapeutic properties without complete alteration of
genes, protein and functions. For example, rat fibroblasts
reversibly permeabilized with SLO and exposed to extract of an
insulin-producing β cell line were capable of secreting insulin on
day 5 (3). For diabetes care, it
is sufficient to harvest cells able to secrete insulin.
Since only this alteration is detectable, it
indicates a limitation of our study. Thus, the relevant mechanism
involved in this process and the methods of increasing efficiency
and the maintenance time of reprogramming should be
investigated.
In conclusion, this study has shown that reversibly
permeabilized BM-MSCs were differentiated into chondrocytes when
they were co-cultured with the chondrocyte extract. SLO plays a key
role, but not dependently, in the reprogramming process and it sets
the stage for the further action of more essential components in
the cell extract. Nevertheless, culturing cells with a high-density
protocol is likely to prolong the phenotypic changes of
differentiated cells. The method used in this study provides tissue
engineering with a novel way of obtaining seeding cells.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (Nos. 30900308 and 81071580).
References
|
1
|
Wong CC, Chiu LH, Lai WF, et al:
Phenotypic re-expression of near quiescent chondrocytes: The
effects of type II collagen and growth factors. J Biomater Appl.
25:75–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hakelien AM, Landsverk HB, Robl JM,
Skalhegg BS and Collas P: Reprogramming fibroblasts to express
T-cell functions using cell extracts. Nat Biotechnol. 20:460–466.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hakelien AM, Gaustad KG and Collas P:
Transient alteration of cell fate using a nuclear and cytoplasmic
extract of an insulinoma cell line. Biochem Biophys Res Commun.
316:834–841. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gaustad KG, Boquest AC, Anderson BE,
Gerdes AM and Collas P: Differentiation of human adipose tissue
stem cells using extracts of rat cardiomyocytes. Biochem Biophys
Res Commun. 314:420–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyamoto K, Furusawa T, Ohnuki M, et al:
Reprogramming events of mammalian somatic cells induced by
Xenopus laevis egg extracts. Mol Reprod Dev. 74:1268–1277.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Y, Chen L, Scott PG and Tredget EE:
Mesenchymal stem cells enhance wound healing through
differentiation and angiogenesis. Stem Cells. 25:2648–2659. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Zan T, Li Y, et al: Transplantation
of adipose-derived stem cells promotes formation of prefabricated
flap in a rat model. Tohoku J Exp Med. 222:131–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Indrawattana N, Chen G, Tadokoro M, et al:
Growth factor combination for chondrogenic induction from human
mesenchymal stem cell. Biochem Biophys Res Commun. 320:914–919.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson AD, Betz MW, Yoon DM and Fisher
JP: Osteogenic differentiation of bone marrow stromal cells induced
by coculture with chondrocytes encapsulated in three-dimensional
matrices. Tissue Eng Part A. 15:1181–1190. 2009. View Article : Google Scholar
|
|
11
|
Liu X, Sun H, Yan D, et al: In vivo
ectopic chondrogenesis of BMSCs directed by mature chondrocytes.
Biomaterials. 31:9406–9414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HJ, Choi BH, Min BH, Son YS and Park
SR: Low-intensity ultrasound stimulation enhances chondrogenic
differentiation in alginate culture of mesenchymal stem cells.
Artif Organs. 30:707–715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajasingh J, Lambers E, Hamada H, et al:
Cell-free embryonic stem cell extract-mediated derivation of
multipotent stem cells from NIH3T3 fibroblasts for functional and
anatomical ischemic tissue repair. Circ Res. 102:e107–e117. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Walev I, Bhakdi SC, Hofmann F, et al:
Delivery of proteins into living cells by reversible membrane
permeabilization with streptolysin-O. Proc Natl Acad Sci USA.
98:3185–3190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palmer M, Vulicevic I, Saweljew P, Valeva
A, Kehoe M and Bhakdi S: Streptolysin O: a proposed model of
allosteric interaction between a pore-forming protein and its
target lipid bilayer. Biochemistry. 37:2378–2383. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Palmer M, Harris R, Freytag C, Kehoe M,
Tranum-Jensen J and Bhakdi S: Assembly mechanism of the oligomeric
streptolysin O pore: the early membrane lesion is lined by a free
edge of the lipid membrane and is extended gradually during
oligomerization. EMBO J. 17:1598–1605. 1998. View Article : Google Scholar
|
|
17
|
Bru T, Clarke C, McGrew MJ, Sang HM,
Wilmut I and Blow JJ: Rapid induction of pluripotency genes after
exposure of human somatic cells to mouse ES cell extracts. Exp Cell
Res. 314:2634–2642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyamoto K, Tsukiyama T, Yang Y, et al:
Cell-free extracts from mammalian oocytes partially induce nuclear
reprogramming in somatic cells. Biol Reprod. 80:935–943. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taranger CK, Noer A, Sorensen AL, Hakelien
AM, Boquest AC and Collas P: Induction of dedifferentiation,
genomewide transcriptional programming, and epigenetic
reprogramming by extracts of carcinoma and embryonic stem cells.
Mol Biol Cell. 16:5719–5735. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hansis C, Barreto G, Maltry N and Niehrs
C: Nuclear reprogramming of human somatic cells by Xenopus
egg extract requires BRG1. Curr Biol. 14:1475–1480. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yin S, Cen L, Wang C, et al: Chondrogenic
transdifferentiation of human dermal fibroblasts stimulated with
cartilage-derived morphogenetic protein 1. Tissue Eng Part A.
16:1633–1643. 2010. View Article : Google Scholar : PubMed/NCBI
|