Introduction
Astrocytoma arises from neural stem or progenitor
cells in the central nervous system and is the most common primary
brain tumor, accounting for ~60% of all brain tumors. Despite
combined treatment strategies, including surgery, radiotherapy and
chemotherapy, the prognosis for high-grade astrocytoma remains
poor, with a median survival of ~1 year (1). The clinical symptoms and prognosis
are closely correlated with tumor location, size and histological
grade. Although the histological grade, in part, reflects the
malignant features of astrocytoma, it is not able to provide an
indication of the exact mechanism of tumor progression and
recurrence. Thus, it is important to understand the molecular
mechanism of astrocytoma cell progression and identify effective
markers of tumorigenesis and progression.
Cancerous inhibitor of protein phosphatase 2A
(CIP2A), originally named KIAA1524 or P90, has been cloned from
hepatocellular carcinoma patients (2). CIP2A has been demonstrated to inhibit
the activity of PP2A toward the oncogenic transcription factor,
c-Myc, thereby preventing the proteolytic degradation of c-Myc,
which is important for cell transformation and tumorigenesis in
vivo and in vitro(3).
In addition, CIP2A has been reported to reduce the apoptotic effect
of bortezomib in breast cancer, hepatocellular carcinoma and head
and neck squamous cell carcinoma (4–6).
Moreover, CIP2A has been found to be overexpressed in various types
of human cancer, including breast, gastric, lung, prostate,
hepatocellular, ovarian, colon and renal cancers (6–17).
The expression pattern of CIP2A in astrocytoma is not clear and the
biological roles of CIP2A in astrocytoma cells remain to be
examined.
In the present study, the expression pattern of
CIP2A was investigated in 135 astrocytoma specimens and the
correlations between CIP2A expression and clinicopathological
factors were analyzed. Furthermore, to clarify the roles of CIP2A
in astrocytoma, the effects of CIP2A on proliferation and apoptosis
were investigated in astrocytoma cell lines.
Materials and methods
Patients and specimens
The study was approved by the institutional review
board of Liaoning Medical University (Jinzhou, China). Primary
tumor specimens were obtained from 135 patients diagnosed with
astrocytoma who underwent resection in the First Affiliated
Hospital of Liaoning Medical University between 2000 and 2005. The
histological diagnosis was evaluated for sections stained with
hematoxylin and eosin according to the World Health Organization
classification guidelines. Clinical and histopathological data,
including histopathological diagnosis and tumor grade, were
extracted from medical records.
Cell culture and transfection
The U87 cell line was obtained from the American
Type Culture Collection (Manassas, VA, USA) and the A172 cell line
was from the Shanghai Cell Bank (Shanghai, China). Cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen
Life Technologies, Carlsbad, CA, USA) containing 10% fetal bovine
serum (FBS; Invitrogen Life Technologies), 100 IU/ml penicillin
(Sigma-Aldrich, St. Louis, MO, USA) and 100 μg/ml streptomycin
(Sigma-Aldrich). Cells were grown on sterilized culture dishes and
were passaged every 2 days with 0.25% trypsin (Invitrogen Life
Technologies).
On-TargetPlus SMARTpool CIP2A siRNA (#L-014135-01)
and On-TargetPlus siControl (D-001810-01-20) were purchased from
Dharmacon (Thermo Fisher Scientific, Waltham, MA, USA). For
transfections, cells were seeded in plates 24 h prior to the
experiment. Cells were transfected with siRNA using DharmaFECT 1
(0.20 μl/well; Thermo Fisher Scientific) according to the
manufacturer’s instructions.
Immunohistochemistry
Sections (4-μm thick) were prepared from
paraffin-embedded tissues. Immunostaining was performed by the
streptavidin-peroxidase method (Ultrasensitive; MaiXin, Fuzhou,
China). Sections were deparaffinized in xylene, rehydrated with
graded alcohol and then boiled in citrate buffer (pH 6.0) for 2 min
in an autoclave. Next, 0.3% hydrogen peroxide was applied to block
endogenous peroxidase activity and the sections were incubated with
normal animal serum to reduce nonspecific binding. Tissue sections
were incubated with CIP2A rabbit polyclonal antibody (1:300
dilution; Novus Biologicals, LCC, Littleton, CO, USA) for 2 h at
room temperature. Rabbit immunoglobulin (at the same concentration
as the antigen-specific antibody) was used as a negative control.
Staining was followed by incubation with biotinylated secondary
antibodies. The peroxidase reaction was developed with
3,3′-diaminobenzidine tetrahydrochloride. Counterstaining was
performed lightly with hematoxylin and the sections were dehydrated
in alcohol prior to mounting.
Immunostaining of CIP2A was scored on a
semiquantitative scale by evaluating the intensity and percentage
of tumor cells as described previously (10). We counted 400 tumor cells and
calculated the percentage of positively stained cells. The
intensity of CIP2A staining was scored as 0 (no signal), 1 (weak),
2 (moderate) and 3 (marked). Percentage scores were assigned as 1,
1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. The scores of each
tumor sample were multiplied to produce a final score of 0–12 and
the tumors were finally determined as negative (−), 0; lower
expression (+), ≤4; moderate expression (++), 5–8; and high
expression (+++), ≥9. Tumor samples that scored (+) to (+++) were
considered to overexpress CIP2A.
Quantitative real-time PCR
Quantitative real-time PCR was performed using the
SYBR-Green PCR master mix in a total volume of 20 μl on a 7900HT
Fast Real-Time PCR System (both Applied Biosystems, Bedford, MA,
USA) as follows: 95°C for 30 sec and 40 cycles at 95°C for 5 sec
and 60°C for 30 sec. A dissociation step was performed to generate
a melting curve to confirm the specificity of the amplification.
Expression levels of the analyzed genes were normalized against
β-actin expression. Relative levels of gene expression were
determined using the following formula: ΔCt = Ctgene −
Ctref and the fold change of gene expression was
calculated by the 2−ΔΔCt method. The primer sequences
were as follows: CIP2A, 5′-ATACTTCAGGACCCACGTTTGAT-3′ (forward) and
5′-TCTCCAAGTACTAAAGCAGGA AAATCT-3′ (reverse); β-actin,
5′-ATAGCACAGCCTGGA TAGCAACGTAC-3′ (forward) and 5′-CACCTT
CTACAATGAGCTGCGTGTG-3′ (reverse). Experiments were repeated in
triplicate.
Western blot analysis
Total protein from tissue and cells was extracted in
lysis buffer (Pierce Biotechnology, Inc., Rockford, IL, USA) and
quantified using the Bradford method. Samples were separated by
SDS-PAGE and transferred to polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA) and incubated overnight at 4°C with
antibodies against CIP2A (1:1,000; Novus Biologicals, LCC),
caspase-3, cleaved caspase-3, c-Myc, phospho-Akt, Akt, Bcl-2
(1:800, Cell Signaling Technology, Inc., Danvers, MA, USA) and
β-actin (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). Following incubation with peroxidase-coupled anti-mouse IgG
(Santa Cruz Biotechnology, Inc.) at 37°C for 2 h, bound proteins
were visualized using an ECL detection system (Pierce
Biotechnology, Inc.) and detected using a BioImaging System (UVP
Inc., Upland, CA, USA). Relative protein levels were quantified
using β-actin as a loading control.
Colony formation, anchorage-independent
colony formation and thiazolyl blue (MTT) assays
For the colony formation assay, A172 and U87 cells
were transfected with siRNA for 48 h and plated into three 6-cm
cell culture dishes (1,000 cell/dish). Cells were incubated for 12
days in medium containing 10% FBS. The plates were washed with
phosphate-buffered saline (PBS) and stained with Giemsa. The number
of colonies with >50 cells was counted manually using a
microscope.
For the anchorage-independent colony growth assay
~2,000 cells/well were seeded in medium containing 0.5% agarose on
top of bottom agar containing 1% low-melting agar in regular
medium. After 14–21 days, colonies were stained with Giemsa and
counted using a microscope.
For the MTT assay, cells were plated in 96-well
plates in medium containing 10% FBS at ~3,000 cells/well 24 h
following transfection. For quantification of cell viability, 20 μl
MTT (5 mg/ml) solution was added to each well and incubated for 4 h
at 37°C. The medium was removed from each well and the resulting
MTT formazan was solubilized in 150 μl DMSO. Each solution was
measured spectrophotometrically at 490 nm.
Apoptosis analysis
Cells (5×105) were seeded into 6-cm
tissue culture dishes. After 12 h, the cells were transfected with
siRNA using DharmaFECT 1 (0.20 μl/well). For the detection of
apoptosis, adherent cells were collected and resuspended in cold
PBS for analysis. The cells were stained with the Annexin V-FITC
Apoptosis kit (BD Pharmingen, San Diego, CA, USA) to monitor
apoptotic cells and propidium iodide (PI) to detect dead cells.
Data were collected using a BD FACSCalibur flow cytometer (San
Jose, CA, USA).
Statistical analysis
SPSS version 11.5 for Windows was used for all
statistical analyses (SPSS, Inc., Chicago, IL, USA). A
χ2 test was used to examine the possible correlations
between CIP2A expression and clinicopathological factors. The
Student’s t-test was used to compare densitometry data on focus
numbers between control and CIP2A-transfected cells. All P-values
are based on a two-sided statistical analysis and P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of CIP2A in astrocytoma
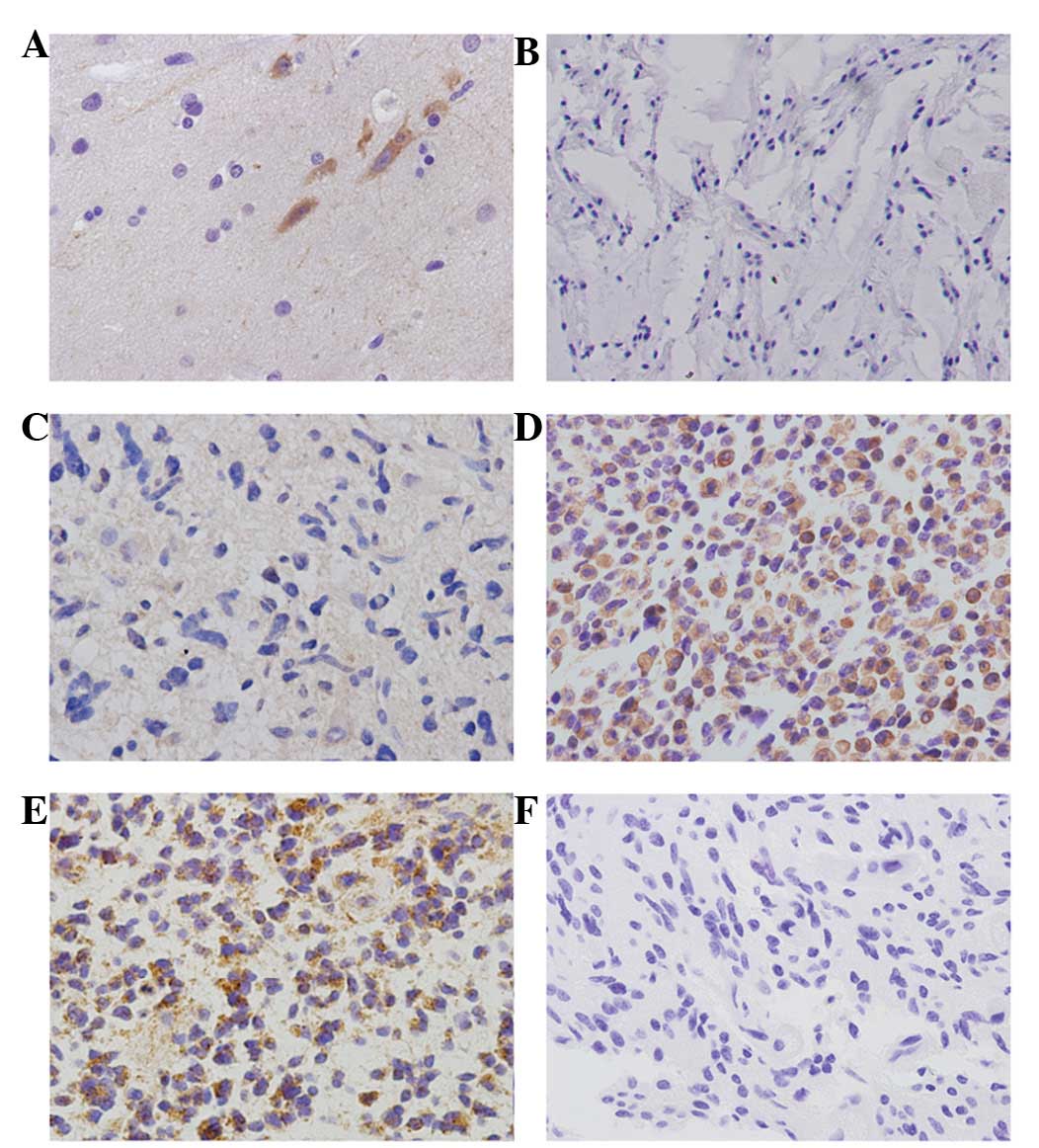
In normal brain tissues, negative expression of
CIP2A in astrocytes and positive cytoplasmic CIP2A expression in
neurons was observed (Fig. 1A).
Positive cytoplasmic CIP2A staining was observed in 75/135 (55.6%)
human astrocytomas (Fig. 1B-E),
while no staining was detected in sections from the same samples
subjected to immunohistochemical analysis using non-immune rabbit
immunoglobulin (Fig. 1F). The
correlations between CIP2A protein expression and
clinicopathological factors was investigated and no relationship
was found between CIP2A expression and age and gender. The positive
rates of CIP2A overexpression in grades I (Fig. 1B), II (Fig. 1C) and III astrocytomas (Fig. 1D) and grade IV
astrocytoma/glioblastoma (Fig. 1E)
were 11.1 (1/9), 47.0 (31/66), 76.2 (32/42) and 61.1% (11/18),
respectively (Table I). The
positive rate of CIP2A was identified to be significantly higher in
high-grade astrocytomas than in those of low-grade
(P<0.001).
 | Table IRelationship between cancerous
inhibitor of protein phosphatase 2A (CIP2A) and clinicopathological
features |
Table I
Relationship between cancerous
inhibitor of protein phosphatase 2A (CIP2A) and clinicopathological
features
| Clinical
parameters | n | CIP2A | P-value |
|---|
|
|---|
| Negative | Positive |
|---|
| Age |
| <45 years | 67 | 33 | 34 | 0.301 |
| ≥45 years | 68 | 27 | 41 | |
| Gender |
| Male | 82 | 37 | 45 | 0.861 |
| Female | 53 | 23 | 30 | |
| Grading |
| I | 9 | 8 | 1 | <0.001 |
| II | 66 | 36 | 31 | |
| III | 42 | 10 | 32 | |
| IV | 18 | 7 | 11 | |
CIP2A depletion in astrocytoma cell lines
inhibits cell proliferation and increases cell apoptosis
To determine whether CIP2A enhances the
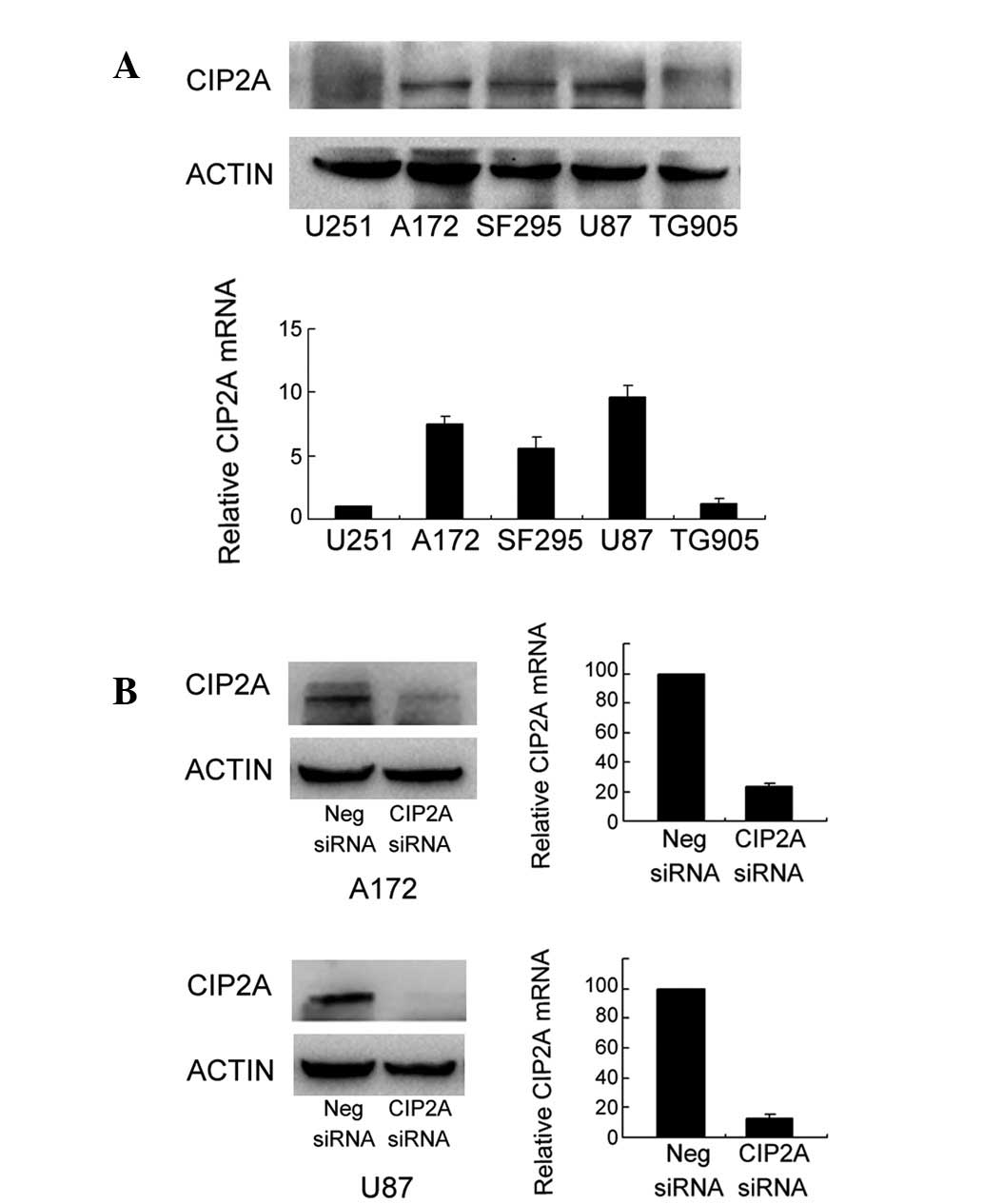
proliferation of astrocytoma, CIP2A expression levels were analyzed
in several astrocytoma cell lines and A172 and U87 cells were found
to exhibit high levels of CIP2A expression (Fig. 2A). siRNA-mediated knockdown of
CIP2A was performed in these cell lines. As demonstrated in
Fig. 2B, CIP2A siRNA decreased the
levels of CIP2A protein and mRNA in the A172 and U87 cells. The
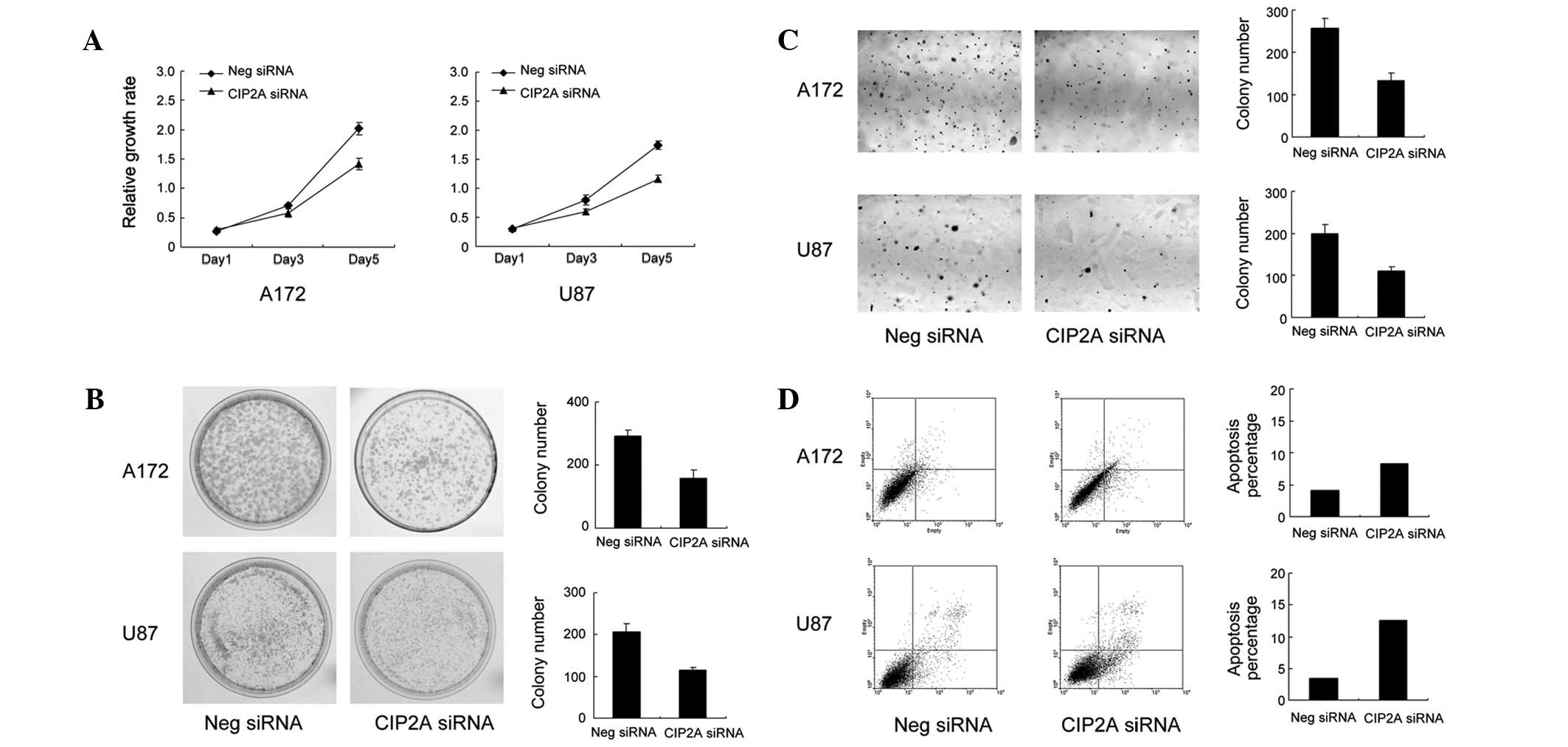
proliferation rate of the cells was determined by MTT assay. A172
and U87 cells treated with CIP2A siRNA exhibited a significantly
slower growth rate than the vector control cells (Fig. 3A). Consistent with MTT results, the
colony formation assay revealed that CIP2A-knockdown in A172 and
U87 cells led to a marked decrease in focus numbers (A172: control
292±17 vs. CIP2A siRNA 157±26, P<0.05; U87: control 207±19 vs.
CIP2A siRNA 119±6, P<0.05; Fig.
3B). To examine the impact of CIP2A on anchorage-independent
cell growth, a soft agar colony formation assay was performed.
CIP2A-knockdown reduced the colony numbers in soft agar (A172:
control 257±22 vs. CIP2A siRNA 133±16, P<0.05; U87: control
199±21 vs. CIP2A siRNA 109±11, P<0.05)
Annexin V/PI analysis was employed to characterize
the rate of apoptosis. As demonstrated in Fig. 3D, the populations of cells with
CIP2A-knockdown that were observed to be undergoing early and late
apoptosis (A172: 8.7%; U87: 13.2%) were significantly larger
compared with scramble controls (A172: 4.6%; U87: 3.9%),
demonstrating that CIP2A-knockdown results in the apoptosis of
astrocytoma cells.
CIP2A depletion increases caspase-3
cleavage, downregulates c-Myc and Bcl-2 expression and inhibits Akt
phosphorylation in astrocytoma cells
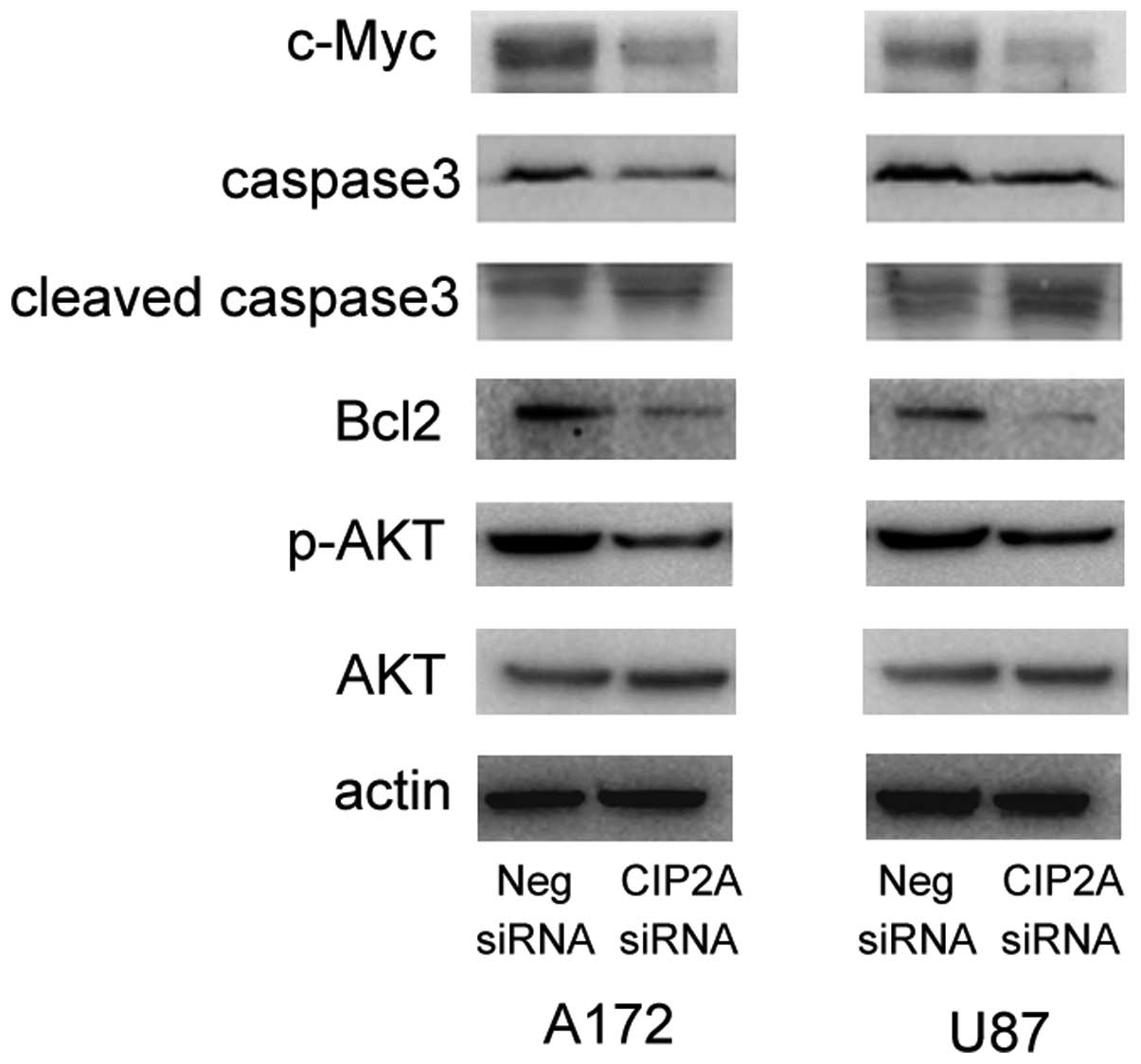
To investigate the underlying mechanism by which
CIP2A affects proliferation and apoptosis, the effect of
CIP2A-knockdown on several potential molecular targets was
analyzed. As revealed in Fig. 4,
western blot analysis demonstrated that the knockdown of CIP2A
decreased the expression levels of c-Myc protein in the two cell
lines. In addition, the levels of apoptosis-related caspase-3 and
Bcl-2 expression were determined and it was observed that CIP2A
depletion led to reductions in caspase-3 and Bcl-2 protein levels
and increased the levels of cleaved caspase-3. In addition, Akt
phosphorylation levels were markedly lower in the cells treated
with CIP2A siRNA. Together, these results indicate that CIP2A
regulates cell proliferation and apoptosis through modulation of
c-Myc, Bcl-2 and Akt levels.
Discussion
The present study demonstrated that CIP2A was
overexpressed in 55.6% of human astrocytoma and correlated with
increased tumor grade. In addition, CIP2A depletion in two
astrocytoma cell lines was demonstrated to inhibit cell growth and
anchorage-independent cell growth and increase apoptosis, by
downregulating c-Myc, Bcl-2 and phospho-Akt protein and
upregulating caspase-3 cleavage.
CIP2A has been reported to be overexpressed in
various types of cancer, including breast, gastric, lung, prostate,
hepatocellular, colon and renal cancers (4,6–10,14,17).
A previous study reported that the overexpression of CIP2A
increases and siRNA silencing of CIP2A decreases the self-renewal
and proliferation of mouse neural progenitor cells (18). However, the expression patterns and
biological roles of CIP2A in human astrocytoma remain largely
unknown. In the current study, marked cytoplasmic CIP2A expression
was found in neurons and negative staining was identified in human
glial cells, including astrocytes. CIP2A overexpression was found
in 55.6% of the astrocytoma tissues examined. In addition, a close
association between CIP2A overexpression and the astrocytoma grade
was identified.
Different gene expression patterns have been
proposed in the development of low- vs. high-grade astrocytomas,
which reflects the malignant potential of high invasive capability
and growth rate (19). The higher
expression rate of CIP2A in high-grade (III–IV) compared with
low-grade carcinomas indicates its potential association with the
aggressiveness of astrocytoma cells.
To determine the role of CIP2A in astrocytoma cells,
CIP2A expression was knocked down in A172 and U87 cell lines.
Consistent with previous studies, CIP2A depletion was found to
significantly decrease the proliferation rate, colony formation
ability and anchorage-independent growth of A172 and U87 cell
lines. To examine the potential mechanism, the effect of
CIP2A-knockdown on c-Myc expression, a target protein of CIP2A, was
examined. CIP2A depletion markedly downregulated c-Myc expression.
c-Myc is a cellular proto-oncogene associated with a variety of
types of human cancer and is associated with control of cellular
proliferation (20). Loss of c-Myc
is associated with reduction of cyclin D1-Cdk4 and cyclin D1-Cdk6
complexes during the cell cycle transition (21). Overexpression of CIP2A has been
demonstrated to upregulate Akt and protect cells from
bortezomib-induced apoptosis in hepatocellular and head and neck
squamous cell carcinoma cells (4,5). In
the present study, CIP2A-knockdown was observed to facilitate the
apoptosis of astrocytoma cells.
Caspase-3 is a critical executioner of apoptosis.
Activation of caspase-3 requires proteolytic processing of its
inactive zymogen into activated p17 and p12 fragments (22). In the current study, CIP2A
depletion increased caspase-3 cleavage, consistent with increased
apoptosis.
Bcl-2 prevents cells from undergoing apoptosis in
response to a variety of stimuli and is hypothesized to be involved
in resistance to conventional cancer treatment. It has been
reported that activation of the Akt pathway modulates Bcl-2
expression. In the present study, CIP2A depletion reduced Bcl-2
protein levels and Akt phosphorylation (23). These results indicate that CIP2A
regulates cell apoptosis via Bcl-2 and Akt activation.
CIP2A is overexpressed in astrocytomas and
correlates with tumor grade. CIP2A depletion attenuates cell
proliferation and facilitates apoptosis. In addition, CIP2A
depletion increases caspase-3 cleavage and inhibits c-Myc, Bcl-2
and phospho-Akt expression. These results are likely to provide
insight into the functional importance of CIP2A in the progression
of human astrocytoma. The observations of the present study
indicate that CIP2A represents a molecular target closely
associated with cell proliferation and apoptosis and may provide a
basis for the future development of cancer therapeutics.
References
|
1
|
Bondy ML, Scheurer ME, Malmer B, et al:
Brain tumor epidemiology: consensus from the Brain Tumor
Epidemiology Consortium. Cancer. 113:1953–1968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soo Hoo L, Zhang JY and Chan EK: Cloning
and characterization of a novel 90 kDa ‘companion’ auto-antigen of
p62 overexpressed in cancer. Oncogene. 21:5006–5015. 2002.
|
|
3
|
Junttila MR, Puustinen P, Niemela M, et
al: CIP2A inhibits PP2A in human malignancies. Cell. 130:51–62.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen KF, Liu CY, Lin YC, et al: CIP2A
mediates effects of bortezomib on phospho-Akt and apoptosis in
hepatocellular carcinoma cells. Oncogene. 29:6257–6266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin YC, Chen KC, Chen CC, Cheng AL and
Chen KF: CIP2A-mediated Akt activation plays a role in
bortezomib-induced apoptosis in head and neck squamous cell
carcinoma cells. Oral Oncol. 48:585–593. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Come C, Laine A, Chanrion M, et al: CIP2A
is associated with human breast cancer aggressivity. Clin Cancer
Res. 15:5092–5100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Basile JR and Czerninski R: The role of
CIP2A in oral squamous cell carcinoma. Cancer Biol Ther.
10:700–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bockelman C, Koskensalo S, Hagstrom J,
Lundin M, Ristimaki A and Haglund C: CIP2A overexpression is
associated with c-Myc expression in colorectal cancer. Cancer Biol
Ther. 13:289–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bockelman C, Lassus H, Hemmes A, et al:
Prognostic role of CIP2A expression in serous ovarian cancer. Br J
Cancer. 105:989–995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong QZ, Wang Y, Dong XJ, et al: CIP2A is
overexpressed in non-small cell lung cancer and correlates with
poor prognosis. Ann Surg Oncol. 18:857–865. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang LP, Adelson ME, Mordechai E and
Trama JP: CIP2A expression is elevated in cervical cancer. Cancer
Biomark. 8:309–317. 2011.PubMed/NCBI
|
|
12
|
Katz J, Jakymiw A, Ducksworth MK, et al:
CIP2A expression and localization in oral carcinoma and dysplasia.
Cancer Biol Ther. 10:694–699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qu W, Li W, Wei L, Xing L, Wang X and Yu
J: CIP2A is overexpressed in esophageal squamous cell carcinoma.
Med Oncol. 29:113–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren J, Li W, Yan L, et al: Expression of
CIP2A in renal cell carcinomas correlates with tumour invasion,
metastasis and patients’ survival. Br J Cancer. 105:1905–1911.
2011.PubMed/NCBI
|
|
15
|
Teng HW, Yang SH, Lin JK, et al: CIP2A is
a predictor of poor prognosis in colon cancer. J Gastrointest Surg.
16:1037–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vaarala MH, Vaisanen MR and Ristimaki A:
CIP2A expression is increased in prostate cancer. J Exp Clin Cancer
Res. 29:1362010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Ge Z, Liu C, et al: CIP2A is
overexpressed in gastric cancer and its depletion leads to impaired
clonogenicity, senescence, or differentiation of tumor cells. Clin
Cancer Res. 14:3722–3728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kerosuo L, Fox H, Perala N, et al: CIP2A
increases self-renewal and is linked to Myc in neural progenitor
cells. Differentiation. 80:68–77. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chow LM, Endersby R, Zhu X, et al:
Cooperativity within and among Pten, p53 and Rb pathways induces
high-grade astrocytoma in adult brain. Cancer Cell. 19:305–316.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gordan JD, Thompson CB and Simon MC: HIF
and c-Myc: sibling rivals for control of cancer cell metabolism and
proliferation. Cancer Cell. 12:108–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mateyak MK, Obaya AJ and Sedivy JM: c-Myc
regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle
progression at multiple independent points. Mol Cell Biol.
19:4672–4683. 1999.PubMed/NCBI
|
|
22
|
D’Amelio M, Cavallucci V and Cecconi F:
Neuronal caspase-3 signaling: not only cell death. Cell Death
Differ. 17:1104–1114. 2010.
|
|
23
|
Pugazhenthi S, Nesterova A, Sable C, et
al: Akt/protein kinase B up-regulates Bcl-2 expression through
cAMP-response element-binding protein. J Biol Chem.
275:10761–10766. 2000. View Article : Google Scholar : PubMed/NCBI
|