Introduction
Gastric cancer is a prevalent health issue and
remains a leading cause of cancer-related mortality worldwide
(1). Cisplatin (CDDP) is an
antineoplastic agent used for the treatment of several types of
human cancer (2,3), including gastric cancer. However, the
efficacy of these drugs is limited by CDDP resistance. To date, a
number of mechanisms have been proposed to explain CDDP resistance,
including AKT overexpression (4–7). A
better understanding of the processes and mechanisms leading to
CDDP resistance in gastric cancer cells is necessary to develop
effective therapies which may provide a pharmacological
intervention for acquired CDDP resistance in cancer.
AKT is a serine/threonine kinase and consists of a
family of proteins, which include AKT1, AKT2 and AKT3 (8). The AKT pathway regulates diverse
cellular processes, including cell proliferation, cell cycle
progression and cell survival (9).
AKT is additionally important in survival when cancer cells are
exposed to various stress stimuli, including cytotoxic anticancer
drugs (6). Previous studies have
shown that AKT hyperactivation may correlate with the resistance of
cancer cells to CDDP (10–12). In the present study, we observed
that AKT expression levels were significantly increased when cells
were treated with CDDP. Further experiments demonstrated that AKT
activation may be associated with the Janus kinase 2 (JAK2)/signal
transducer and activator of transcription 3 (STAT3) pathway. The
data presented in this study may provide useful information with
regard to the development of potential new therapeutic approaches
for the treatment of gastric cancer by targeting the JAK2/STAT3/AKT
pathway to overcome CDDP resistance.
Materials and methods
Cell lines and animals
BGC823 gastric cancer cells were obtained from the
Cell Bank of Chinese Academy of Sciences (Shanghai, China) and
maintained in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS), 100 mg/l penicillin and 100 mg/l streptomycin at 37°C
in a humidified atmosphere of 5% CO2. The following
antibodies were used: anti-AKT, anti-STAT3, anti-phospho-JAK2,
anti-phospho-STAT3, STAT3 inhibitor (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), anti-phospho-AKT (Millipore, Billerica,
MA, USA), anti-JAK2 (Cell Signaling Technology, Inc., USA),
dichlorodihydrofluorescein diacetate (DCFDA; Sigma, St. Louis, MO,
USA) and N-acetylcysteine (NAC; Sigma).
A total of 9 BALB/c-nu mice, 4–5 weeks old and 18–20
g in weight, provided by Beijing HFK Bioscience Co., Ltd. (Beijing,
China), were bred in laminar flow cabinets and kept at a constant
humidity and temperature (25–28°C) according to standard guidelines
outlined in a protocol approved by Wuhan University, Wuhan,
China.
Measurement of reactive oxygen species
(ROS) production
BGC823 cells were treated with CDDP for 6 h. After
washing with PBS, the cells were incubated with 33 mg/ml DCFDA in
PBS for 30 min at 37°C. The excess probe was washed off and the
labeled cells were measured using a microscope.
JAK2 siRNA transfection
BGC823 cells were transfected with JAK2 siRNA and
control siRNA (Santa Cruz Biotechnology) according to the
manufacturer’s instructions. Fresh medium without antibiotics was
applied following 8 h of incubation, and following an additional 48
h, the medium was replaced with 10% FBS, 100 mg/l penicillin and
100 mg/l streptomycin. Cell growth was maintained.
Western blot analysis
The cells were solubilized in ice-cold lysis buffer
(1X PBS, 1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% SDS and
10 mg/ml phenylephrine). Following 30 min of centrifugation at
13,000 g at 4°C, the supernatants were transferred to new
microcentrifuge tubes and the protein concentration of the
supernatant was measured using the BCA protein assay (Pierce
Biotechnology, Inc., Rockford, IL, USA) and then stored at −80°C.
Cell lysate (50 μg) was separated on an SDS-polyacrylamide gel.
Following SDS-PAGE, the proteins were transferred onto
nitrocellulose membranes. For detection of the proteins, the
membranes were blocked using 10% non-fat dry milk in Tris-buffer
containing 0.1% Tween (TBS-T) and then incubated at 4°C overnight
with anti-AKT, anti-phospho-JAK2, anti-phospho-STAT3,
anti-phospho-AKT antibodies (Millipore), which were diluted in
TBS-T containing 5% non-fat dry milk.
In vivo tumor growth assay
All animal experiments were conducted with the
approval of the Beijing HFK Bioscience Co., Ltd. Following alcohol
preparation of the skin, the nude mice (4 weeks of age) were
subcutaneously inoculated with 200 μl of cell suspension
(2×106 cells/ml) into the dorsal flank using a sterile
22-gauge needle. The animals were monitored daily and tumor volume
(mm3) was calculated as follows: volume = (shortest
diameter)2 x (longest diameter)/2. When the diameters of
the tumors were >10 mm, the mice were treated by an
intraperitoneal (i.p.) injection of 1 mg/kg CDDP, 2 g/kg NAC, and
solvent once a week for 14 days. The nude mice were divided into
the following groups: treated with solvent (control), CDDP and
CDDP/NAC. Each group contained 3 mice. Bidimensional tumor
measurements were analyzed with calipers 3 times a week; the mice
were euthanized on the 7th day following drug treatment. The
expression levels of AKT were examined by immunohistochemistry as
previously described (13,14).
Statistical analysis
Statistical analyses were performed using SPSS
version 11.3 software (SPSS, Inc. Chicago, IL, USA). Values are
expressed as the means ± SD. Differences were analyzed by the
χ2 test for incidence data and by the Student’s t-test
for comparison of the means. P<0.05 was considered to indicate a
statistically significant difference.
Results
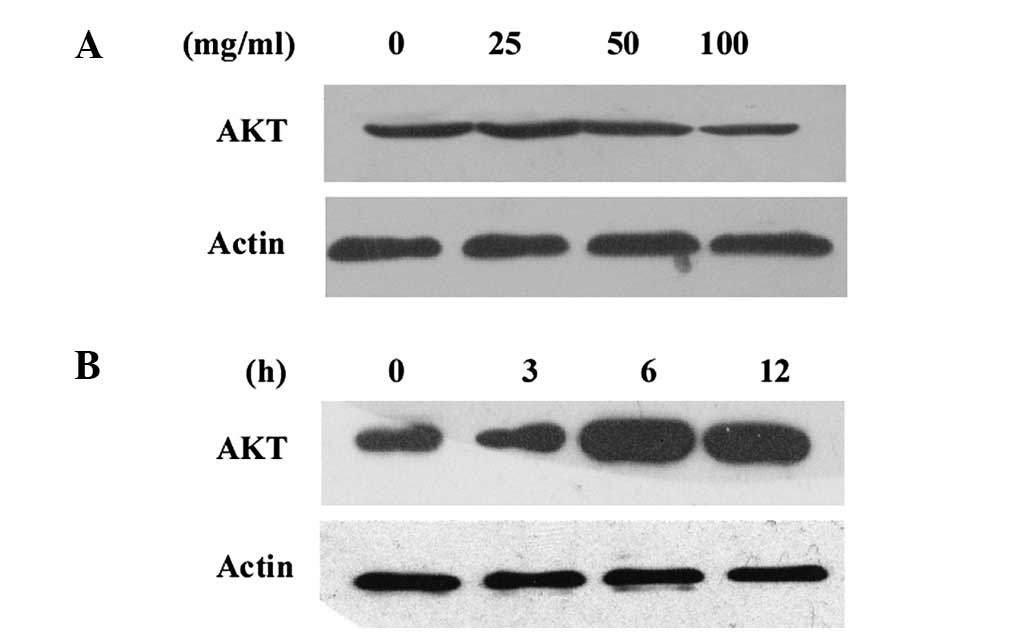
Expression levels of AKT in gastric
cancer cells
The AKT protein levels in the cancer cells were
measured by western blot analysis. The cells were treated with
various concentrations of CDDP for different periods of time
(Fig. 1).
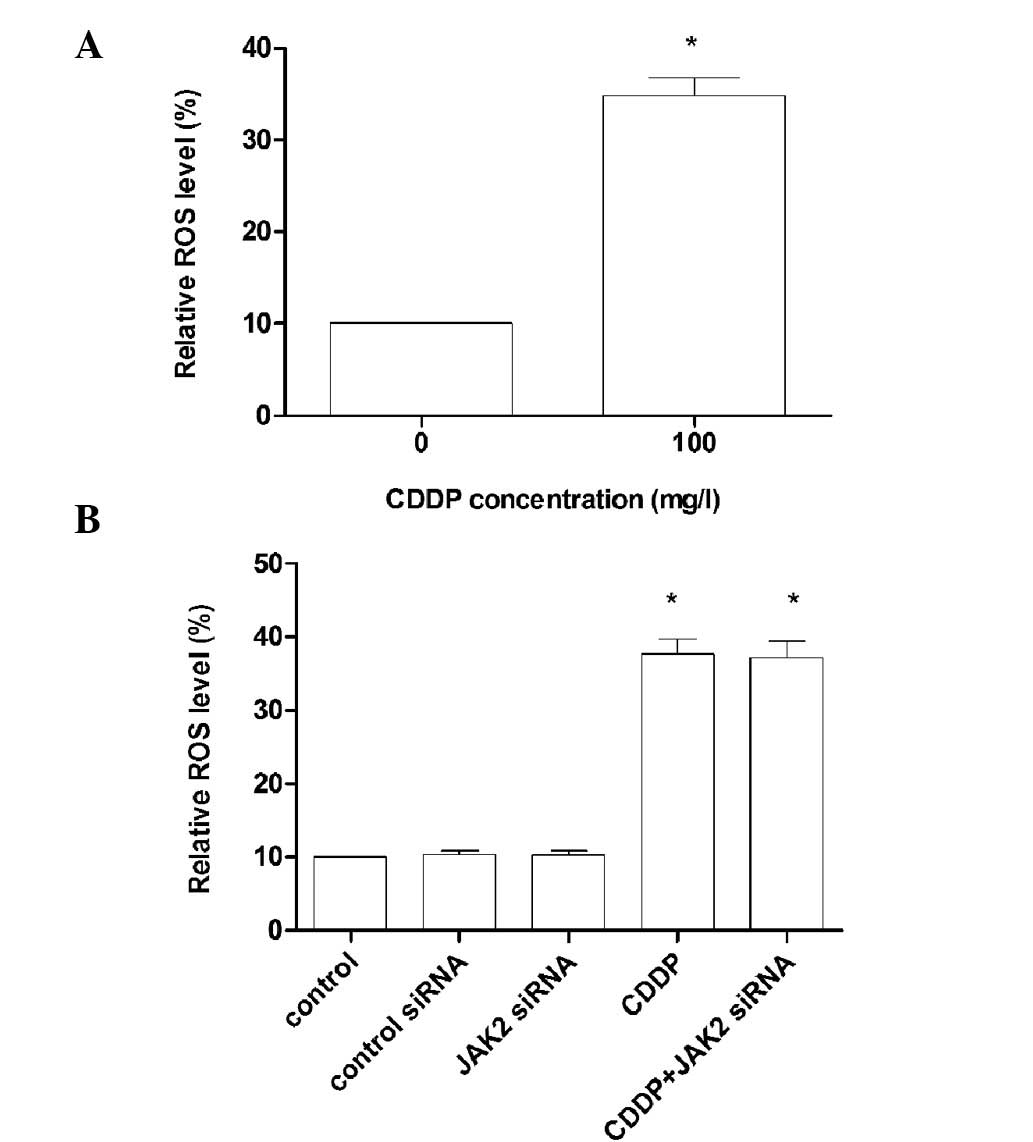
ROS production in gastric cancer
cells
BGC823 cells were treated with 30 mg/ml CDDP for 6 h
and the production of ROS was measured by flow cytometry. (Fig. 2A). BGC823 cells were then treated
with CDDP, control siRNA and JAK2 siRNA. After washing with PBS,
the cells were incubated with 33 mg/ml DCFDA in PBS for 30 min at
37°C to measure the prodution of ROS (Fig. 2B).
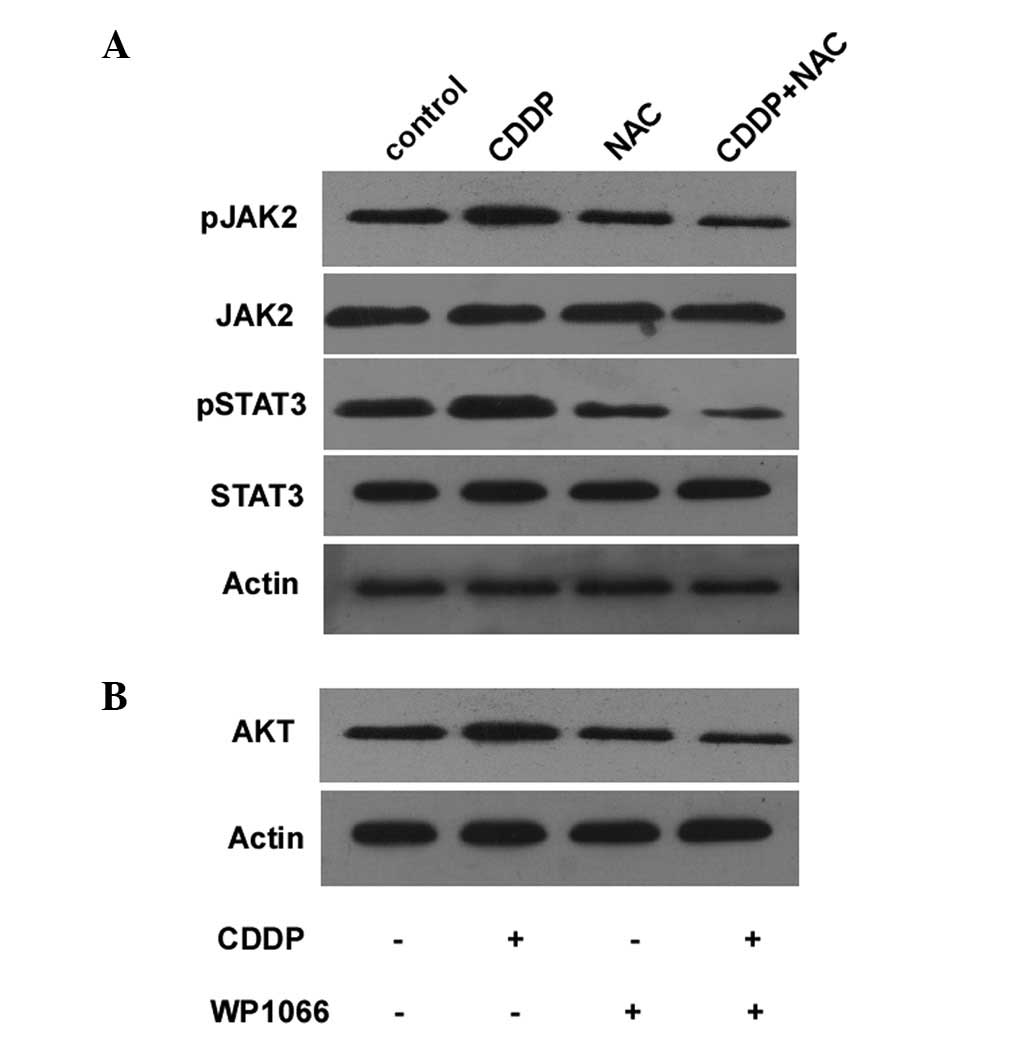
Effect of downregulation of the
JAK2/STAT3 signaling pathway in gastric cancer cells
To determine whether the AKT-induced CDDP resistance
is dependent on the activation of the JAK2/STAT3 signaling pathway,
the cells were treated with 33 mg/ml NAC and 30 mg/ml CDDP. This
significantly suppressed the activation of the JAK2/STAT3 signaling
pathway (Fig. 3A). The expression
of AKT was also decreased in the cells treated with CDDP in
combination with WP1066, a STAT3 inhibitor (Fig. 3B). These results demonstrate that
NAC increases the sensitivity of BGC823 cells to CDDP and that the
JAK2/STAT3 pathway is involved in the AKT-induced CDDP resistance
of cancer cells. Combination treatment with NAC and CDDP also
decreased the levels of phospho-JAK2 and phospho-STAT3 in the
BGC823 cells (Fig. 3A).
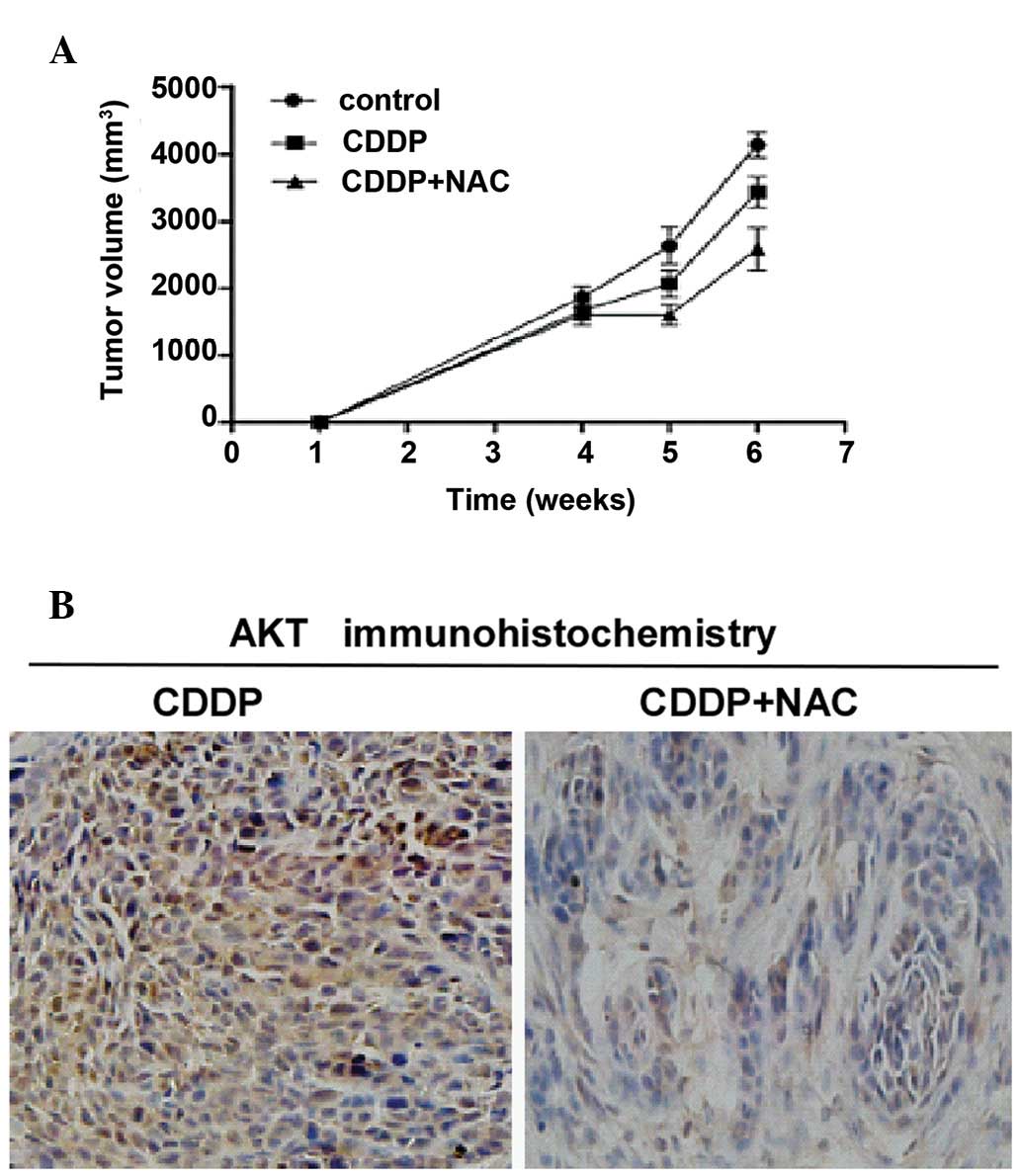
In vivo studies
Tumor development was induced by an injection of 200
μl of cell suspension (2×106 cells/ml) into the dorsal
flank of athymic nude mice at 4 weeks of age. When the diameters of
the tumors were >10 mm, the mice were treated by an
intraperitoneal (i.p.) injection of 1 mg/kg CDDP, 2 g/kg NAC and
solvent once a week for 14 days. The mice were sacrificed at 6
weeks post inoculation and tumors were excised and photographed to
measure tumor size. The tumors from the mice treated with CDDP and
NAC were significantly smaller in size compared to those from the
mice treated with CDDP alone or solvent (control) (Fig. 4A). These results supported those
obtained by immunohistochemistry assay, which showed that
combination treatment with NAC and CDDP exerted a significantly
greater inhibitory effect on AKT activation in gastric cancer cells
compared to treatment with CDDP alone (Fig. 4B)
Discussion
Previous studies have demonstrated that the
upregulation of AKT is a potential target for chemoresistance and
AKT is overexpressed in certain types of cancer cells following
treatment with CDDP (15–17). AKT is considered as an attractive
target for chemotherapy and it has been postulated that the
inhibition of AKT may preferentially kill cancer cells (18). Certain studies have reported the
association of AKT gene amplification with drug resistance
(19–22). In this study, we demonstrated that
the AKT gene is amplified in BGC823 gastric cancer cells following
treatment with CDDP (Fig. 1).
These data suggest that the AKT gene is a potential target for CDDP
resistance in BGC823 human gastric cancer cells.
The present data indicate that treatment with CDDP
significantly increases AKT expression in BGC823 gastric cancer
cells, as shown by western blot analysis. Furthermore, our data
demonstrate that AKT expression notably reaches a plateau in BGC823
gastric cancer cells. This indicates that AKT may be a positive
regulator of drug resistance in human gastric cancer cells.
Previous studies have reported that CDDP is capable of inducing ROS
production (16). Our results were
consistent with these findings. Thus, we hypothesized that ROS may
be an important component of the cellular stress signal
transduction network. In the present study, we demonstrated that
the inhibition of ROS activation inhibits AKT activation, while no
changes in the levels of ROS were observed when the cells were
treated with JAK2 siRNA (Fig. 2B),
suggesting that the decreased expression of ROS renders AKT
inactive at upstream signaling pathways. Thus, the production of
ROS induced by CDDP activates AKT, thus promoting the development
of resistance to chemotherapeutic agents, indicating the important
role of AKT in BGC823 cells.
We also determined whether the AKT-induced CDDP
resistance is dependent on the activation of the JAK2/STAT3
signaling pathway. The combined treatment of the BGC823 cells with
NAC and CDDP significantly decreased the activation of the
JAK2/STAT3 (Fig. 3A). In addition,
AKT activation was decreased when the cells were treated with CDDP
and WP1066, a STAT3 inhibitor (Fig.
3B). These results indicate that NAC increases the sensitivity
of the BGC823 cells to CDDP. Thus, the JAK2/STAT3 pathway is
involved in the AKT-induced CDDP resistance of cancer cells. This
is consistent with AKT activation through phosphorylation that is
dependent on JAK2/STAT3 activity. NAC and CDDP combination
treatment decreased the levels of phospho-JAK2 and phospho-STAT3 in
BGC823 cells (Fig. 3A).
However, we have not demonstrated the mechanism
underlying acquired drug resistance. Evidence suggests that the
JAK2/STAT3 signaling pathway is involved in drug resistance and the
regulation of AKT signaling, which may subsequently be associated
with the resistance of cancer cells. By contrast, inhibiting the
activity of ROS may improve the sensitivity and efficacy of
chemotherapeutic drugs in the treatment of gastric cancer as it may
result in the downregulation of the JAK2/STAT3 pathway and AKT,
thus increasing the sensitivity of the cancer cells to treatment.
Thus, it can be hypothesized that the overexpression of the AKT
gene may induce drug resistance in gastric cancer via a multitude
of mechanisms and AKT may be a potential molecular therapeutic
target in gastric cancer.
In conclusion, our results indicate that the
JAK2/STAT3 signaling pathway regulates AKT expression by ROS and is
thus involved in the resistance of gastric cancer cells to
chemotherapeutic agents in vitro and in vivo. The AKT
gene may provide a promising therapeutic target, which may be
targeted to overcome CDDP resistance in human tumors.
Acknowledgements
The authors would like to thank Mr. Hong Xia for
administrative support and technical assistance in this study. This
study was supported by grants from the National Natural Science
Foundation of China (no. 30770967) and the Fundamental Research
Funds for the Chinese Central Universities (no. 201130202020016 and
no. 201130202020017).
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
NAC
|
N-acetylcysteine
|
References
|
1
|
Bonenkamp JJ, Songun I, Hermans J, Sasako
M, Welvaart K, Plukker JT, van Elk P, Obertop H, Gouma DJ, Taat CW,
et al: Randomised comparison of morbidity after D1 and D2
dissection for gastric cancer in 996 Dutch patients. Lancet.
345:745–748. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohtsu A, Shimada Y, Shirao K, Boku N,
Hyodo I, Saito H, Yamamichi N, Miyata Y, Ikeda N, Yamamoto S, et
al: Randomized phase III trial of fluorouracil alone versus
fluorouracil plus cisplatin versus uracil and tegafur plus
mitomycin in patients with unresectable, advanced gastric cancer:
The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol.
21:54–59. 2003. View Article : Google Scholar
|
|
3
|
Yun J, Lee J, Park SH, Park JO, Park YS,
Lim HY and Kang WK: A randomised phase II study of combination
chemotherapy with epirubicin, cisplatin and capecitabine (ECX) or
cisplatin and capecitabine (CX) in advanced gastric cancer. Eur J
Cancer. 46:885–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu W, Pak BJ, Bani MR, Kapoor M, Lu SJ,
Tamir A, Kerbel RS and Ben-David Y: Tyrosinase-related protein 2 as
a mediator of melanoma specific resistance to
cis-diamminedichloroplatinum(II): therapeutic implications.
Oncogene. 19:395–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizutani Y and Bonavida B: Overcoming
cis-diamminedichloroplatinum (II) resistance of human ovarian tumor
cells by combination treatment with cis-diamminedichloroplatinum
(II) and tumor necrosis factor-alpha. Cancer. 72:809–818. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu LZ, Zhou XD, Qian G, Shi X, Fang J and
Jiang BH: AKT1 amplification regulates cisplatin resistance in
human lung cancer cells through the mammalian target of
rapamycin/p70S6K1 pathway. Cancer Res. 67:6325–6332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meijer C, Mulder NH, Timmer-Bosscha H,
Sluiter WJ, Meersma GJ and de Vries EG: Relationship of cellular
glutathione to the cytotoxicity and resistance of seven platinum
compounds. Cancer Res. 52:6885–6889. 1992.PubMed/NCBI
|
|
8
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
VanderWeele DJ, Zhou R and Rudin CM: Akt
up-regulation increases resistance to microtubule-directed
chemotherapeutic agents through mammalian target of rapamycin. Mol
Cancer Ther. 3:1605–1613. 2004.
|
|
11
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells andpromotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
12
|
Falasca M: PI3K/Akt signalling pathway
specific inhibitors: a novel strategy to sensitize cancer cells to
anti-cancer drugs. Curr Pharm Des. 16:1410–1416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beckmann JS, Ye YZ, Anderson PG, et al:
Extensive nitration of protein tyrosines in human atherosclerosis
detected by immunohistochemistry. Biol Chem Hoppe Seyler.
375:81–88. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu QS, Zhang J, Liu M and Dong WG:
Lentiviral-mediated miRNA against liver-intestine cadherin
suppresses tumor growth and invasiveness of human gastric cancer.
Cancer Sci. 101:1807–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Page C, Lin HJ, Jin Y, Castle VP, Nunez G,
Huang M and Lin J: Overexpression of Akt/AKT can modulate
chemotherapy-induced apoptosis. Anticancer Res. 20:407–416.
2000.PubMed/NCBI
|
|
16
|
Pommier Y, Sordet O, Antony S, Hayward RL
and Kohn KW: Apoptosis defects and chemotherapy resistance:
molecular interaction maps and networks. Oncogene. 23:2934–2949.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sekharam M, Zhao H, Sun M, Fang Q, Zhang
Q, Yuan Z, Dan HC, Boulware D, Cheng JQ and Coppola D: Insulin-like
growth factor 1 receptor enhances invasion and induces resistance
to apoptosis of colon cancer cells through the Akt/Bcl-x(L)
pathway. Cancer Res. 63:7708–7716. 2003.PubMed/NCBI
|
|
18
|
Kandasamy K and Srivastava RK: Role of the
phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in tumor
necrosis factor-related apoptosis-inducing ligand-induced apoptosis
in non-small cell lung cancer cells. Cancer Res. 62:4929–4937.
2002.
|
|
19
|
Liu Y, Chen L, Ko TC, Fields AP and
Thompson EA: Evi1 is a survival factor which conveys resistance to
both TGFbeta- and taxol-mediated cell death via PI3K/AKT. Oncogene.
25:3565–3575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Campbell RA, Bhat-Nakshatri P, Patel NM,
Constantinidou D, Ali S and Nakshatri H: Phosphatidylinositol
3-kinase/AKT- mediated activation of estrogen receptor alpha: a new
model for anti-estrogen resistance. J Biol Chem. 276:9817–9824.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamasaki F, Johansen MJ, Zhang D,
Krishnamurthy S, Felix E, Bartholomeusz C, Aguilar RJ, Kurisu K,
Mills GB, Hortobagyi GN and Ueno NT: Acquired resistance to
erlotinib in A-431 epidermoid cancer cells requires down-regulation
of MMAC1/PTEN and up-regulation of phosphorylated Akt. Cancer Res.
67:5779–5788. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia W, Mullin RJ, Keith BR, Liu LH, Ma H,
Rusnak DW, Owens G, Alligood KJ and Spector NL: Anti-tumor activity
of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation
ofEGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene.
21:6255–6263. 2002. View Article : Google Scholar : PubMed/NCBI
|