Introduction
A number of environmental and genetic factors
combined with a dysregulated immune system response contributes to
allergic diseases, including atopic dermatitis, asthma and allergic
rhinitis (1–3). The house dust mite,
Dermatophagoides pteronissinus (DpE), induces the production
of immunoglobulin E (IgE), stimulates cytokine expression by
activating immune cells and exploits defects in the skin barrier
proteins (4,5). Regulation of cytokine production is
important in the pathogenesis of allergic diseases. Cytokines,
including interleukin (IL)-6, IL-8 and monocyte chemotactic
protein-1 (MCP-1)/CCL2, participate in the shift from acute to
chronic phases of allergy and in the attraction of neutrophils and
monocytes, culminating in allergic inflammation (6–8). The
thymus and activation-regulated chemokine (TARC)/CCL17, which is a
Th2 chemokine associated with allergy, specifically atopic
dermatitis, is primarily produced in keratinocytes (9). Keratinocytes also produce skin
barrier proteins, including filaggrin, involucrin and loricrin and
defects in the skin barrier evoke atopic dermatitis (10,11).
Since the exact pathogenic mechanism of allergic diseases has not
yet been determined, general therapy for atopic dermatitis depends
on anti-inflammatory or immunosuppressive drugs. However, a number
of drugs elicit toxic side effects.
Arazyme is a novel metalloprotease, produced and
secreted in the culture medium by Aranicola proteolyticus,
also known as Serratia proteamaculans, an aerobic
gram-negative symbiotic bacterium that was isolated from the
intestine of the spider Nephila clavata(12,13).
Arazyme protects against acute hepatic injury by enhancing SMP30
expression, which suppresses the transforming growth factor-β
(TGF-β)/Smad pathway and by increasing the expression of
anti-oxidant proteins (14).
The identification of new drug candidates for the
treatment of allergic diseases using an in vitro screening
system has previously been reported (15–17).
The development of therapies for the treatment of allergic diseases
has been unsuccessful thus far. Therefore, the development of a new
screening system was beneficial. In the present study, the effect
of arazyme on cytokine and skin barrier protein production in
immune cells and skin keratinocytes was investigated, with the aim
to explore arazyme therapeutically for the treatment of allergies,
including atopic dermatitis.
Materials and methods
Enzyme purification
Arazyme was purified as previously described
(13). Briefly, extracellular
fractions were collected by centrifugation of the culture medium or
by filtration using a 0.2 μl membrane filter (Pall Life Sciences,
Port Washington, NY, USA). Chromatography was performed on a
DEAE-cellulose column equilibrated with 50 mM potassium phosphate
buffer (pH 7.6). Bound proteins were eluted with a concentration
gradient of sodium chloride ranging between 0.1 and 0.5 M at a flow
rate of 400 ml/h and each fraction was concentrated with a 10 kDa
cassette membrane (Pall Life Sciences). The protein solution was
loaded at a flow rate of 20 ml/h onto a Sephadex G-75 column
previously equilibrated with 50 mM potassium phosphate buffer (pH
7.8). Fractions containing proteolytic activity were concentrated
with the 10 kDa cassette membrane and stored at -20°C.
Cell culture
The THP-1 human monocytic cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA). The
EoL-1 human eosinophilic leukemia cell line was obtained from the
Riken Cell Bank (Tsukuba, Japan). The two cell types were cultured
in RPMI-1640 medium. HMC-1 human mast cells and human keratinocytic
HaCaT cells were cultured in Iscove’s medium and Dulbecco’s
modified Eagle’s medium, respectively, supplemented with 10%
heat-inactivated fetal bovine serum, penicillin (100 U/ml) and
streptomycin (100 μg/ml).
Cell viability assay
Cell viability was assayed based on the conversion
of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) using a cell proliferation kit (Roche Korea, Seoul, Korea).
THP-1, EoL-1, HMC-1 and HaCaT cells in 100 μl culture medium were
seeded into a 96-well plate. Arazyme was added to the wells at a
concentration ranging between 1 and 50 μg/ml. Following incubation
for 24 h at 37°C, 10 μl MTT solution was added and incubated for 4
h. Solubilization solution (100 μl) was added to the wells.
Following 24-h incubation, absorbance was measured at 550 nm using
an ELx808 enzyme-linked immunosorbent assay (ELISA) reader (Bio-Tek
Instruments Inc., Winooski, VT, USA).
ELISA
Following pre-treatment with arazyme for 30 min,
THP-1, EoL-1, HMC-1 and HaCaT cells were treated with DpE, supplied
by the Korea National Arthropods of Medical Importance Resource
Bank (Seoul, Korea). The concentrations of MCP-1, IL-6, IL-8, TARC
and tumor necrosis factor-α (TNF-α) in the supernatant were
measured by sandwich ELISA using an OptEIA Set (BD Biosciences, San
Jose, CA, USA) according to the manufacturer’s instructions. The
cytokine concentration was calculated using a linear-regression
equation obtained from the standard absorbance values.
Western blotting
HaCaT cells were seeded into a six-well plate at a
cell density of 5×106 cells/ml. Following treatment with
TNF-α and interferon-γ (IFN-γ) in the absence or presence of
arazyme, the cells were harvested and lysed in 50 μl lysis buffer
(20 mM HEPES, 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 25% glycerol, 1 mM
dithiothreitol, 0.1 mM Na3VO4 and protease
inhibitors). Samples were centrifuged at 12,000 × g for 15 min at
4°C. The protein samples (50 μg/lane) were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
to nitrocellulose filters. Blots were incubated with antibodies
against filaggrin, involucrin or loricrin (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) and were developed using the
enhanced chemiluminescence detection system (Amersham Pharmacia
Biotech., Piscataway, NJ, USA). The membrane was stripped and
reprobed with anti-ERK2 antibody as an internal control.
Statistical analysis
Data were presented as the mean ± SD. The
statistical differences were analyzed using a one-way ANOVA. The
SPSS statistical software (version 10.0; SPSS Inc., Chicago, IL,
USA) was used for statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Arazyme inhibits the secretion of MCP-1
and IL-8 in THP-1 and EoL-1 cells
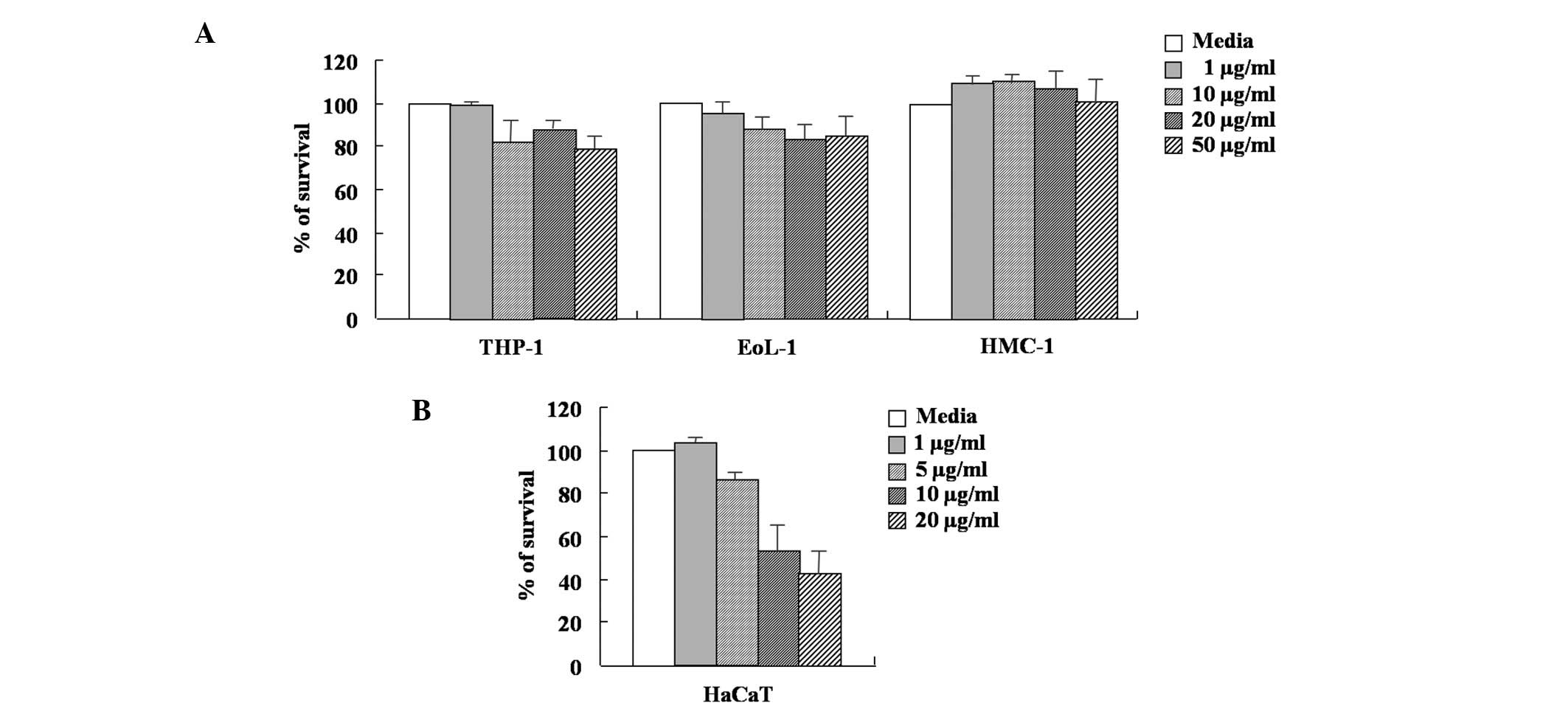
A MTT-based assay was used to determine the effect
of arazyme on the viability of THP-1, EoL-1, HMC-1 and HaCaT cells.
As shown in Fig. 1, the survival
rate of HMC-1 cells was not affected by arazyme concentration
ranging between 1 and 50 μg/ml. The viability of THP-1 and EoL-1
cells was weakly inhibited by arazyme concentration ranging between
10 and 50 μg/ml. In HaCaT cells, arazyme at a concentration of 5
μg/ml weakly inhibited cell viability. Arazyme at concentrations
ranging between 10 and 50 μg/ml was toxic. The secretion of MCP-1,
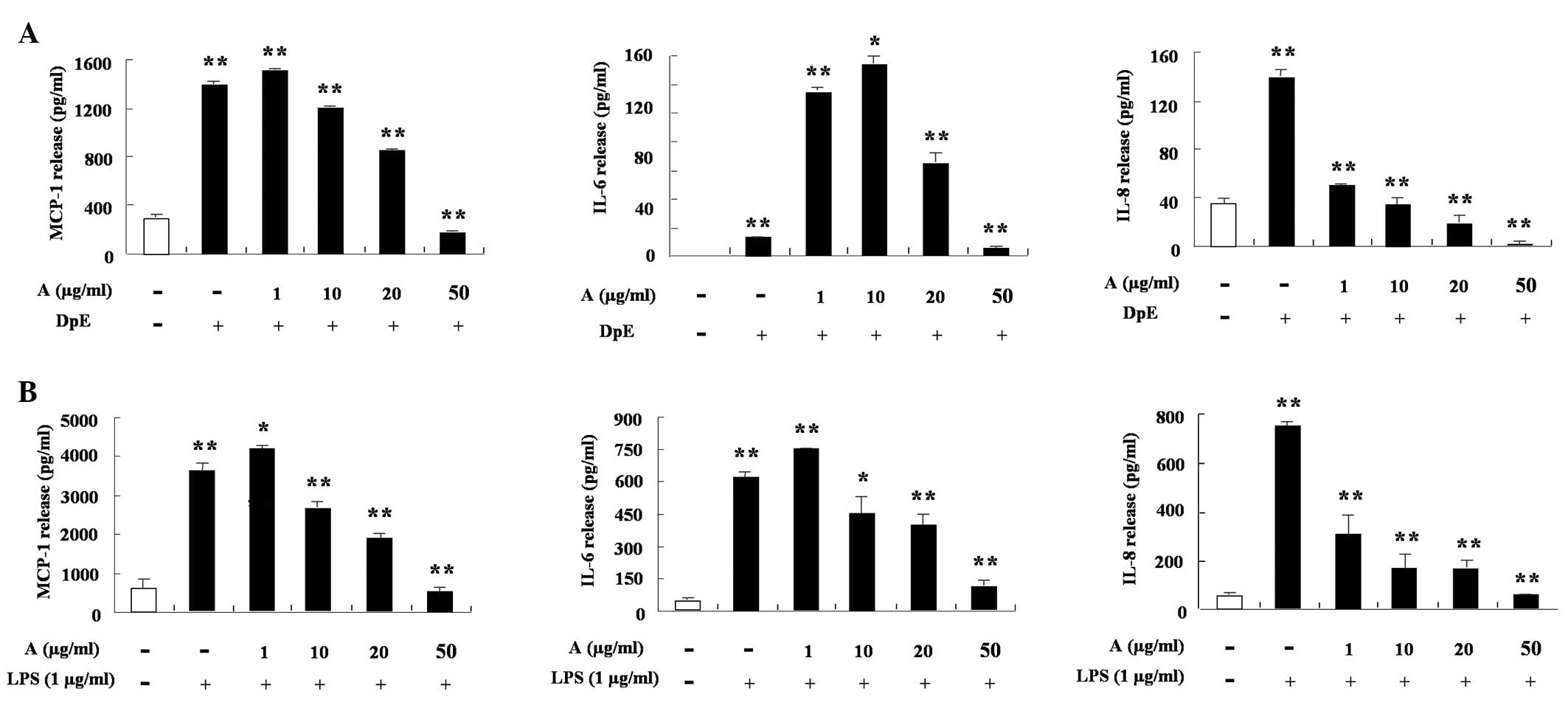
IL-6 and IL-8 increased following treatment with an extract of DpE
and lipopolysaccharide (LPS) in THP-1 cells (Fig. 2A and B). Arazyme significantly
suppressed the production of MCP-1, while IL-8 increased following
treatment with DpE in a dose-dependent manner, despite different
inhibition depending on the cytokine type (P<0.05). Arazyme also
inhibited the LPS-mediated increased production of MCP-1, IL-6 and
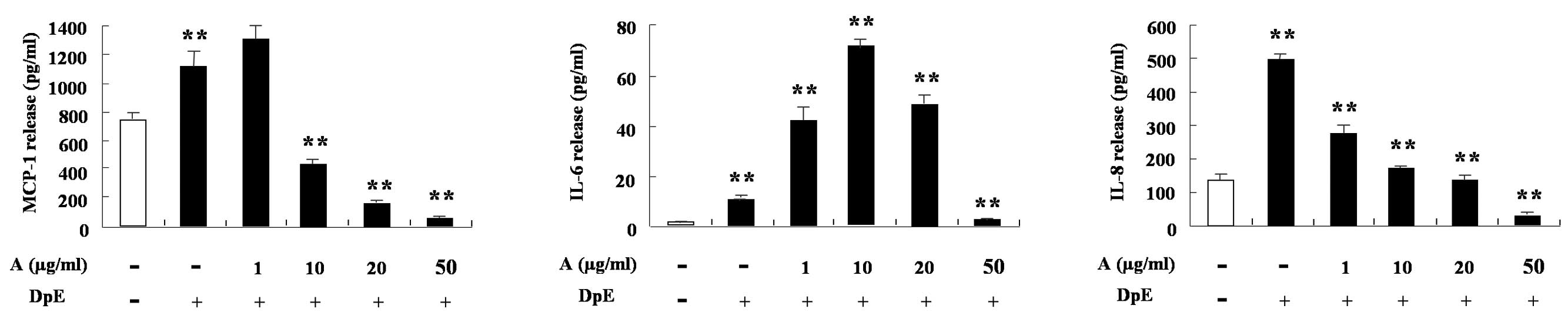
IL-8 (Fig. 2B). In EoL-1 cells,
DpE enhanced the expression of MCP-1, IL-6 and IL-8. MCP-1 and IL-8
expression decreased following treatment with arazyme in a
dose-dependent manner (Fig. 3).
IL-6 expression increased following treatment with a low
concentration of arazyme, but decreased following treatment with a
high concentration when compared with mite treatment alone.
Alteration of IL-6 by arazyme in EoL-1 cells was similar to that in
THP-1 cells (Figs. 2A and 3).
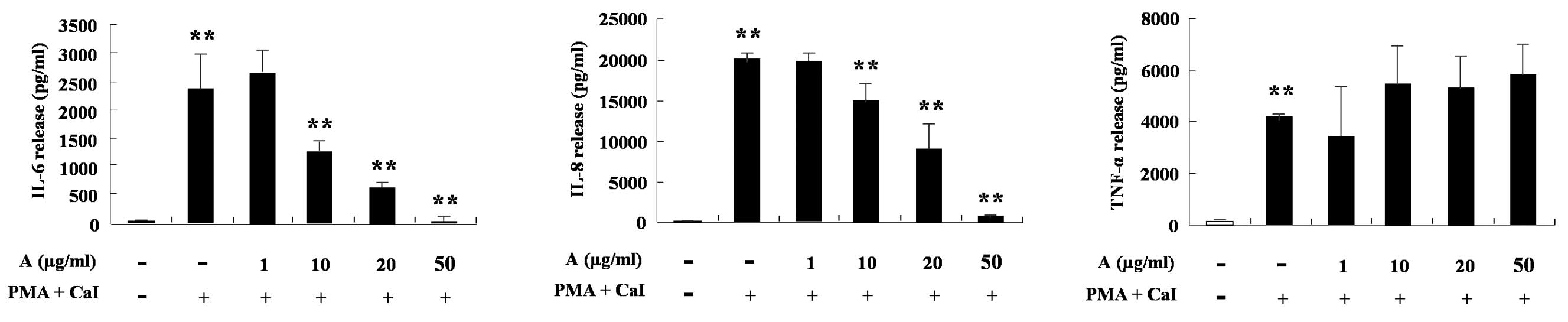
Arazyme inhibits the secretion of IL-6
and IL-8 in HMC-1 cells and the production of TARC, MCP-1, IL-6 and
IL-8 in HaCaT cells
HMC-1 cells produce IL-6, IL-8 and TNF-α following
treatment with phorbol 12-myristate 13-acetate (PMA) and calcium
ionophore (CaI). Arazyme was found to significantly inhibit the
increase of IL-6 and IL-8 induced by PMA and CaI in a
dose-dependent manner (P<0.05; Fig.
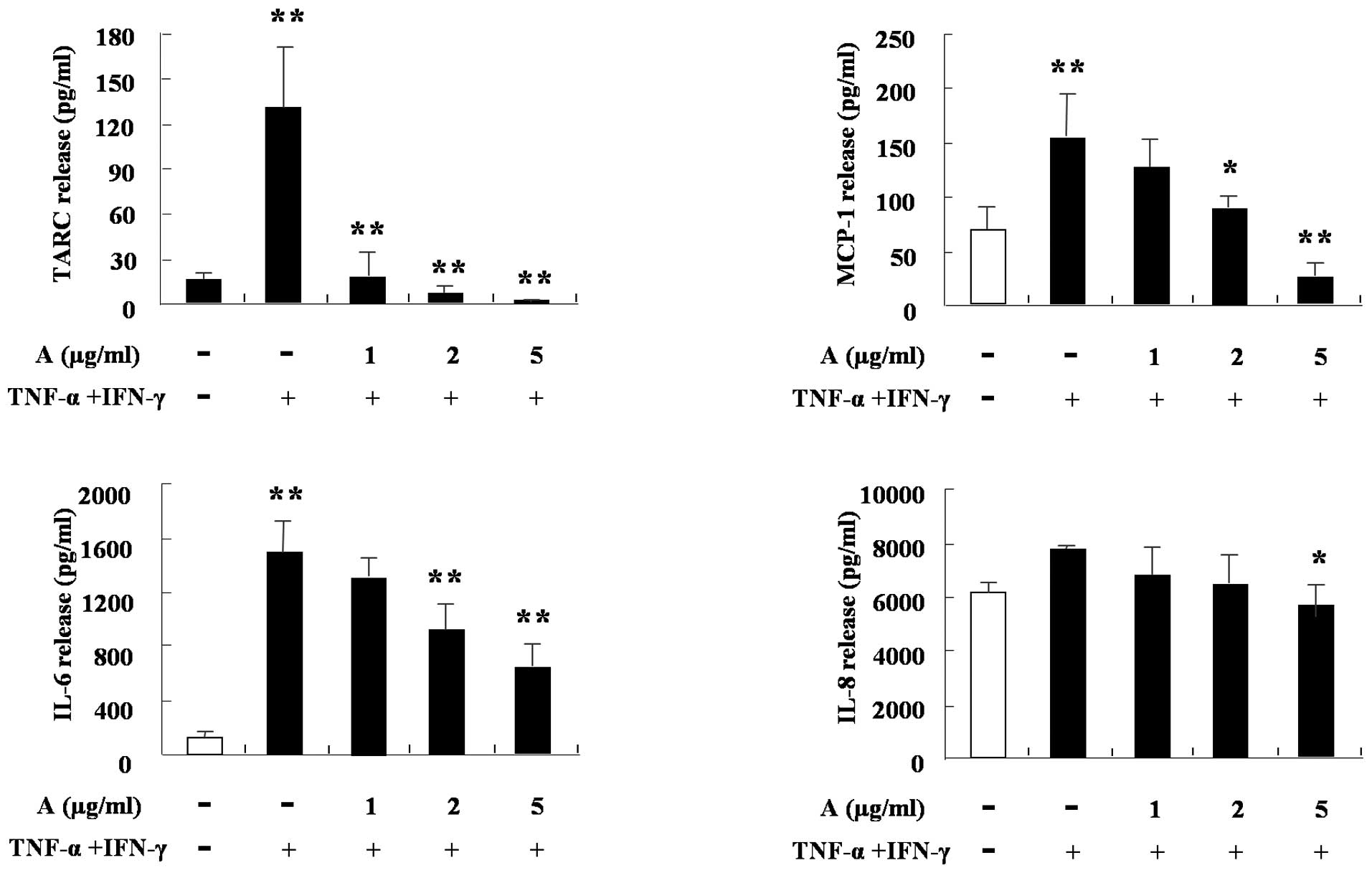
4). The cytokine production of HaCaT cells was also
investigated. TNF-α and IFN-γ increased the production of TARC,
MCP-1, IL-6 and IL-8 in the cells. IL-8 was weakly blocked by
arazyme, however, TARC, MCP-1 and IL-6 were markedly inhibited by
arazyme. The results shown in Figs.
1–5 are consistent with the
hypothesis that arazyme inhibits the cytokine production of various
cells, including monocytes, eosinophils, mast cells and
keratinocytes, thus suggesting arazyme as a possible candidate
factor for the treatment of inflammation, including allergic
diseases.
Arazyme increases the expression of
filaggrin, involucrin and loricrin in HaCaT cells
The extent of barrier dysfunction correlates with
the degree of allergy, in particular atopic dermatitis, since the
skin barrier blocks the penetration of microbes, allergens and
other environmental toxins. Skin barrier proteins include
filaggrin, involucrin and loricrin and are produced by
keratinocytes. Therefore, the effect of arazyme on the regulation
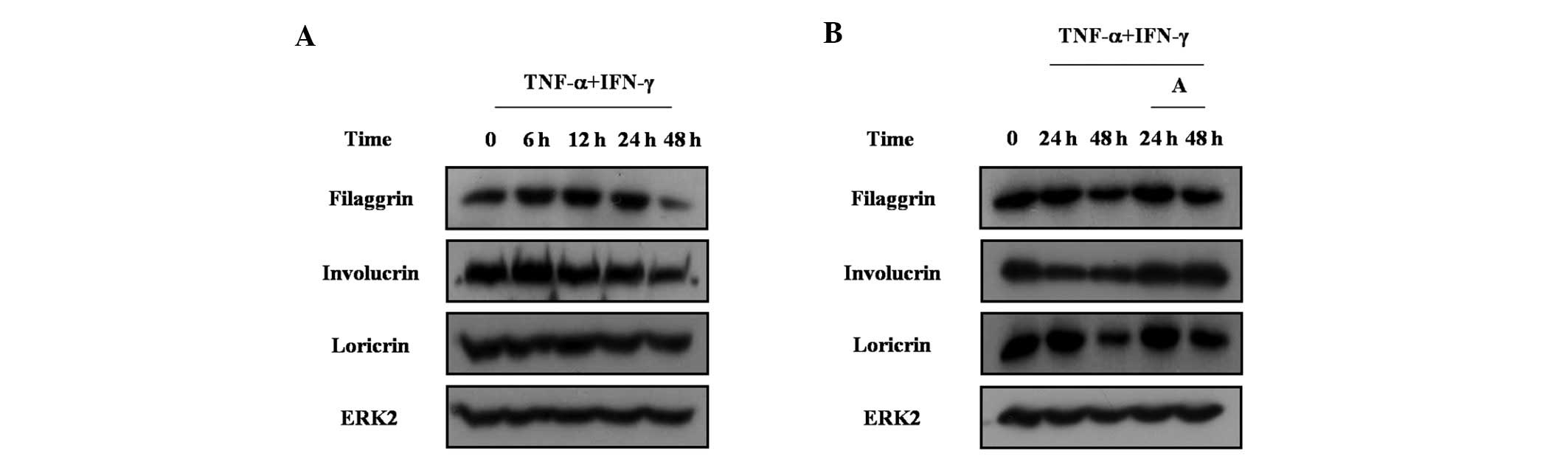
of skin barrier proteins in HaCaT cells was investigated. TNF-α and
IFN-γ treatment inhibited the expression of filaggrin, involucrin
and loricrin in HaCaT cells (Fig.
6A). Arazyme reversed the decrease of filaggrin, involucrin and
loricrin in HaCaT cells (Fig. 6B).
These results indicate that arazyme increases the expression of
skin barrier proteins under conditions where skin barrier proteins
are decreased.
Discussion
In the present study, the efficacy of arazyme as an
anti-inflammatory or anti-atopic dermatitis drug was examined for
the first time using human inflammatory-associated cells. Arazyme
was observed to inhibit the production of MCP-1, IL-6 and IL-8 in
THP-1 and EoL-1 cells, suppress the secretion of IL-6 and IL-8 in
HMC-1 cells, reduce TARC, MCP-1, IL-6 and IL-8 in HaCaT cells and
upregulate the production of filaggrin, involucrin and loricrin in
HaCaT cells.
Arazyme is a metalloprotease and its effect on the
pathogenesis of allergic inflammation is unclear, however, it is
known to protect hepatocytes that have been injured by CCl4
(14). The efficacy of arazyme as
an inhibitor of inflammation was determined by evaluating the
alteration of cytokines in inflammatory cells and skin barrier
proteins in keratinocytes. Although arazyme differentially
inhibited cytokine production, depending on the effector cells, the
enzyme had an inhibitory effect on cytokine production in THP-1,
EoL-1, HMC-1 and HaCaT cells. Arazyme blocked IL-8 expression in
all the cells used in this study and inhibited MCP-1 expression in
THP-1, EoL-1 and HaCaT cells. Since MCP-1 acts as a potent
chemoattractant of monocytes and IL-8 functions as an essential
molecule in the survival, migration and activation of neutrophils,
arazyme may inhibit the inflammatory responses by regulation of the
immune responses involved in monocytes and neutrophils (18). In the present study, arazyme also
suppressed IL-6 expression in HMC-1 and HaCaT cells in a
dose-dependent manner. In THP-1 and EoL-1 cells, arazyme increased
IL-6 expression at a low concentration and decreased the expression
at a high concentration. These observations are consistent with our
previous study (17), however, the
mechanism remains unknown. The release of IL-6 and IL-8 following
treatment with DpE in our previous studies (15,16)
was higher than that in the present study. This inconsistency may
be caused by a variety of factors, including cell culture
conditions and variations in the skills of the different
investigators. However, arazyme clearly reveals an inhibitory trend
of cytokine production similar to anti-inflammatory chemicals or
extracts (17,19,20).
Since arazyme is a protease with strong cleavage
activity, it may hydrolyze pro-inflammatory molecules, including
bradykinin and histamine, as previously observed (21,22).
This is important for determining how arazyme induces an
anti-inflammatory effect. Based on the present results, a number of
hypotheses were considered. Firstly, arazyme cleaves extracellular
inflammatory stimulators, including mite extract, LPS, TNF-α and
IFN-γ. Therefore, the stimulators do not transduce inflammatory
signals associated with production of cytokines and skin barrier
proteins. Secondly, arazyme directly cleaves cytokines, including
MCP-1, IL-6, IL-8 and TARC. Arazyme also binds to a novel and as of
yet unidentified receptor and tranduces an anti-inflammatory signal
associated with inhibition of the cytokine production and skin
barrier proteins. The exact mechanism of arazyme remains to be
elucidated and is the subject of ongoing studies.
Atopic dermatitis is an allergic skin disease
characterized by inappropriate epidermal-barrier function,
relapsing skin inflammation and IgE-mediated sensitization to
environmental allergens, including house dust mites. Filaggrin,
involucrin and loricrin are major proteins that form the epidermal
skin barrier and defects in the production and/or installation of
these proteins is important in the pathogenesis of atopic
dermatitis (23). In the present
study, filaggrin, involucrin and loricrin decreased following
treatment with TNF-α and IFN-γ in HaCaT cells and arazyme increased
expression of these molecules. Defects in skin barrier protein
production evoke or aggravate atopic dermatitis by facilitating
microbe penetration and contact of allergen and toxic chemicals
(10). A loss of function mutation
of filaggrin is associated with other allergic diseases, as well as
atopic dermatitis (24). TARC is
produced by keratinocytes and functions as a Th2 chemokine, which
induces skin inflammation in atopic dermatitis (9). In the present study, arazyme potently
decreased TARC expression in HaCaT cells. These results may
indicate that arazyme alleviates the severity of atopic dermatitis
by regulating the expression of TARC and skin barrier proteins in
keratinocytes.
Although drugs for allergy treatment, including
atopic dermatitis, are being actively developed, steroids are
broadly used as an effective drug for allergy or inflammation
therapy. However, steroids elicit a variety of side effects. To
investigate a new candidate for allergy treatment, the effect of
arazyme derived from A. proteolyticus was investigated and
arazyme was observed to induce an anti-inflammatory effect and
increase the expression of filaggrin, involucrin and loricrin in
keratinocytes. In conclusion, arazyme may be promising in the
treatment of allergic diseases.
Acknowledgements
This research was supported by a grant from KRIBB
Research Initiative Program.
References
|
1
|
Cookson WO and Moffatt MF: The genetics of
atopic dermatitis. Curr Opin Allergy Clin Immunol. 2:383–387. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bieber T: Atopic dermatitis. N Engl J Med.
358:1483–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holgate ST and Polosa R: Treatment
strategies for allergy and asthma. Nat Rev Immunol. 8:218–230.
2008. View
Article : Google Scholar
|
|
4
|
Marsella R and Samuelson D: Unravelling
the skin barrier: a new paradigm for atopic dermatitis and house
dust mites. Vet Dermatol. 20:533–540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thomas WR, Hales BJ and Smith WA: House
dust mite allergens in asthma and allergy. Trends Mol Med.
16:321–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hirano T: Interleukin 6 and its receptor:
ten years later. Int Rev Immunol. 16:249–284. 1998.PubMed/NCBI
|
|
7
|
Rossi D and Zlotnik A: The biology of
chemokines and their receptors. Annu Rev Immunol. 18:217–242. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shakoory B, Fitzgerald SM, Lee SA, Chi DS
and Krishnaswamy G: The role of human mast cell-derived cytokines
in eosinophil biology. J Interferon Cytokine Res. 24:271–281. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tokura Y: Extrinsic and intrinsic types of
atopic dermatitis. J Dermatol Sci. 58:1–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Benedetto A, Agnihothri R, McGirt LY,
Bankova LG and Beck LA: Atopic dermatitis: a disease caused by
innate immune defects. J Invest Dermatol. 129:14–30.
2009.PubMed/NCBI
|
|
11
|
Kim BE, Howell MD, Guttman-Yassky E,
Gilleaudeau PM, Cardinale IR, Boguniewicz M, Krueger JG and Leung
DY: TNF-α downregulates filaggrin and loricrin through c-Jun
N-terminal kinase: role for TNF-α antagonists to improve skin
barrier. J Invest Dermatol. 131:1272–1279. 2011.
|
|
12
|
Bersanetti PA, Park HY, Bae KS, Son KH,
Shin DH, Hirata IY, Juliano MA, Carmona AK and Juliano L:
Characterization of arazyme, an exocellular metalloprotease
isolated from Serratia proteamaculans culture medium. Enzyme
Microb Technol. 37:574–581. 2005. View Article : Google Scholar
|
|
13
|
Kwak J, Lee K, Shin DH, Maeng JS, Park DS,
Oh HW, Son KH, Bae KS and Park HY: Biochemical and genetic
characterization of arazyme, an extracellular metalloprotease
produced from Serratia proteamaculans HY-3. J Microbiol
Biotechnol. 17:761–768. 2007.PubMed/NCBI
|
|
14
|
Park JK, Jeong DH, Park HY, Son KH, Shin
DH, Do SH, Yang HJ, Yuan DW, Hong IH, Goo MJ, Lee HR, Ki MR,
Ishigami A and Jeong KS: Hepatoprotective effect of Arazyme on
CCl4-induced acute hepatic injury in SMP30 knock-out mice.
Toxicology. 246:132–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee JS, Kim IS, Ryu JS and Yun CY: House
dust mite, Dermatophagoides pteronissinus increases
expression of MCP-1, IL-6 and IL-8 in human monocytic THP-1 cells.
Cytokine. 42:365–371. 2008.
|
|
16
|
Lee JS, Kim IS, Ryu JS and Yun CY: House
dust mite, Dermatophagoides pteronissinus increases
expression of MCP-1, IL-6 and IL-8 in human eosinophilic leukemia
EoL-1 cells. Animal Cells Syst. 13:391–397. 2009.
|
|
17
|
Kim IS, Song GY, Kim DH, Cho SH, Yun CY
and Lee JS: Effect of (E)-2-(3,4-dimethoxyphenyl)-4-oxo-4H-chrom-
en-7-yl-3-(3,4-dimethoxyphenyl) acrylate on the development of
atopic dermatitis-like lesions. Life Sci. 91:338–344. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murphy PM, Baggiolini M, Charo IF, Hébert
CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ and Power CA;
International union of pharmacology. XXII Nomenclature for
chemokine receptors. Pharmacol Rev. 52:145–176. 2000.PubMed/NCBI
|
|
19
|
Yang EJ, Lee JS, Song BB, Yun CY, Kim DH
and Kim IS: Anti-inflammatory effects of ethanolic extract from
Lagerstroemia indica on airway inflammation in mice. J
Ethnopharmacol. 136:422–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JS, Kim IS, Ryu JS, Kim JH, Kim JS,
Kim DH and Yun CY: The inhibitory effect of Duchesnea
chrysantha extract on the development of atopic dermatitis-like
lesions by regulating IgE and cytokine production in Nc/Nga mice.
Phytother Res. 26:284–290. 2012.
|
|
21
|
Hauck G: Proceedings: Vitalmicroscopic
investigations of the effects of thrombin, a snake venom enzyme and
histamine effect on the mesenteric microvasculature of rabbit and
cat. Arzneimittelforschung. 26:12331976.
|
|
22
|
Wolz RL and Bond JS:
Phe5(4-nitro)-bradykinin: a chromogenic substrate for assay and
kinetics of the metalloendopeptidase meprin. Anal Biochem.
191:314–320. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Candi E, Schmidt R and Melino G: The
cornified envelope: a model of cell death in the skin. Nat Rev Mol
Cell Biol. 6:328–340. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Holloway JW, Yang IA and Holgate ST:
Genetics of allergic disease. J Allergy Clin Immunol. 125(2 Suppl
2): S81–S94. 2010. View Article : Google Scholar : PubMed/NCBI
|