Introduction
Muscle atrophy is a disease that may be caused by
denervation (1,2), joint immobilization (3,4),
hindlimb unloading (5,6) and spinal cord injury (7–9).
Resistance exercise training is a straightforward therapeutic
method that is used to prevent muscle atrophy. In particular,
isometric contraction exercise has significant protective effects
against muscle atrophy since it is a high-intensity activity
compared with other types of exercise (10,11).
However, exercise training is not suitable for patients with severe
disease or who are unable to perform voluntary limb movements.
Therefore, electrical stimulation has also been used in the
treatment of patients with muscle atrophy in a clinical setting
(22).
Electrical stimulation includes transcutaneous
electrical stimulation, as well as electrical stimulation by
implantable and semi-implantable electrodes. Transcutaneous
electrical stimulation is straightforward and easy to perform.
However, the stimulation is attenuated by the skin, which may
result in the diffusion of signals. Electrical stimulation by
implantable electrodes is characterized by precise stimulation;
however, the electrodes have to be implanted into the muscles and
eventually removed. Furthermore, stimulation cannot be easily
adjusted since the electrodes are implanted. With regard to
electrical stimulation by semi-implantable electrodes, the
electrodes are implanted into the muscles; however, they remain
linked to the equipment, which enables the intensity of the
stimulation to be easily adjusted. The effectiveness of electrical
stimulation depends on the intensity of the current, which is
affected by the frequency (12,13),
number of contractions (1,14) and chronaxie (2).
Electrical stimulation attenuates the decrease in
muscle mass and muscle fiber cross-sectional area (15). Three major protein degradation
pathways have been reported to be involved in such muscle
atrophy-associated alterations (16): i) The ubiquitin-proteasome pathway,
which includes conjugation of multi-ubiquitin moieties to the
substrate and degradation of the tagged protein by the 26S
proteasome (17). The
muscle-specific ubiquitin ligases, including atrogin-1/MAFbx
(atrogin-1) and muscle ring finger-1 (MuRF-1) are overexpressed in
atrophied muscles (16); ii) the
lysosomal protease pathway involving cathepsins, which have been
shown to increase activity in atrophied muscles (18) and iii) the calpain pathway
involving calpain 1 and calpain 2, which are cytosolic
calcium-dependent proteases with an increased expression in
atrophied muscles (18).
Examination of the electrical stimulation-induced
alterations in the expression levels of factors involved in the
above-mentioned pathways may improve our understanding of
therapeutic approaches to muscle atrophy. In the present study, we
established Sprague Dawley (SD) rat models with damaged sciatic
nerves. Electrical stimulation by semi-implantable electrodes was
administered to these SD rat models during the daytime alone,
nighttime alone and both the daytime and nighttime. Evaluation of
the muscle wet weight and the number of muscle satellite cells, as
well as western blot analysis was performed. Electrical stimulation
was demonstrated to reduce the expression levels of the cellular
proteins that contribute to muscle atrophy, including cathepsin L,
calpain 1 and the ubiquitinated MuRF-1 protein.
Materials and methods
Experimental groups
Male SD rats with damaged sciatic nerves (age, 46–49
days old; weight, 200–225 g) were maintained in a 12-h light/dark
cycle. Thirty-two rats were randomly allocated into 4 groups (each
group, n=8) and housed individually in standard wire mesh cages.
Food and sterile tap water were made freely available. The rats
received electrical stimulation by semi-implantable electrodes
during the daytime alone (group D), nighttime alone (group N) or
during the daytime and nighttime (group DN). The rats in group C
received no electrical stimulation and served as the control group.
All of the experiments in this study were approved by the Animal
Care Committee of the Affiliated Nanhua Hospital, University of
South China (Hengyang, Hunan, China).
Electrical stimulation
The electrical stimulation equipment was provided by
the South China Hospital Affiliated to the University of South
China.
The male SD rats were anesthetized. The sciatic
nerve was sharply cut at a site 5 mm from the lower edge of the
piriformis. Under a microscope, by performing epineurium suture
with a non-invasive suture needle, one end of an insulated wire was
implanted into the proximal sciatic nerve. The middle part of the
wire was fixed on the starting point of the iliac muscle tendon.
The other end of the wire was left outside of the skin. The wire
was then connected to the electrical stimulation equipment, which
was modulated to the correct parameters. The continuous electrical
stimulation parameters used were as follows: voltage, 1.5 V;
frequency, 50 Hz; pulse width, 0.5 ms and stimulus interval, 1/4,
1/3 or 1/2 sec. The same stimulation parameters were used for each
of the 3 groups (D, N and DN) in which the rats received electrical
stimulation.
Sample preparation and histological
analysis
Twelve hours following the last stimulation, all of
the animals were anesthetized by an intraperitoneal injection of
sodium pentobarbital, and then the gastrocnemius muscle was removed
and weighed using an electronic balance. The animals were then
sacrificed by an overdose of sodium pentobarbital. Isolated parts
from the muscle sample (~10 mg) were immediately frozen in acetone,
cooled in dry ice, and maintained at −80°C for further histological
examination and western blot analysis. Serial sections were cut
from the middle part of the muscle belly in the gastrocnemius
muscle and stained for lead and uranium. The number of satellite
cells was determined with transmission electron microscopy.
Western blot analysis
The total protein was harvested from muscle tissues,
separated on 10% SDS-PAGE gel and then examined by immunoblot
analysis. The primary antibodies against cathepsin L (~38 kDa),
calpain 1 (large units, ~80 kDa), MuRF-1 (~44 kDa) and β-actin were
purchased from Santa Cruz Biotechnology, Inc. [Santa Cruz, CA, USA;
anti-cathepsin L (H-80), cat. no. sc-10778, 1:200; anti-calpain 1
(D-11), cat. no. sc-271313, 1:200; anti-MuRF1 (SW-53), cat. no.
sc-134397; anti-β-actin, cat. no. sc-130301, 1:10,000]. The
secondary antibodies used in this study were goat anti-mouse
IgG-horseradish peroxidase (HRP; cat. no. sc-2005, 1:10,000; Santa
Cruz Biotechnology, Inc.) and goat anti-rabbit IgG-HRP (cat. no.
sc-2004, 1:5,000; Santa Cruz Biotechnology, Inc.). Bound antibodies
were detected using the ECL system (Pierce Biotechnology, Inc.,
Rockford, IL, USA). The immunoblot assays were repeated at least 3
times. The mean normalized optical density (OD) of the cathepsin L,
calpain 1 and MuRF-1 protein bands relative to the OD of the
β-actin band from the same animal was calculated.
Statistical analysis
The experimental data are expressed as the means ±
standard error of the mean (SEM). P<0.05 was considered to
indicate a statistically significant difference.
Results
Electrical stimulation increases muscle
wet weight
Thirty-two SD rats were randomly allocated into 4
groups (each group, n=8). The rats in group C received no
electrical stimulation; the rats in groups D, N and DN received
electrical stimulation by semi-implantable electrodes during the
daytime alone, nighttime alone and both the daytime and nighttime,
respectively. The values of body weight and the gastrocnemius
muscle wet weight are shown in Table
I. The values of body weight in the 4 groups were similar,
without statistical differences. The mean value of the
gastrocnemius muscle wet weight in groups D, N and DN were
significantly increased compared with that in group C (P<0.05).
The mean value of muscle wet weight in group DN was significantly
increased compared with those in groups D and N (P<0.05),
although there was no significant difference between groups D and
N.
 | Table IBody weight, wet weight of the
gastrocnemius muscle and the wet muscle weight/body weight ratio of
all groups (mean ± SEM). |
Table I
Body weight, wet weight of the
gastrocnemius muscle and the wet muscle weight/body weight ratio of
all groups (mean ± SEM).
| Group (n=8) |
|---|
|
|
|---|
| Weight | C | D | N | DN |
|---|
| Muscle wet weight
(mg) | 329.2±6.8 | 411.5±4.6a | 428.8±2.9a | 492.3±10.2a,b |
| Body weight (g) | 218.1±4.2 | 216.3±4.1 | 212.4±3.9 | 215.3±3.8 |
| Muscle wet
weight/body weight (mg/g) | 1.54±0.02 | 1.91±0.03a | 2.02±0.01a | 2.31±0.01a,b |
The mean value of the ratio of wet muscle weight to
body weight in group DN was also significantly increased compared
with the ratio in groups D and N (P<0.05). These results suggest
that electrical stimulation increases the muscle wet weight.
Electrical stimulation increases the
number of muscle satellite cells
In the experiments described above using electrical
stimulation by semi-implantable electrodes, muscle satellite cells
were isolated from the rats in each of the 4 groups. The number of
muscle satellite cells was counted using a microscope. The mean
number of muscle satellite cells and the ratio of the cell number
to body weight are shown in Table
II. The number of muscle satellite cells in groups D, N and DN
was significantly increased compared with that in group C
(P<0.05). The number of muscle satellite cells in group DN was
significantly increased compared with that in groups D and N
(P<0.05), although there was no significant difference between
the number of satellite cells in groups D and N. The ratio of cell
number to body weight in group DN was also significantly increased
compared with those in groups D and N (P<0.05), although there
was no significant difference between the ratios in groups D and N.
These results suggest that electrical stimulation increases the
number of muscle satellite cells.
 | Table IINumbers of muscle satellite cells in
the cross-sectional area and ratios of cell number to body weight
in all the groups (mean ± SEM). |
Table II
Numbers of muscle satellite cells in
the cross-sectional area and ratios of cell number to body weight
in all the groups (mean ± SEM).
| Group (n=8) |
|---|
|
|
|---|
| Characteristic | C | D | N | DN |
|---|
| Number of muscle
satellite cells (μm2) | 112±19 | 326±34a | 402±53a | 823±91a,b |
| Body weight (g) | 218.1±4.2 | 216.3±4.1 | 212.4±3.9 | 215.3±3.8 |
| Number of muscle
satellite cells/body weight (μm2/g) | 0.52±0.01 | 1.51±0.03a | 1.90±0.03a | 3.83±0.05a,b |
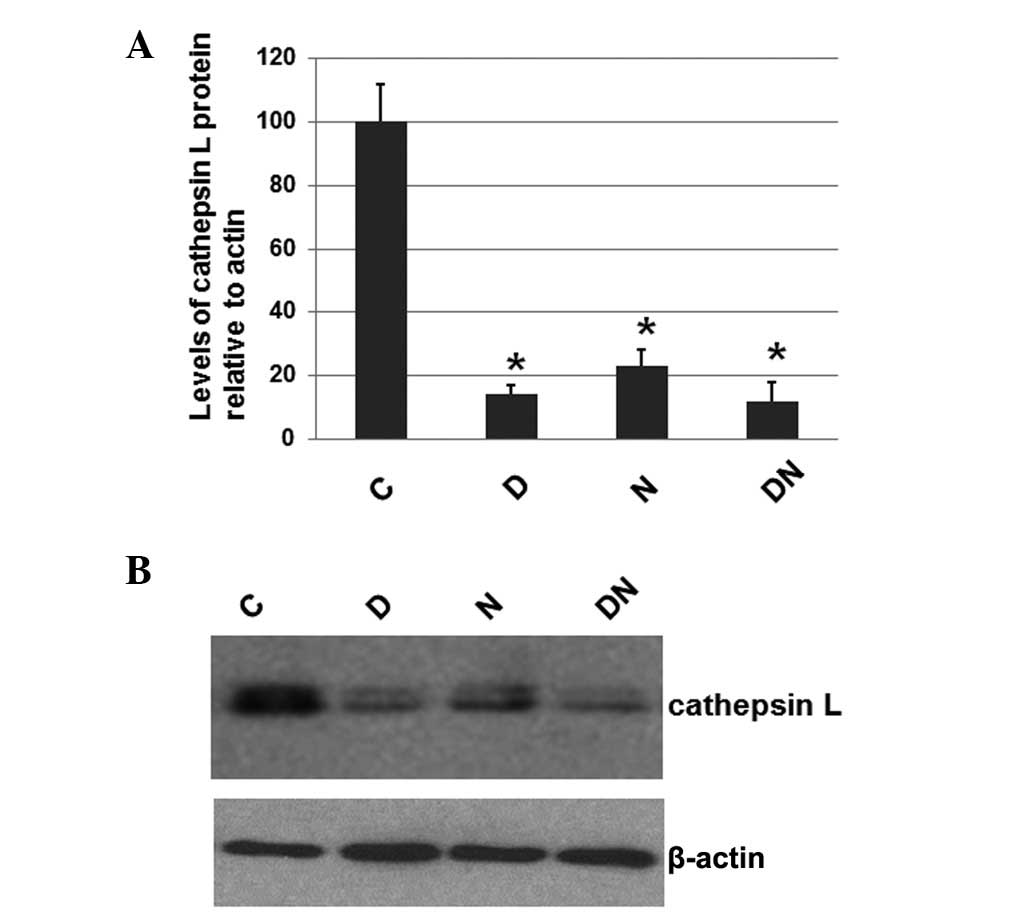
Electrical stimulation reduces the level
of cathepsin L
To investigate whether electrical stimulation by
semi-implantable electrodes affects the expression levels of
cathepsin L, a protein that is often expressed at elevated levels
in atrophied muscles, the total protein was extracted from the
muscle tissue of rats from all 4 groups. The expression level of
cathepsin L was determined by western blot analysis, with cellular
β-actin serving as a loading control. The mean normalized OD of the
cathepsin L protein bands relative to the OD of the β-actin band
from each group was calculated and subjected to statistical
analysis. The error bars show the SEM (P<0.05; Fig. 1A). Representative blots are shown
in Fig. 1B.
As shown in Fig. 1,
the expression levels of cathepsin L in the rats of groups D, N and
DN were significantly decreased compared with group C, which
received no electrical stimulation. However, the cathepsin L
expression levels in groups D, N and DN were similar. These results
suggest that electrical stimulation by semi-implantable electrodes
during the daytime, nighttime or both the daytime and nighttime
decreases the expression levels of cathepsin L.
Electrical stimulation reduces the
expression levels of calpain 1
To investigate whether electrical stimulation by
semi-implantable electrodes affects the expression levels of
calpain 1, the total protein was extracted from the muscle tissue
of rats from each group. The expression levels of calpain 1 were
determined by western blot analysis, with the cellular β-actin
protein serving as a loading control. The mean normalized OD of the
calpain 1 protein bands relative to the OD of the β-actin band from
each group was calculated and subjected to statistical analysis.
Error bars show the SEM (P<0.05; Fig. 2A). Representative blots were shown
in Fig. 2B.
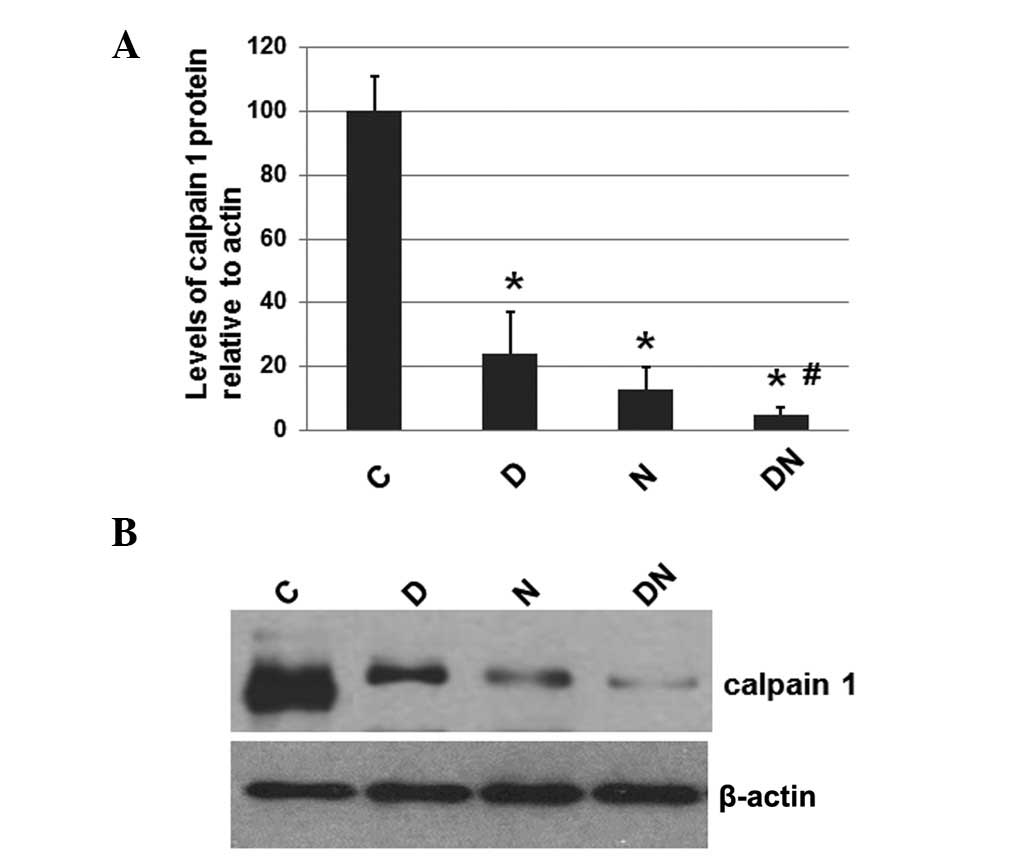 | Figure 2Immunoblot analysis of calpain 1 in
the rats of all 4 groups. The rats in group C received no
electrical stimulation; the rats in groups D, N and DN received
electrical stimulation by semi-implantable electrodes during the
daytime, the nighttime and both the daytime and nighttime,
respectively. (A) Total protein was extracted from the muscle
tissue of rats, separated on an SDS-PAGE gel and subjected to
immunoblot analysis. Primary antibodies against calpain 1 and
β-actin were used. The secondary antibody used was goat anti-mouse
IgG-HRP (cat. no. sc-2005, 1:10,000). Bound antibodies were
detected using the ECL system. The calpain 1 protein was ~80 kDa.
Histograms show the mean normalized OD of the calpain 1 protein
bands relative to the OD of the β-actin band from the rats in each
group. Error bars show the SEM. *P<0.05 compared with
the calpain 1 protein levels in the rats of group C;
#P<0.05 compared with the calpain 1 protein levels in
the rats of groups D and N. (B) Representative blots. HRP,
horseradish peroxidase; OD, optical density; SEM, standard error of
the mean; MuRF-1, muscle ring finger-1. |
As shown in Fig. 2,
the expression levels of calpain 1 in the rats of the groups which
received electrical stimulation (D, N and DN) by semi-implantable
electrodes were significantly decreased compared with the
expression levels in the rats of group C, which received no
electrical stimulation. Furthermore, the calpain 1 expression
levels in the rats of group DN were lower compared with those in
the rats of groups D and N. These results suggest that electrical
stimulation by semi-implantable electrodes during the daytime,
nighttime and both the daytime and nighttime decreases the levels
of calpain 1. Additionally, electrical stimulation during the
daytime and nighttime was more effective compared with electrical
stimulation during the daytime and nighttime alone.
Electrical stimulation decreases the
levels of the ubiquitinated MuRF-1 protein
To investigate whether electrical stimulation by
semi-implantable electrodes affects the expression levels of
MuRF-1, a protein that is often elevated in atrophied muscles, the
total protein was extracted from the muscle tissue of rats from all
4 groups. The expression levels of MuRF-1 were determined by
western blot analysis, with the cellular β-actin protein serving as
a loading control. The mean normalized OD of the MuRF-1 protein
bands relative to the OD of the β-actin band from rats in each
group was calculated and subjected to statistical analysis. Error
bars show the SEM (P<0.05; Fig.
3A). Representative blots are shown in Fig. 3B.
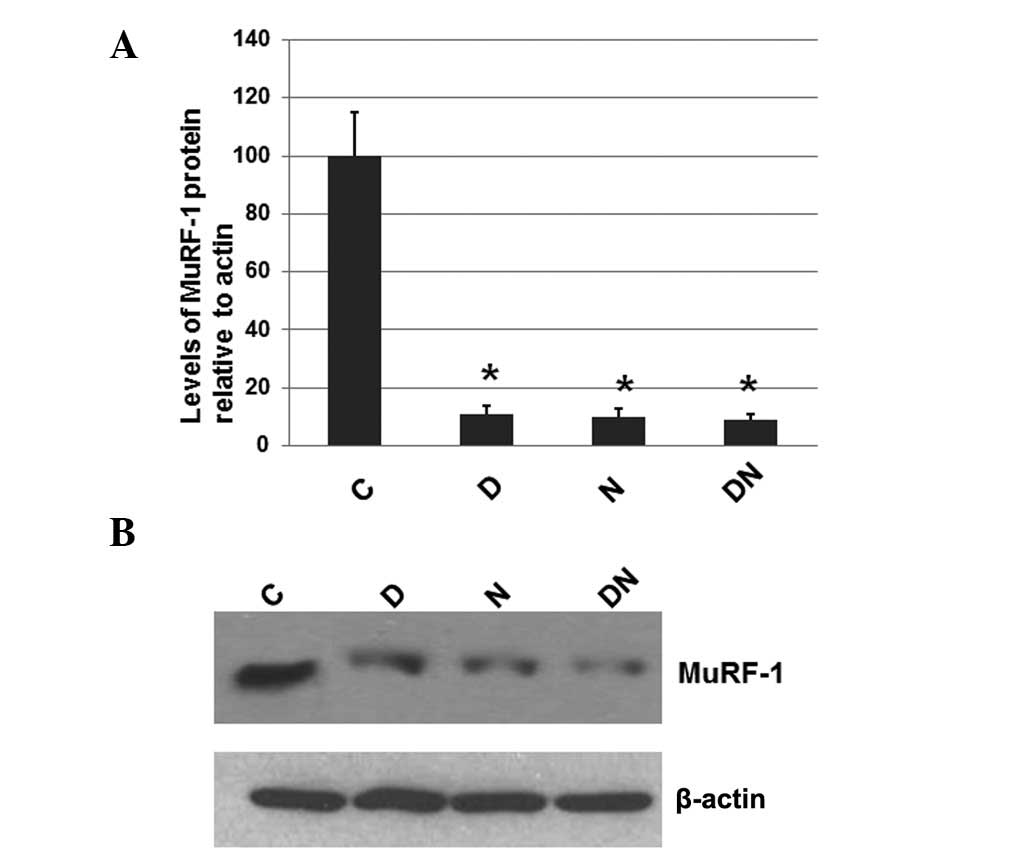 | Figure 3Immunoblot analysis of MuRF-1 in the
rats of all 4 groups. The rats in group C received no electrical
stimulation; the rats in groups D, N and DN received electrical
stimulation by semi-implantable electrodes during the daytime, the
nighttime and both the daytime and nighttime, respectively. (A)
Total protein was extracted from the muscle tissue of rats,
separated on an SDS-PAGE gel and subjected to immunoblot analysis.
Primary antibodies against MuRF-1 and β-actin were used. The
secondary antibody used was goat anti-mouse IgG-HRP (cat no.
sc-2005, 1:10,000). Bound antibodies were detected using the ECL
system. The MuRF-1 protein was ~44 kDa. Histograms show the mean
normalized OD of the MuRF-1 protein bands relative to the OD of the
β-actin band from the rats in each group. Error bars show the SEM.
*P<0.05 compared with the MuRF-1 protein levels in
the rats of group C. (B) Representative blots. HRP, horseradish
peroxidase; OD, optical density; SEM, standard error of the mean;
MuRF-1, muscle ring finger-1. |
As shown in Fig. 3,
the expression levels of MuRF-1 in the rats of all groups receiving
electrical stimulation by semi-implantable electrodes during the
daytime (group D), nighttime (group N) or both the daytime and
nighttime (group DN) were significantly decreased compared with the
rats of group C, which received no electrical stimulation. However,
the MuRF-1 expression levels among groups D, N and DN were similar.
These results suggest that electrical stimulation by
semi-implantable electrodes during the daytime, nighttime or both
the daytime and nighttime decreases the levels of MuRF-1.
Discussion
Muscle atrophy is a disease that is usually caused
by denervation (1,2), joint immobilization (3,4),
hindlimb unloading (5,6) and spinal cord injury (7–9).
Recently, several studies have reported that electrical stimulation
may be used in the treatment of muscle atrophy-related diseases
(19–24). In particular, neuromuscular
electrical stimulation has been shown to increase muscle protein
synthesis in elderly type 2 diabetic male patients (22). Electrical stimulation provides a
degree of protection against the degeneration of target and
non-target muscles during treatment with botulinum toxin type A, a
treatment modality commonly used in various neuromuscular disorders
(19).
In the present study, male SD rats were randomly
allocated into 4 groups. Depending on the group, the rats received
either no electrical stimulation (group C) or electrical simulation
by semi-implantable electrodes during the daytime (group D),
nighttime (group N) or during the daytime and nighttime (group DN).
The body weight and the gastrocnemius muscle wet weight of the rats
were measured. The number of muscle satellite cells was also
detected using a microscope. Electrical stimulation was
demonstrated to increase the number of muscle satellite cells.
Electrical stimulation during the daytime and nighttime was
demonstrated to be more effective compared with the other treatment
strategies.
Stimulation cannot be easily adjusted when the
electrodes are implanted. With regard to electrical stimulation by
semi-implantable electrodes, the electrodes are implanted into the
muscles; however, they remain linked to the equipment, allowing the
intensity of the stimulation to be more easily adjusted. The
effectiveness of electrical stimulation is determined by the
intensity of the current, which is affected by the frequency
(12,13), number of contractions (1,14)
and chronaxie (2). To investigate
the effect of electrical stimulation at the molecular level, the
expression levels of three cellular proteins involved in the muscle
atrophy process were determined. Immunoblot assay results indicated
that electrical stimulation significantly reduces the expression
levels of cathepsin L, calpain 1 and ubiquitinated protein
MuRF-1.
Although the expression levels of cathepsin L,
calpain 1 and ubiquitinated MuRF-1 protein were decreased by
electrical stimulation compared with the expression levels in the
rats of group C, which received no electrical stimulation, only
calpain 1 expression levels in group DN were lower compared with
those in groups D and N. These results indicate that the expression
levels of other unknown cellular proteins may also be altered due
to electrical stimulation.
Taken together, electrical stimulation by
semi-implantable electrodes may constitute a potential method for
the treatment of sciatic nerve injury-induced muscle atrophy. The
decreased expression levels of the cellular proteins cathepsin L
and calpain 1, as well as the ubiquitinated MuRF-1 protein are
associated with the attenuation of sciatic nerve injury-induced
muscle atrophy.
References
|
1
|
Dow DE, Cederna PS, Hassett CA,
Kostrominova TY, Faulkner JA and Dennis RG: Number of contractions
to maintain mass and force of a denervated rat muscle. Muscle
Nerve. 30:77–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Russo TL, Peviani SM, Freria CM,
Gigo-Benato D, Geuna S and Salvini TF: Electrical stimulation based
on chronaxie reduces atrogin-1 and myoD gene expressions in
denervated rat muscle. Muscle Nerve. 35:87–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujita N, Fujimoto T, Tasaki H, Arakawa T,
Matsubara T and Miki A: Influence of muscle length on muscle
atrophy in the mouse tibialis anterior and soleus muscles. Biomed
Res. 30:39–45. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujita N, Arakawa T, Matsubara T, Ando H
and Miki A: Influence of fixed muscle length and contractile
properties on atrophy and subsequent recovery in the rat soleus and
plantaris muscles. Arch Histol Cytol. 72:151–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujino H, Ishihara A, Murakami S, et al:
Protective effects of exercise preconditioning on hindlimb
unloading-induced atrophy of rat soleus muscle. Acta Physiol (Oxf).
197:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takeda I, Fujino H, Murakami S, Kondo H,
Nagatomo F and Ishihara A: Thermal preconditioning prevents fiber
type transformation of the unloading induced-atrophied muscle in
rats. J Muscle Res Cell Motil. 30:145–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roy RR, Zhong H, Hodgson JA, Grossman EJ,
Siengthai B, Talmadge RJ and Edgerton VR: Influences of
electromechanical events in defining skeletal muscle properties.
Muscle Nerve. 26:238–251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SJ, Roy RR, Zhong H, Suzuki H, et al:
Electromechanical stimulation ameliorates inactivity-induced
adaptations in the medial gastrocnemius of adult rats. J Appl
Physiol. 103:195–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SJ, Roy RR, Kim JA, Zhong H, Haddad F,
Baldwin KM and Edgerton VR: Gene expression during
inactivity-induced muscle atrophy: effects of brief bouts of a
forceful contraction countermeasure. J Appl Physiol. 105:1246–1254.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fitts RH: Effects of regular exercise
training on skeletal muscle contractile function. Am J Phys Med
Rehabil. 82:320–331. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hurst JE and Fitts RH: Hindlimb
unloading-induced muscle atrophy and loss of function: protective
effect of isometric exercise. J Appl Physiol. 95:1405–1417. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Misawa A, Shimada Y, Matsunaga T and Sato
K: The effects of therapeutic electric stimulation on acute muscle
atrophy in rats after spinal cord injury. Arch Phys Med Rehabil.
82:1596–1603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boonyarom O, Kozuka N, Matsuyama K and
Murakami S: Effect of electrical stimulation to prevent muscle
atrophy on morphologic and histologic properties of hindlimb
suspended rat hindlimb muscles. Am J Phys Med Rehabil. 88:719–726.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dennis RG, Dow DE and Faulkner JA: An
implantable device for stimulation of denervated muscles in rats.
Med Eng Phys. 25:239–253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita N, Murakami S, Arakawa T, Miki A
and Fujino H: The combined effect of electrical stimulation and
resistance isometric contraction on muscle atrophy in rat tibialis
anterior muscle. Bosn J Basic Med Sci. 11:74–79. 2011.PubMed/NCBI
|
|
16
|
Jackman RW and Kandarian SC: The molecular
basis of skeletal muscle atrophy. Am J Physiol Cell Physiol.
287:C834–C843. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Glickman MH and Ciechanover A: The
ubiquitin-proteasome proteolytic pathway: destruction for the sake
of construction. Physiol Rev. 82:373–428. 2002.PubMed/NCBI
|
|
18
|
Taillandier D, Aurousseau E, Meynial-Denis
D, et al: Coordinate activation of lysosomal,
Ca2+-activated and ATP-ubiquitin-dependent proteinases
in the unweighted rat soleus muscle. Biochem J. 316:65–72.
1996.
|
|
19
|
Fortuna R, Horisberger M, Vaz MA, Van der
Marel R and Herzog W: The effects of electrical stimulation
exercise on muscles injected with botulinum toxin type-A (botox). J
Biomech. 46:36–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clair-Auger JM, Lagerquist O and Collins
DF: Depression and recovery of reflex amplitude during electrical
stimulation after spinal cord injury. Clin Neurophysiol.
124:723–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lagerquist O, Mang CS and Collins DF:
Changes in spinal but not cortical excitability following combined
electrical stimulation of the tibial nerve and voluntary
plantar-flexion. Exp Brain Res. 222:41–53. 2012. View Article : Google Scholar
|
|
22
|
Wall BT, Dirks ML, Verdijk LB, et al:
Neuromuscular electrical stimulation increases muscle protein
synthesis in elderly type 2 diabetic men. Am J Physiol Endocrinol
Metab. 303:E614–E623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang J, Lu L, Zhang J, et al: Electrical
stimulation to conductive scaffold promotes axonal regeneration and
remyelination in a rat model of large nerve defect. PLoS One.
7:e395262012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Santoro GA, Infantino A, Cancian L,
Battistella G and Di Falco G: Sacral nerve stimulation for fecal
incontinence related to external sphincter atrophy. Dis Colon
Rectum. 55:797–805. 2012. View Article : Google Scholar : PubMed/NCBI
|