Introduction
Liver disease is one of the most serious health
problems worldwide, yet despite tremendous advances in modern
medicine, prevention and treatment options remain limited. The
pathogenesis of hepatic diseases, as well as the role of oxidative
stress and inflammation, is well established (1). Therefore, blocking or retarding the
chain reactions involved in these oxidation and inflammatory
processes is a promising therapeutic strategy for the prevention
and treatment of liver injury.
There is increasing evidence that the subcutaneous
injection of high doses of D-Galactosamine (D-Gal) induces severe
oxidative stress, as indicated by elevated levels of malonaldehyde
(MDA) and decreased levels of antioxidant enzymes in a murine model
(2,3,4).
D-Gal-induced liver injury is a well-established experimental model
that closely resembles the morphological and functional features of
human hepatitis. This model has provided insights into the
pathogenesis of clinical hepatitis that are useful for developing
novel liver-protective reagents.
The Inula britannica flower (IBF) is well
known in traditional Chinese herbal medicine. Numerous studies have
reported that the IBF has multiple biological and pharmacological
effects, including antitumor, antidiabetic (5,6,7),
immunomodulatory (8) and
hepatoprotective functions (9,10,11).
However, the mechanism by which IBF flavonoids (IBFF) exert their
hepatoprotective effect has not yet been reported.
The aim of this study was to explore whether IBFF
protects the mouse liver from D-Gal-induced injury by attenuating
oxidative stress and suppressing the inflammatory response.
Materials and methods
Materials and reagents
The IBF was obtained from Jishen Pharmacy
(Changchun, China). D-Gal was obtained from Qidong Jiufeng Industry
and Trade Co., Ltd. (Jiangsu, China). Alanine aminotransferase
(ALT), aspartate aminotransferase (AST), superoxide dismutases
(SODs), MDA, glutathione peroxidase (GSH-PX), catalase (CAT) and
inducible nitric oxide synthase (iNOS) kits were obtained from
Nanjing Jiancheng Institute of Biotechnology (Nanjing, China).
ELISA kits were obtained from R&D Systems (Shanghai, China).
The real time-PCR (RT-PCR) kit was obtained from Takara
Biotechnology (Dalian) Co., Ltd (Shiga, Japan).
Animals
Male Kunming mice (20±2 g each) were obtained from
the Jilin University Laboratory Animal Center (Changchun, China).
The mice were maintained under a constant 12-h light and dark cycle
at an environmental temperature of 25°C and 45–55% relative
humidity. All experiments were performed according to the
Guidelines of the Experimental Laboratory Animal Committee of the
Jilin Province. The study was approved by the ethics committee of
Jilin university, Changchun, China.
Preparation of IBFF
The IBF was soaked in 85% ethanol solvent (1:20 w/v)
for 3 h, placed in an ultrasonic bath and sonicated at 200 kHz at
55°C for 30 min. The samples were filtered through a 0.45 μm
microporous membrane (Shanghai Wanzi Shiye Co., Ltd., Shanghai,
China). The IBF was extracted two additional times with the same
volume of fresh solvent and the filtrate was collected. The
filtrates were mixed and concentrated under reduced pressure in a
rotary evaporator to yield dried crude extracts. Distilled water
was added to the extracts, which were then defatted using petroleum
ether and ethyl acetate. The total flavonoid extracts were purified
using AB-8 resin under the following conditions: the extracted
sample concentration was 20 mg/ml and washing was performed with
70% ethanol at a flow velocity of 2 ml/min.
The total flavonoid content of the extracts was
determined by the method described in the Chinese Pharmacopoeia
(2005, p211–212). The extracts (500 μl) were diluted and mixed with
1 ml 5% NaNO2. After standing for 6 min, 1 ml 10%
AlCl3 and 10 ml NaOH (1 M) were added to the mixture.
The mixture was adjusted to 25 ml by adding 70% ethanol and allowed
to rest for 15 min. Absorbance (A) was measured at 510 nm with 70%
ethanol as a blank control. Rutin was used as a reference standard
and the total flavonoid content was expressed relative to rutin.
The result revealed that the IBFF content of an IBF was 86.54%.
Animal grouping and treatment
The mice were divided into 5 groups (10 mice in each
group) as follows: the control, D-Gal-treated, low-dose (IBFF 125
mg/kg body weight plus D-Gal), middle-dose (IBFF 250 mg/kg body
weight plus D-Gal) and high-dose groups (IBFF 500 mg/kg body weight
plus D-Gal). All IBFF groups received the indicated concentration
of IBFF orally every 24 h for 7 consecutive days. To evaluate the
effects of IBFF in the liver injury mouse model, D-Gal was
intraperitoneally injected once at a dose of 850 mg/kg on day 7, 1
h after oral administration of IBFF. An equal volume of saline
instead of D-Gal was injected into the mice of the control
group.
Determination of liver enzyme
activity
Liver injury was assessed 24 h after D-Gal injection
by measuring the enzyme levels of ALT and AST in the serum. The
levels of ALT and AST were expressed as an international unit
(U/l). Liver enzyme levels were determined according to the
manufacturer's instructions for the ALT and AST kits.
Measurement of lipid peroxidation
level
The lipid peroxidation level of MDA was determined
by kits from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). All procedures were performed according to the
manufacturer's instructions. MDA in the liver was determined by
measuring the level of thiobarbituric acid-reactive substances
spectrophotometrically at 535 nm.
Measurement of antioxidant enzyme
activity
The activity of the antioxidant enzymes SOD, GSH-PX
and CAT were determined by kits obtained from Nanjing Jiancheng
Bioengineering Institute. All procedures were performed according
to the manufacturer's instructions. SOD activity in liver
homogenates was detected using the xanthine oxidase method. The
levels of GSH-PX in the liver homogenates were determined using a
colorimetric dithiobis(2-nitrobenzoic acid) assay. CAT activity was
assayed by measuring the decomposition of
H2O2.
Measurement of serum TNF-α, COX-2 and
iNOS levels
The serum TNF-α and COX-2 levels were measured using
ELISA kits (R&D Systems) according to the manufacturer's
instructions. The iNOS level was determined by a kit from the
Nanjing Jiancheng Bioengineering Institute. iNOS activity was
determined by measuring the level of NO generated by sodium
nitroprusside.
Histological evaluation
The livers were perfused transcardially with 25 ml
of normal saline (0.9%). Formalin-fixed specimens were embedded in
paraffin and HE-stained and images were captured under a light
microscope. The liver morphology was assessed based on the
following parameters: vacuolization, nuclear condensation,
lymphocyte infiltration and erythrocyte stasis.
RT-PCR analysis
Total RNA was isolated from liver tissues using
TRIzol reagent. First-strand complementary DNA (cDNA) was
synthesized using oligo(dT) primers and M-MLV reverse
transcriptase. RT-PCR was performed using PCR Master Mix. The
following primers were used to amplify TNF-α, COX-2 and iNOS:
TNF-α, sense 5′-TCTCAAGTCTCCACAAGAGG-3′ and antisense
5′-TGAGTTGTAACCAGGTCAG-3′; COX-2, sense 5′-AGACATCCTGATCCTGGTTT-3′
and antisense 5′-GTTCAATGGGCTGGAAGACA-3′; iNOS, sense
5′-GCTCGGGTTGAAGTGGTAT-3′ and antisense 5′-TGAAGGACTCTGAGGCTGT-3′;
and β-actin, sense 5′-ATATCGCTGCGCTGGTCGTC-3′ and antisense
5′-AGGATGGCGTGAGGGAGAGC-3′. The TNF-α PCR conditions were as
follows: denaturation at 94°C for 30 sec, annealing at 52°C for 30
sec and elongation at 72°C for 60 sec. For COX-2 and iNOS, the PCR
conditions were as follows: denaturation at 94°C for 30 sec,
annealing at 53°C for 30 sec and elongation at 72°C for 60 sec.
β-actin was used as a reference gene to normalize the expression
level of each gene.
Statistics
The data are expressed as the mean ± SE. The
statistical significance of the differences detected for each
parameter among the groups was evaluated using one-way analysis of
variance (ANOVA), followed by Fisher's protected least significant
difference (PLSD) comparison test for post hoc t-tests.
Results
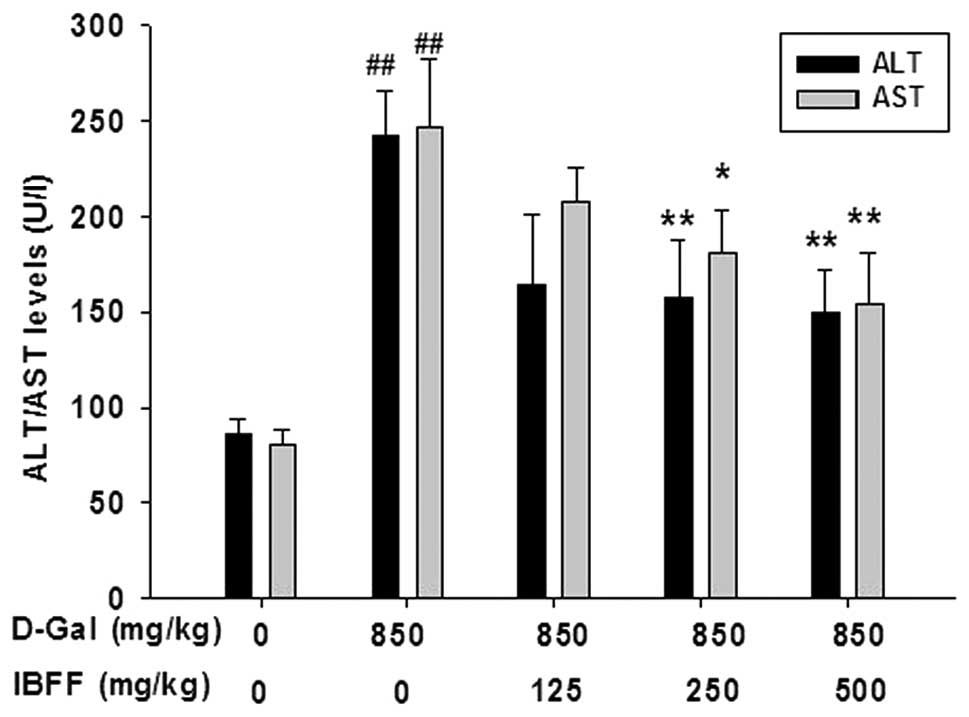
Effects of IBFF on serum ALT and AST
levels in D-Gal-induced liver injury
Several hepatic enzymes, including AST and ALT in
the serum were used as biochemical markers for acute hepatic
damage. The levels of AST and ALT were measured in the serum to
evaluate hepatic tissue damage. D-Gal administration resulted in a
statistically significant (P<0.01) increase in the AST and ALT
levels compared with the control group. Groups treated with IBFF
(125, 250, 500 mg/kg) did not show an increase in the levels of AST
and ALT in the serum (Fig. 1).
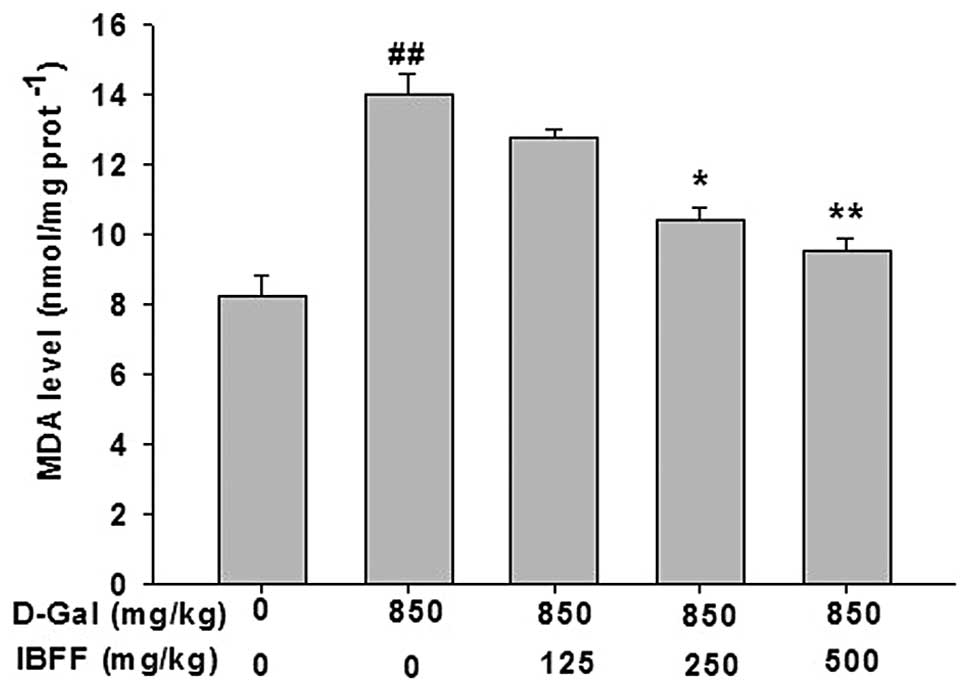
Effect of IBFF on the lipid peroxidation
level in D-Gal-induced liver injury
Hepatic levels of MDA were assessed as an indicator
of liver tissue lipid peroxidation. An increase (P<0.01) in the
levels of MDA was observed in the D-Gal-treated group when compared
with the control group. The D-Gal-induced elevation of the tissue
MDA concentration was reduced (P<0.01) by the administration of
IBFF at three different doses, as shown in Fig. 2.
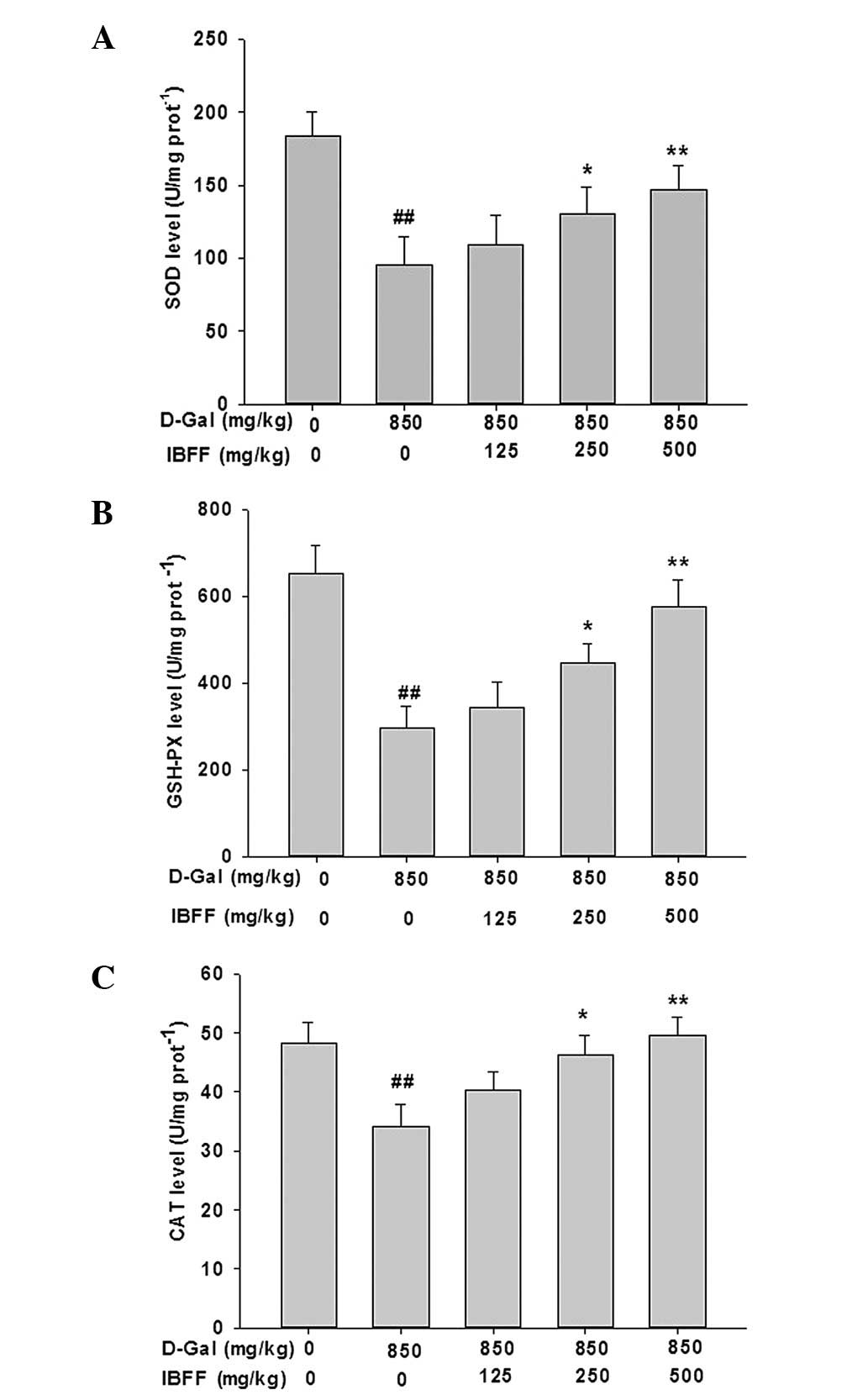
Effects of IBFF on the antioxidative
status in D-Gal-induced liver injury
To determine whether IBFF attenuates the increased
oxidative damage in the livers of D-Gal-treated mice, we determined
the levels of SOD, GSH-PX and CAT in the liver tissue. IBFF
treatment inhibited the decrease in activity of antioxidant enzymes
induced by D-Gal (Fig. 3).
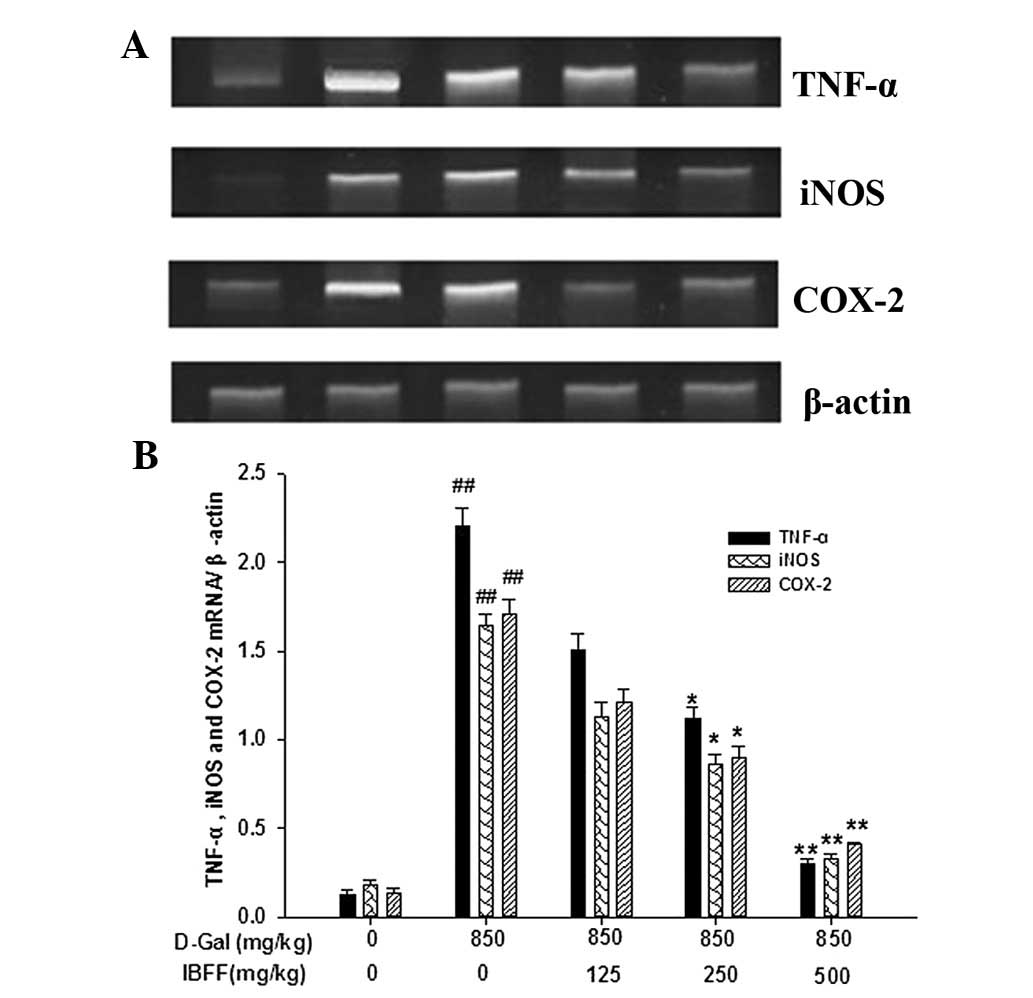
Effect of IBFF on TNF-α, COX-2 and iNOS
levels in D-Gal-induced liver injury
TNF-α and COX-2 are critical mediators of liver
injury induced by D-Gal; injection of D-Gal induced the elevation
of hepatic TNF-α and COX-2 levels. The serum TNF-α levels were
8.7±1.9 pg/ml in the control group. By contrast, the serum TNF-α
levels were increased ~4-fold by D-Gal and were attenuated by IBFF.
The changes in the COX-2 and iNOS levels were similar to that of
TNF-α (Fig. 4).
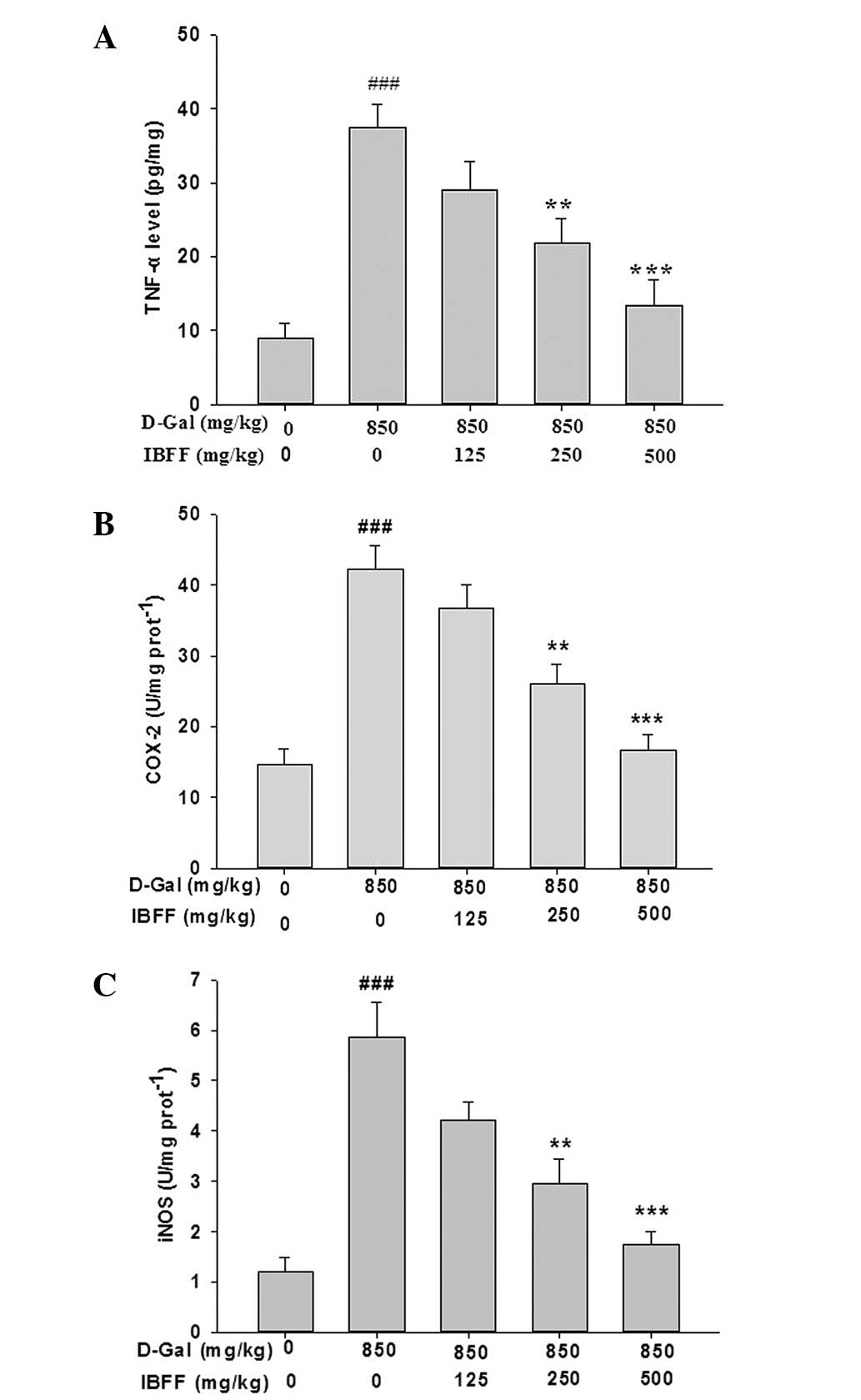 | Figure 4(A) Effect of IBFF on the TNF-α level
in D-Gal-induced liver injury. The values are reported as the means
± SE. ###P<0.001 compared with the control group;
**P<0.01, ***P<0.001 compared with the
model group. (B) Effect of IBFF on the COX-2 level in D-Gal-induced
liver injury. Values are reported as the means ± SE,
###P<0.001 compared with the control group;
**P<0.01, ***P<0.001 compared with the
model group. (C) Effect of IBFF on the iNOS level in D-Gal-induced
liver injury. Values are reported as the means ± SE,
###P<0.01 compared with the control group;
**P<0.01, ***P<0.001 compared with the
model group. D-Gal, D-Galactosamine; IBFF, Inula britannica
flower flavonoids; TNF-α, tumor necrosis factor-α; COX-2,
cyclooxygenase-2; iNOS, inducible nitric oxide synthase. |
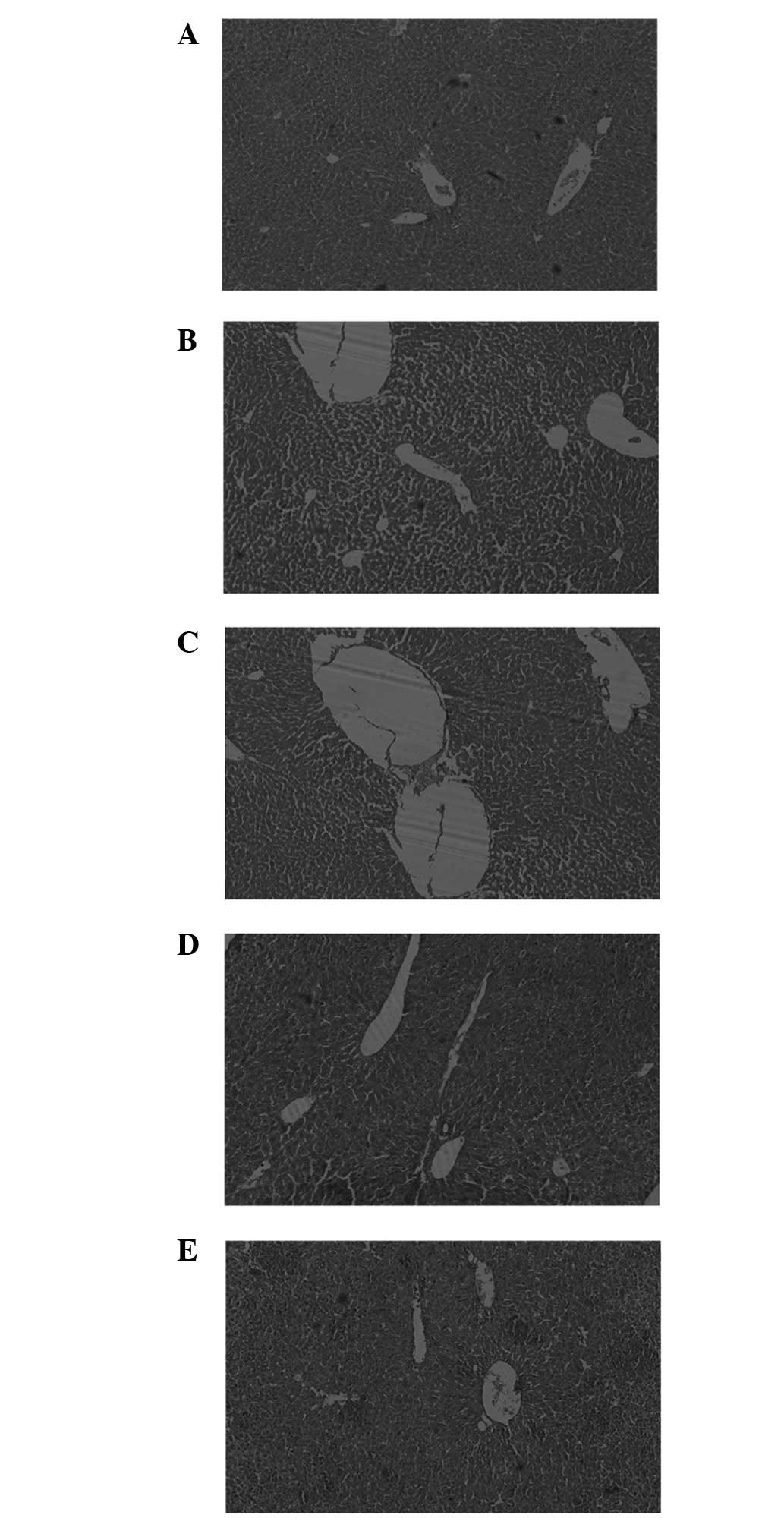
Effects of IBFF on histopathological
changes in D-Gal-induced mouse liver injury
The liver tissues revealed multiple
histopathological changes under an optical microscope (Fig. 5). Normal hepatic cells were
arranged regularly and radially around the central vein, with an
intact hepatic lobule structure. The liver cells of the mice
treated with 850 ml/kg D-Gal appeared to have degenerated, the
vacuoles underwent necrosis and an abnormal level of eosinophil
infiltration existed in the portal tracts and liver lobules. After
the administration of IBFF for 7 continuous days, the alleviation
of degeneration and necrosis in the liver tissue was observed,
accompanied by a reduction in the infiltration of inflammatory
cells. The results of the histopathological evaluation revealed
that IBFF exhibited a hepatoprotective effect against D-Gal-induced
liver injury.
Effects of IBFF on TNF-α, iNOS and COX-2
levels in D-Gal-induced mouse liver injury
Expression levels of hepatic TNF-α, iNOS and COX-2
mRNA were low in the control group. However, the mRNA expression
levels of these genes were increased by the administration of
D-Gal. The expression of TNF-α, iNOS and COX-2 mRNA was suppressed
by all three doses of IBFF (Fig.
6).
Discussion
In this study, we investigated the protective
effects of IBFF and its underlying mechanism on D-Gal-induced
experimental liver injury in mice.
During hepatic injury, the deterioration of the
integrity of the cellular membrane leads to the release of enzymes
into the circulation, reflecting damage to the hepatic cells
(12). Thus, abnormally high
levels of serum hepatospecific enzymes, including ALT and AST, are
considered to be good biomarkers for liver injury. We identified
that IBFF inhibited the levels of ALT and AST in D-Gal-induced
liver injury. The attenuated increase in serum ALT and AST levels
suggests that IBFF improved the structural integrity of the
hepatocellular membrane.
It has been demonstrated that oxidative stress is
important in hepatotoxin-mediated liver injury and the same
mechanism is involved in D-Gal-induced liver injury (13,14).
The level of MDA, which indicates the degree of lipid peroxidation,
is an oxidative stress marker. In our study, IBFF inhibited the
D-Gal-induced increase in MDA content in the mouse liver. Our
findings suggest that IBFFs attenuate oxidative stress by
decreasing the lipid peroxide level in the D-Gal-treated mouse
liver.
The defense system of antioxidant enzymes includes
SOD, CAT and GSH-PX. This study demonstrated that the activities of
antioxidant enzymes in the mouse liver were markedly decreased by
treatment with D-Gal. However, IBFF renewed the activities of the
antioxidant enzymes in the livers of D-Gal-treated mice.
Furthermore, in addition to inducing direct cellular
damage, oxidative stress activates the expression of various
inflammatory genes implicated in hepatotoxicity. D-Gal activated
monocytes to produce TNF-α, a pro-inflammatory cytokine. It has
been reported that increased levels of pro-inflammatory cytokines
and neutrophils in the liver are associated with liver cell damage
(15). TNF-α also stimulates the
release of cytokines from macrophages and induces phagocyte
oxidative metabolism and NO production. NO, a highly reactive
oxidant produced through the action of iNOS, plays a role in a
number of physiological processes (16,17).
This study confirmed an increase in the levels of TNF-α protein in
the serum and mRNA expression in liver tissue after the
intraperitoneal injection of D-Gal. These alterations were
attenuated after pretreatment by oral administration of IBFF. IBFF
also decreased the expression level of iNOS mRNA in liver
tissue.
A number of studies have suggested that the NO
released from inflammatory cells increases COX-2 activity (18). COX-2, which catalyzes the formation
of prostaglandins and other eicosanoids from arachidonic acid, was
induced at the site of inflammation. The production of prostanoids
by COX-2 has often been implicated in inflammatory diseases
characterized by edema and tissue injury (19). The present study demonstrated that
IBFF attenuated the upregulation of COX-2 expression in the livers
of D-Gal-treated mice, suggesting that IBFF attenuates inflammatory
processes by suppressing the inflammatory gene expression.
In conclusion, IBFF inhibited the increase in AST
and ALT levels, renewed the activities of antioxidant enzymes and
attenuated the upregulation of TNF-α, iNOS and COX-2 in the
D-Gal-treated mouse liver. Our findings suggest that IBFF
alleviates liver injury caused by D-Gal through antagonizing
oxidative stress and the inflammatory response.
References
|
1
|
Malhi H and Gores GJ: Cellular and
molecular mechanisms of liver injury. Gastroenterology.
134:1641–1654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ho SC, Liu JH and Wu RY: Establishment of
the mimetic aging effect in mice caused by D-galactose.
Biogerontology. 4:15–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Zhang A, Jiang B, Bao Y, Wang J
and An L: Further pharmacological evidence of the neuroprotective
effect of catalpol from Rehmannia glutinosa. Phytomedicine.
15:484–490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramana BV, Kumar VV, Krishna PN, Kumar CS,
Reddy PU and Raju TN: Effect of quercetin on galactose-induced
hyperglycaemic oxidative stress in hepatic and neuronal tissues of
Wistar rats. Acta Diabetol. 43:135–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rafi MM, Bai NS, Chi-Tang-Ho, Rosen RT,
White E, Perez D and Dipaola RS: A sesquiterpenelactone from
Inula britannica induces anti-tumor effects dependent on
Bcl-2 phosphorylation. Anticancer Res. 25:313–318. 2005.
|
|
6
|
Bai N, Lai CS, He K, Zhou Z, Zhang L, Quan
Z, Zhu N, Zheng QY, Pan MH and Ho CT: Sesquiterpene lactones from
Inula britannica and their cytotoxic and apoptotic effects
on human cancer cell lines. J Nat Prod. 69:531–535. 2006.
|
|
7
|
Kobayashi T, Song QH, Hong T, Kitamura H
and Cyong JC: Preventative effects of the flowers of Inula
britannica on autoimmune diabetes in C57BL/KsJ mice induced by
multiple low doses of streptozotocin. Phytother Res. 16:377–382.
2002.
|
|
8
|
Song QH, Kobayashi T, Hong T and Cyong JC:
Effects of Inula Britannica on the production of antibodies
and cytokines and on T cell differentiation in C57BL/6 mice
immunized by ovalbumin. Am J Chin Med. 30:297–305. 2002.
|
|
9
|
Song QH, Kobayashi T, Iijima K, Hong T and
Cyong JC: Hepatoprotective effects of Inula britannica on
hepatic injury in mice. Phytother Res. 14:180–186. 2000.
|
|
10
|
Iijima K, Kiyohara H, Tanaka M, Matsumoto
T, Cyong JC and Yamada H: Preventive effect of taraxasteryl acetate
from Inula britannica subsp japonica on experimental
hepatitis in vivo. Planta Med. 61:50–53. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng MS: An experimental study of the
anti-HSV-II action of 500 herbal drugs. J Tradit Chin Med.
9:113–116. 1989.PubMed/NCBI
|
|
12
|
Drotman RB and Lawhorn GT: Serum enzymes
as indicators of chemically induced liver damage. Drug Chem
Toxicol. 1:163–171. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM,
Shan Q and Hu B: Purple sweet potato color attenuates oxidative
stress and inflammatory response induced by d-galactose in mouse
liver. Food Chem Toxicol. 47:496–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM,
Shan Q and Hu B: Troxerutin protects the mouse liver against
oxidative stress-mediated injury induced by D-galactose. J Agric
Food Chem. 57:7731–7736. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsieh CH, Frink M, Hsieh YC, Kan WH, Hsu
JT, Schwacha MG, Choudhry MA and Chaudry IH: The role of MIP-1
alpha in the development of systemic inflammatory response and
organ injury following trauma hemorrhage. J Immunol. 181:2806–2812.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lowenstein CJ and Snyder SH: Nitric oxide,
a novel biological messenger. Cell. 70:705–707. 1992. View Article : Google Scholar
|
|
17
|
Michel T and Feron O: Nitric oxide
synthases: which, where, how, and why? J Clin Invest.
100:2146–2152. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Müller-Decker K, Berger I, Ackermann K,
Ehemann V, Zoubova S, Aulmann S, Pyerin W and Fürstenberger G:
Cystic duct dilatations and proliferative epithelial lesions in
mouse mammary glands upon keratin 5 promoter-driven overexpression
of cyclooxygenase-2. Am J Pathol. 166:575–584. 2005.PubMed/NCBI
|
|
19
|
Tanaka Y, Takahashi M, Kawaguchi M and
Amano F: Delayed release of prostaglandins from arachidonic acid
and kinetic changes in prostaglandin H synthase activity on the
induction of prostaglandin H synthase-2 after
lipopolysaccharide-treatment of RAW264.7 macrophage-like cells.
Biol Pharm Bull. 20:322–326. 1997. View Article : Google Scholar
|