Introduction
Combination chemotherapy is an effective treatment
for cancer and is often more effective than single chemotherapy due
to additive or synergistic effects. In addition, adjuvant therapy
decreases dose-related toxicities. Therefore, identifying nontoxic
chemoadjuvants, including natural compounds, may be an essential
step in advancing cancer treatment. Panax notoginseng
saponins (PNSs) are extracted from the perennial herb, notoginseng.
The main components are ginsenoside Rg1 and Rb1, and
notoginsenoside R1. Previous studies have indicated that PNS and
its components may enhance the cytotoxicities of a number of
chemotherapy agents (1–3).
Gap junctions (GJs) are specialized cell-cell
junctions that directly link the cytoplasm of neighboring cells in
the majority of vertebrate organs. A number of specific functions,
including homeostasis maintenance, morphogenesis, cell
differentiation and growth control in multicellular organisms, have
been associated with GJs.
Previous studies have shown that GJs promote
apoptosis induced by specific chemical agents in normal or tumor
cells (4,5). A study by Wang et al confirmed
that enhancing the cytotoxicities of cisplatin and etoposide
depends on GJ intercellular communication (GJIC) (6). A hypothesis derived from these
studies is that a molecular ‘death signal’ caused by the induction
of apoptotic or necrotic processes in one cell may be transmitted
to neighboring cells via GJs. Thus, a number of studies have shown
that an increase in the intercellular spread of the ‘death signal’
caused by the enhancement of GJ formation or function may
contribute to increased cytotoxic action of cisplatin (4–8). By
contrast, blocking GJ-signaling decreases the intercellular
cytotoxicity of cisplatin (8,9).
Our previous study showed that PNS increases the
cytotoxicity of cisplatin by enhancing GJ formation or function
(7); however, the mechanism of
action remains poorly defined. In the current study, the effects of
the main constituents of PNS, ginsenoside Rg1 and Rb1, and
notoginsenoside R1, on the cytotoxicity of cisplatin, were
investigated, as well as the correlation between the effects and
modulation of GJ function in transfected HeLa cells. The active
compounds in PNS responsible for the enhancement of the cytotoxic
action of cisplatin were investigated and the cellular mechanisms
underlying this action were identified.
Materials and methods
Materials
Ginsenoside Rg1 and Rb1, and notoginsenoside R1 were
purchased from Yunnan Kingpanax (Group) Co., Ltd. (Kunming, Yunnan,
China). Cisplatin, anti-hemagglutinin (HA) mouse IgG, anti-β-actin
and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St.
Louis, MO, USA). Hygromycin B, G418, 2-aminoethoxydiphenyl borate
(2-APB) and doxycycline were purchased from Calbiochem (La Jolla,
CA, USA). Cell culture reagents, calcein-acetoxymethyl ester
(calcein-AM) and TRIzol were obtained from Invitrogen Life
Technologies (Carlsbad, CA, USA). Secondary antibodies for western
blotting were obtained from Amersham Pharmacia Biotech (Piscataway,
NJ, USA). All other reagents were from Sigma-Aldrich unless stated
otherwise.
Cell lines and cell culture
Cell lines expressing heteromeric
connexin32/connexin26 (Cx32/Cx26) were cultured as described
previously (10). In this cell
line, a single bidirectional tetracycline-inducible promoter
controls the expression of the two connexins. The Cx26 has a
thrombin-cleavable C-terminal epitope tag (3.2 kDa) which includes
an HA epitope.
Transfected HeLa cells were grown at 37°C in
Dulbecco’s modified Eagle’s medium supplemented with 10% fetal
bovine serum, 100 μg/ml G418 sulfate and 200 μg/ml hygromycin B.
Connexin expression was induced with 1 μg/ml doxycycline for 48 h
prior to experimental treatments.
‘Parachute’ dye-coupling assay
The assay for GJ function was performed as described
previously (10,11). Briefly, donor and receiver cells
were grown to 70–80% confluency in 12-well plates. Donor cells from
one well were incubated in growth medium supplemented with a
freshly prepared solution of 5 μM calcein-AM for 30 min at 37°C.
Calcein-AM is intracellularly converted into the GJ-permeable dye,
calcein. Subsequently, the donor cells were trypsinized and seeded
onto the receiver cells at a ratio of 1:150. Cells were allowed to
adhere to the monolayer of receiver cells and form GJs for 4 h at
37°C and then examined by fluorescence microscopy. The average
number of receiver cells containing calcein/donor cell was
considered as a measure of the degree of GJIC.
Western blotting
Western blotting was performed as previously
described (7). Mouse anti-HA clone
HA-7 IgG was used at a 1:1,000 dilution and the secondary antibody
was used at a 1:2,000 dilution. Anti-β-actin and the secondary
antibody were used at a 1:8,000 dilution. All western blotting
exposures were in the linear range of detection and the intensities
of the resulting bands were quantified using the Quantity One
software on a GS-800 densitometer (Bio-Rad, Hercules, CA, USA).
Standard colony-forming assay
All exposures to cisplatin and the components of
PNS, i.e. ginsenoside Rg1 and Rb1, and notoginsenoside R1, were
performed for 1 or 4 h, respectively, in the dark. When combined
with cisplatin, the components of PNS were added to cells 3 h prior
to cisplatin. 2-APB, dissolved in DMSO at 2.3 mg/ml and diluted to
a final concentration of 2.3 μg/ml in culture medium, was added to
the cells 1 h prior to cisplatin.
Cisplatin cytotoxicity was assessed as previously
described (12). This assay is a
standard colony-forming assay, adapted for use at high and low cell
density, according to conditions in which junctional channel
formation is permitted or not permitted, respectively. For high
density conditions, cells were seeded at 30,000 cells/ml to obtain
70–100% confluence when exposed to cisplatin. At this density,
there was substantial opportunity for GJ formation since each cell
was in contact with an average of 3–5 other cells. For low-density
conditions, cells were seeded at 100 cells/ml into 6-well plates.
Following 4-h treatment, cells were exposed to cisplatin, washed
with PBS and replenished with fresh media. At low seeding density,
GJ formation was prevented as cells did not contact each other.
Cells were rinsed and assessed for colony formation. Colony
formation was normalized to the colony forming efficiency of
nondrug-treated cells using the following formula: Surviving
fraction = drug-treated cell clone number/nondrug-treated cell
clone number.
To avoid discrepancies in results caused by cells
being in different stages of the cell cycle, serum-free medium was
used for 24 h prior to exposure of cisplatin to maintain cells
synchronously in the G1 phase. There was no significant difference
in plating efficiency between the low- and high-density cultures in
the untreated samples (data not shown).
RNA extraction and reverse transcription
polymerase chain reaction (RT-PCR)
TRIzol reagent was used to extract total RNA
according to the manufacturer’s instructions. Complementary DNA
(cDNA) was synthesized from 1 μg RNA using the standard procedure
with avian myeloblastosis virus reverse transcriptase (Promega
Corporation, Madison, WI, USA). For PCR quantification, 2 μl cDNA
was amplified in a 20 μl standard PCR. PCR was performed by initial
denaturation at 94°C for 3 min, 36 cycles of 94°C for 45 sec, 55°C
for 45 sec and 72°C for 45 sec, and a final extension for 10 min at
72°C, followed by termination at 4°C. RT-PCR was performed using
the following pairs of primers (Invitrogen Life Technologies) for
the semiquantitative assessment: rat Cx26 forward,
5′-TCTCTCACATCCGGCTCTGG-3′ and reverse,
5′-TCCGTTTCTTTTCGTGTCTCC-3′, yielding a 102 bp product; and human
β-actin forward, 5′-CGTGGACATCCGCAAAGAC-3′ and reverse,
5′-GCATTTGCGGTGGACGAT-3′, yielding a 256-bp product. The detection
of β-actin transcripts provided an internal control in PCR,
standardizing the quantity of input cDNA. PCR products were
separated by electrophoresis on a 1.5% agarose gel and visualized
under UV using the gel documentation system (Bio-Rad).
Statistical analysis
Statistical analyses between groups were performed
using an unpaired Student’s t-test with SigmaPlot 10.0 software
(Jandel Scientific, San Rafael, CA, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Differential effects of cell density on
cytotoxicity of cisplatin
Since the formation of GJ channels relies on the
end-to-end docking of two hemichannels in adjacent cells, GJIC may
occur when cells contact each other. At low density, cells were
seeded at 100 cells/ml into 6-well plates. These cells were not in
direct contact with each other and had no opportunities to form
GJs. However, at high density, when cells were seeded at 30,000
cells/ml, GJ formation was possible since the cells were in contact
with one another. To determine the effect of short-term cisplatin
exposure on the survival of HeLa cells expressing Cx32/Cx26, colony
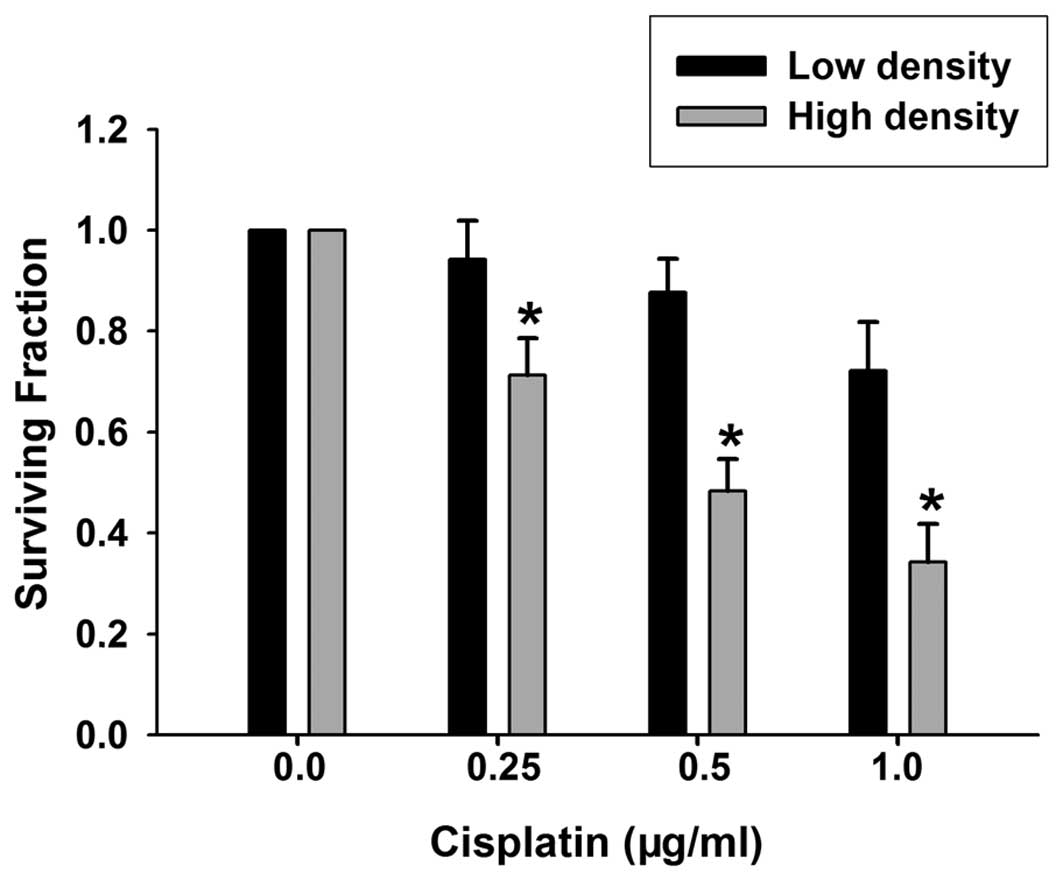
formation was performed in the two culture conditions. As shown in
Fig. 1, the clonogenic survival of
cells at low and high densities was reduced by treatment with
cisplatin (0.25–1 μg/ml) for 1 h. Compared with the samples at low
density, the toxic effect of cisplatin was substantially greater in
the samples at high density, indicating that the cytotoxicity of
cisplatin is cell density dependent. The concentration of cisplatin
used was within the therapeutic range reached in tissue during
chemotherapy (13).
Density dependence of cisplatin response
is mediated by GJIC
From the aforementioned results, cell density was
observed to contribute to the cytotoxicity of cisplatin and GJ
formation was noted to depend on cell density. Thus, GJIC was
hypothesized to mediate the density dependence of the cisplatin
response. To investigate the role of GJIC in cisplatin sensitivity,
GJ function was examined using two methods: the doxycycline
induction of connexin expression and the pharmacological inhibition
of junctional channels by 2-APB (14). HeLa cells transfected with
Cx32/Cx26 were used in the current study and connexin expression
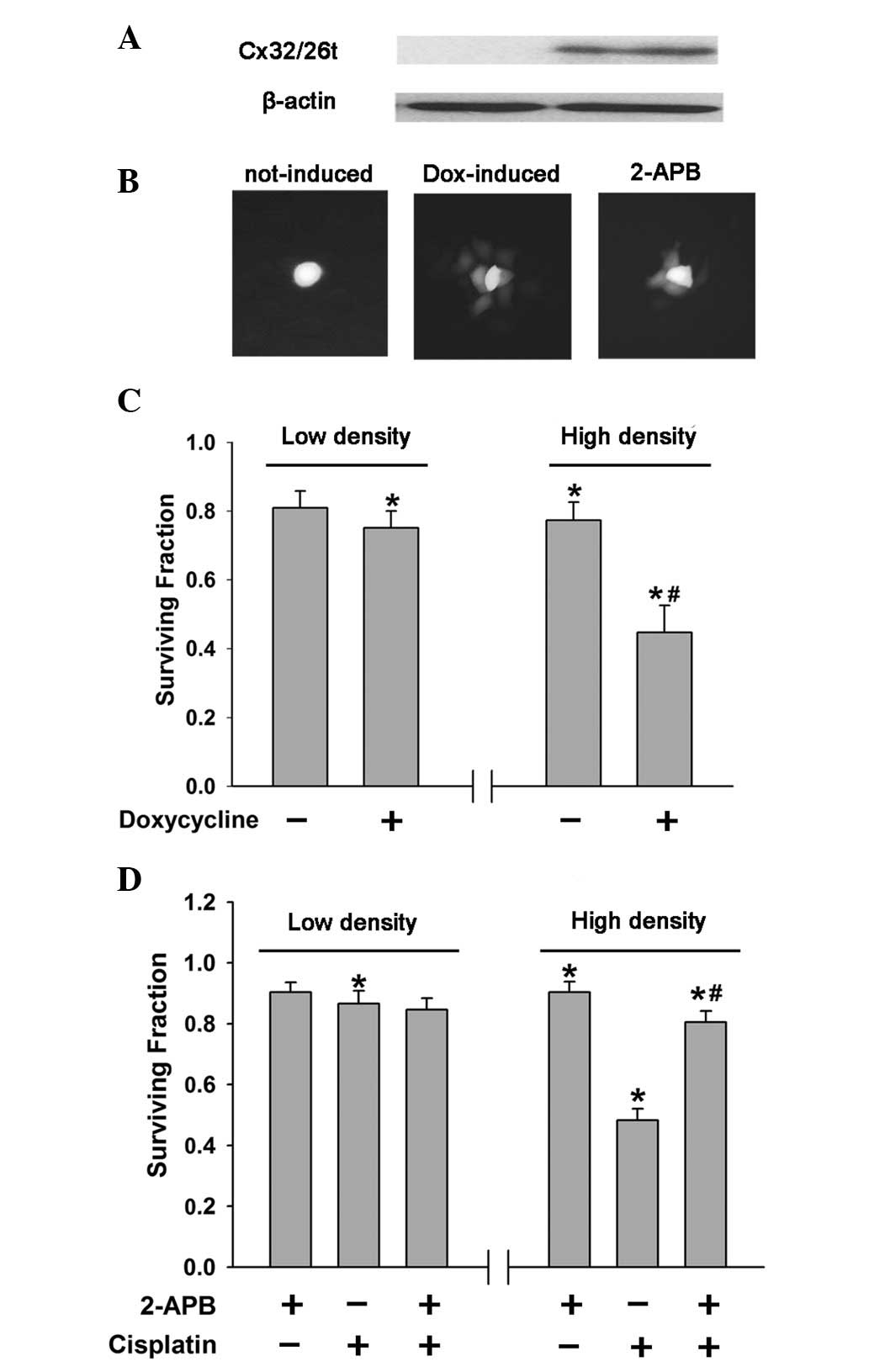
was induced with 1 μg/ml doxycycline for 48 h. The results of
western blotting and ‘parachute’ dye-coupling assay showed that the
expression of Cx32/Cx26 and dye-coupling were significantly induced
by doxycycline (Fig. 2A and B). In
low cell density cultures, without GJIC, there was no significant
difference in cisplatin survival between the doxycycline-induced
and uninduced cells, with and without connexin expression,
respectively, (Fig. 2C). However,
cells treated with doxycycline were more sensitive to cisplatin
compared with untreated cells at high density (Fig. 2C). Incubating the cells with 2-APB
(2.3 μg/ml), a membrane-permeable reagent that was verified to
inhibit dye-coupling in HeLa cells (Fig. 2B) substantially increased cell
survival in high-density cells, with GJIC (Fig. 2D). However, at low cell density,
2-APB had no effect on cisplatin toxicity (Fig. 2D). The 2-APB-mediated reduction of
GJIC and the doxycycline-induced enhancement of GJ function
affected the cytotoxicity of cisplatin at high cell density, which
is in agreement with the hypothesis that GJIC mediates the
cytotoxicity of cisplatin at high cell densities.
Effects of ginsenoside Rg1 and Rb1, and
notoginsenoside R1 on the cytotoxicity of cisplatin
Our previous study showed that PNS enhanced the
function of GJ and the cytotoxicity of cisplatin in high-density
cultures, with GJ formation (7).
Ginsenoside Rg1 and Rb1, and notoginsenoside R1 were shown to be
essential effectors. To assess the role of these components in
modulating the cytotoxicity of cisplatin, the effects of
ginsenoside Rg1 and Rb1, and notoginsenoside R1 on
cisplatin-induced cytotoxicity in HeLa cells were examined.
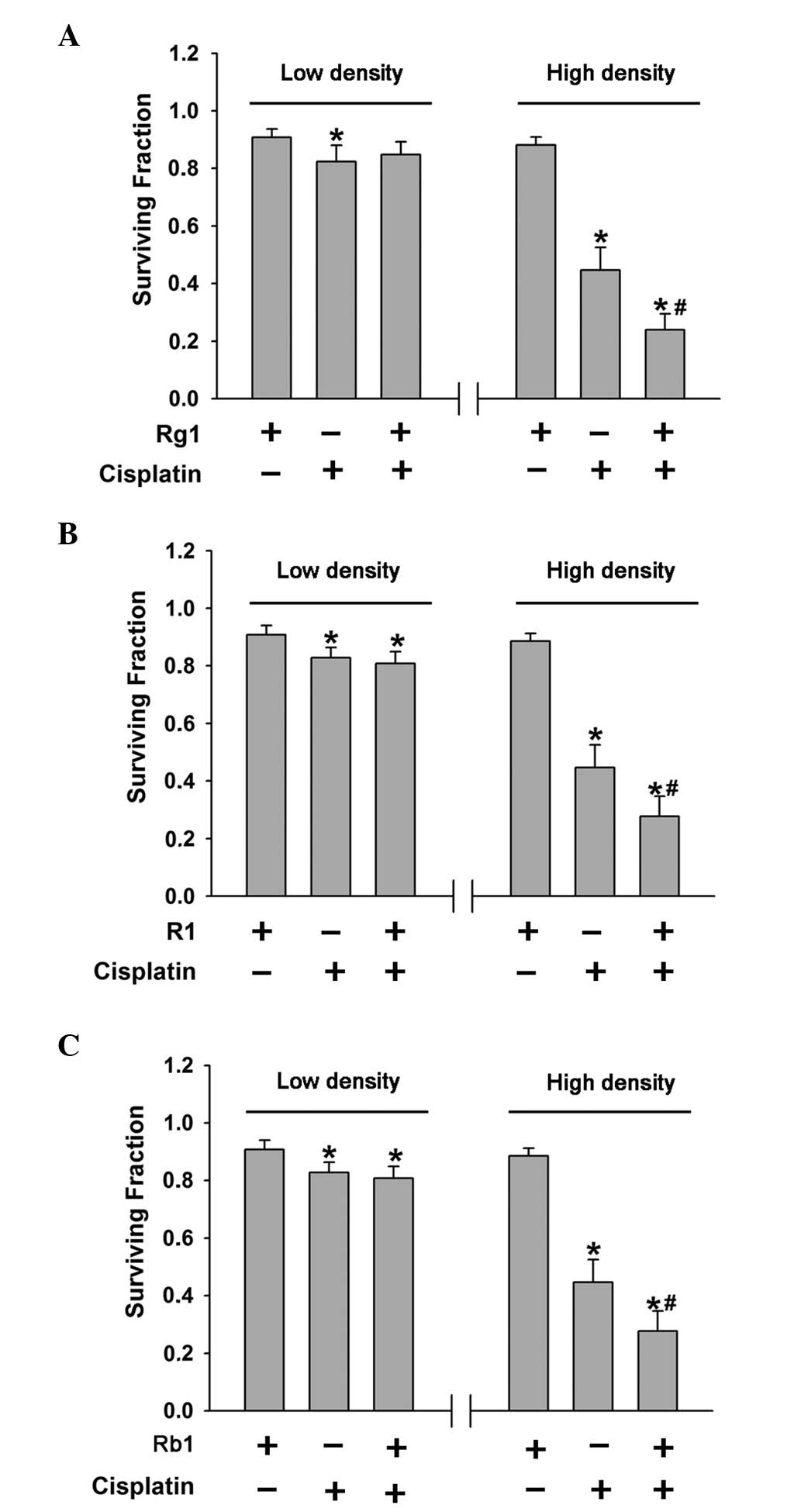
Cells seeded at high or low cell densities were
treated with ginsenoside Rg1 and Rb1, and notoginsenoside R1 for 3
h, followed by exposure to 0.5 μg/ml cisplatin and these components
for 1 h. The clonogenic survival of HeLa cells was examined 7 days
following exposure to cisplatin and ginsenoside Rg1 and Rb1, and
notoginsenoside R1. Ginsenoside Rg1 and notoginsenoside R1 had no
effect on cisplatin toxicity in low-density cultures; however, the
cytotoxicity of cisplatin was enhanced in high-density cultures
(Fig. 3A and B). By contrast,
ginsenoside Rb1 had no effect on low- or high-density cultures
(Fig. 3C). Thus, ginsenoside Rg1
and notoginsenoside R1 enhance the toxicity of cisplatin in
high-density cultures where there is an opportunity for GJ
formation.
Effects of ginsenoside Rg1 and Rb1, and
notoginsenoside R1 on GJ function
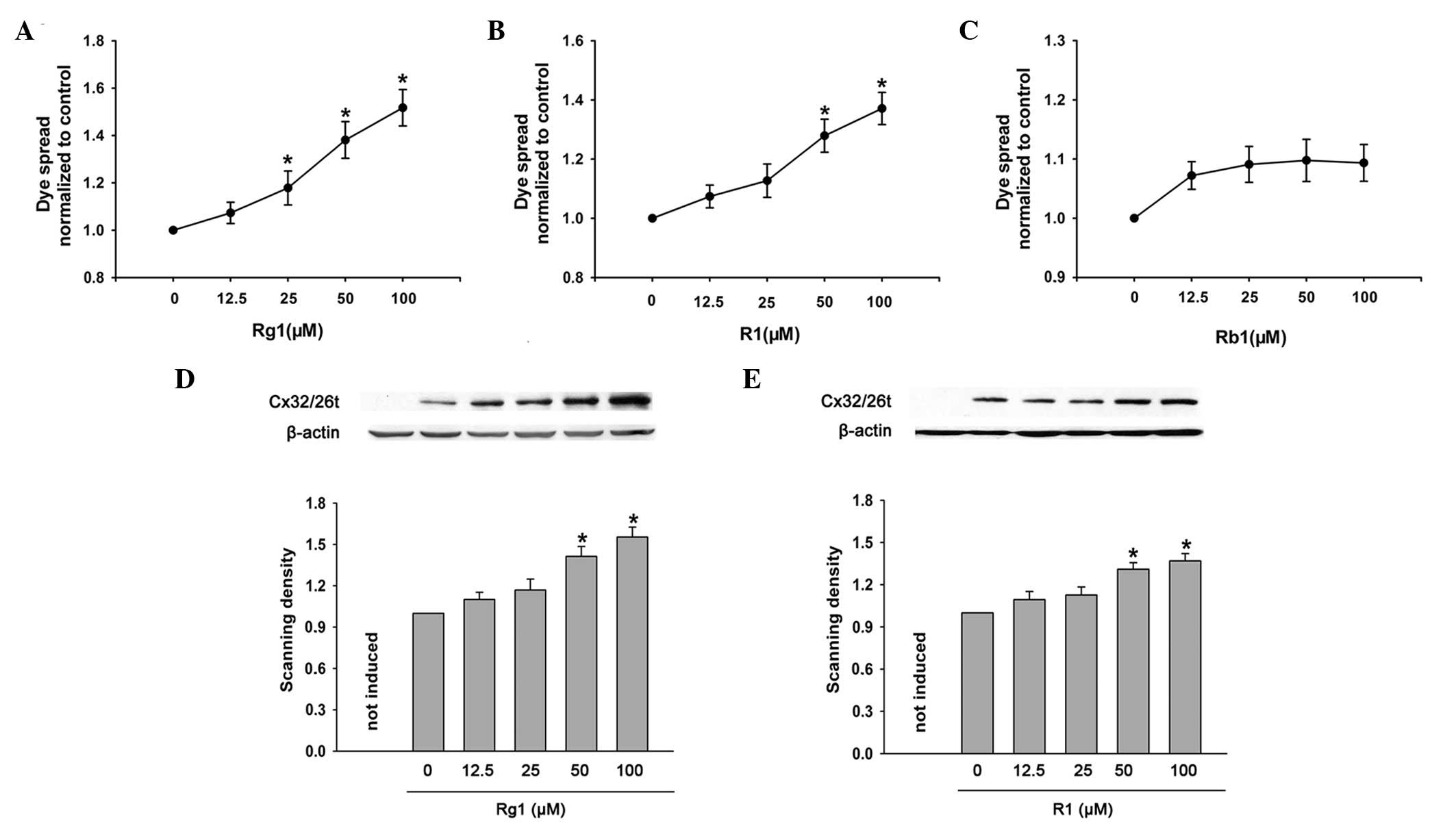
Ginsenoside Rg1 and notoginsenoside R1 affected
cisplatin toxicity at high cell densities indicating that the
protective effects may be mediated by GJ channels. To investigate
this hypothesis, the effects of ginsenoside Rg1 and Rb1, and
notoginsenoside R1 on dye-coupling between cultured cells were
examined by ‘parachute’ dye-coupling assay. The results showed that
ginsenoside Rg1 and notoginsenoside R1 markedly increased the dye
spread from donor to receiver cells in a dose-dependent manner
(Fig. 4A and B); however,
ginsenoside Rb1 had no effect on dye coupling between cultured
cells (Fig. 4C). Thus, ginsenoside
Rb1 did not affect the cytotoxicity of cisplatin, while ginsenoside
Rg1 and notoginsenoside R1 enhanced the toxicity of cisplatin in
the high-density cultures with GJ formation. To determine whether
ginsenoside Rg1 and notoginsenoside R1 affected connexin
expression, the expression of Cx32/Cx26 in cells induced with
doxycycline, followed by exposure to ginsenoside Rg1 and
notoginsenoside R1, was assessed by western blotting. Treatment
with ginsenoside Rg1 and notoginsenoside R1 for 4 h increased
Cx32/Cx26 expression in a dose-dependent manner (Fig. 4D and E), indicating that the
enhancement of GJIC by ginsenoside Rg1 and notoginsenoside R1 is
primarily attributable to increased connexin expression.
Effects of ginsenoside Rg1 and
notoginsenoside R1 on Cx32/Cx26 mRNA levels
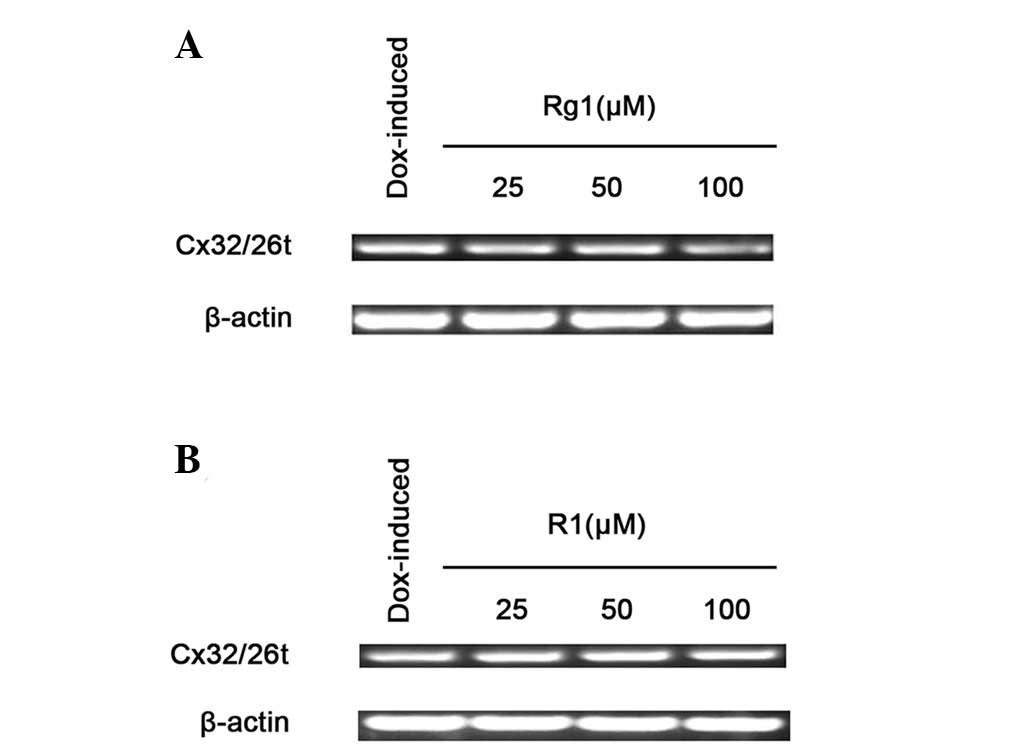
The effects of ginsenoside Rg1 and notoginsenoside
R1 on Cx32/Cx26 mRNA levels were investigated using
semi-quantitative endpoint RT-PCR. Fig. 5 shows that treating HeLa cells with
ginsenoside Rg1 and notoginsenoside R1 for 4 h did not affect the
levels of Cx32/Cx26 mRNA expression. This result indicates that the
enhancement of Cx32/Cx26 expression by ginsenoside Rg1 and
notoginsenoside R1 occurred at the post-transcriptional level.
Discussion
The current results indicate that ginsenoside Rg1
and notoginsenoside R1 are the active compounds responsible for
enhancing the cytotoxic action of cisplatin induced by PNS.
Ginsenoside Rb1 had no effect on the cytotoxicity of cisplatin in
the presence or absence of functional GJs. Our previous study
demonstrated that the enhancement of cisplatin cytotoxicity by PNS
is mediated by upregulating GJ function in HeLa cells (7). Treatment with PNS for 4 h increased
dye coupling via Cx32/Cx26 channels. The present study showed that
pre-treatment of cells with ginsenoside Rg1 and notoginsenoside R1
for 4 h markedly increased the dye spread from donor to receiver
cells in a dose-dependent manner; however, ginsenoside Rb1 had no
effect on dye coupling. Ginsenoside Rg1 and notoginsenoside R1 also
increased Cx32/Cx26 expression; however, Cx32/Cx26 mRNA levels were
not affected. Our previous study confirmed that the long-term
treatment of HeLa cells with cisplatin results in a reduction of
Cx32/Cx26 expression and that the administration of PNS reversed
this reduction. These results indicate that enhancement of
Cx32/Cx26 expression by PNS, ginsenoside Rg1 and notoginsenoside R1
are due to alterations in the stability of the protein, i.e.
inhibition of Cx32/Cx26 degradation and/or modulation of
translation.
Previous studies have demonstrated that cisplatin
toxicity is enhanced by the presence of functional GJs (7,9,12).
GJ expression allows cisplatin to promote apoptosis, cell cycle
arrest and the downregulation of BCL-2 in bladder cancer cell lines
(15). An inference derived from
these studies is that a ‘death signal’ induced by the apoptotic or
necrotic processes of one cell may be transmitted to neighboring
cells via GJs. In the present study, all cells were exposed to the
same dose of drug to mimic drug administration in vivo. In
theory, the presence of GJIC should have no effect if all cells
responded identically. However, there is a range of sensitivities
to toxic agents in cell populations. Therefore, specific cells are
more sensitive and others are less, leading to more or less toxic
signals in response. GJIC transmits a ‘death signal’ from sensitive
to less sensitive cells, thus, enhancing the cytotoxicity of
cisplatin in the entire cell population. In the current study,
cells exposed to 0.5 μg/ml cisplatin at low-density conditions,
without GJ, revealed a 17% cell death rate; however, with the
formation of GJs, i.e. at high-density conditions, there was a cell
death rate of 51%. GJIC increases the cytotoxic activity of
cisplatin by transmitting the ‘death signal’ from sensitive to less
sensitive cells. This indicates that elevating the intercellular
spread of the ‘death signal’ by ginsenoside Rg1 and the
notoginsenoside R1-induced enhancement of GJ formation or function
may be responsible for the increased cytotoxic action of cisplatin.
However, ginsenoside Rb1 had no effect on the cytotoxicity of
cisplatin in the presence or absence of functional GJs, as observed
in the results showing that ginsenoside Rb1 had no effect on
dye-coupling and failed to transmit a ‘death signal’ from sensitive
to less sensitive cells via GJs.
Although the propagation of the ‘death signal’
produced by stimulating pharmacological agents and radiation
through GJIC has been widely investigated, the exact molecules
responsible for this effect have not yet been identified (12,16–18).
A number of possible signals are usually considered, including the
toxic drug itself or its metabolites and molecules involved in the
cellular death pathway. Cisplatin and its cytoplasmic aquated
species have a molecular mass of ~300 Da, which is much less than
the upper limit of GJ-permeable molecules, indicating that these
species may permeate GJs. Since cisplatin exerts its cytotoxic
effects primarily by forming a variety of DNA adducts, intrastrand
and interstrand cross-links (ICLs), Hong et al examined the
effects of GJIC on cisplatin-induced formation of DNA ICLs at low
and high cell densities (8).
However, the results showed that GJIC had no effect on the
formation of DNA ICLs. This result indicates that cisplatin and its
immediate metabolites may not be the responsible toxic signals
transferred among cells. The increase in cytotoxicity is
hypothesized to be caused by the transfer of other toxic factors
and further investigations are required to identify them.
In the present study, 100 μM ginsenoside Rg1 and
notoginsenoside R1 significantly increased the cytotoxicity of
cisplatin via increased GJ formations. Thus, the dosage of
cisplatin may be reduced, resulting in fewer side effects. PNS and
its components may be developed as nontoxic chemoadjuvants that are
used to increase the efficacy of anticancer chemotherapies by the
upregulation or maintenance of GJ function. The current study also
reveals a novel strategy in which the upregulation of GJs may be
utilized to increase the efficacy of chemotherapy.
Acknowledgements
The present study was supported by grants from the
China Postdoctoral Science Foundation (no. 20090461139), the
Natural Science Foundation of the Provincial Education Department
of Anhui (no. KJ2008A167) and the National Natural Science
Foundation of China (no. 81001457).
References
|
1
|
Wang CZ, Luo X, Zhang B, Song WX, Ni M,
Mehendale S, Xie JT, Aung HH, He TC and Yuan CS: Notoginseng
enhances anti-cancer effect of 5-fluorouracil on human colorectal
cancer cells. Cancer Chemother Pharmacol. 60:69–79. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang CZ, Xie JT, Zhang B, Ni M, Fishbein
A, Aung HH, Mehendale SR, Du W, He TC and Yuan CS: Chemopreventive
effects of Panax notoginseng and its major constituents on
SW480 human colorectal cancer cells. Int J Oncol. 31:1149–1156.
2007.
|
|
3
|
Zhang CL, Yu ML, Tao L, Jiang Z and Liu H:
Decreasing toxicity and synergistic effects of Panax
notoginseng to tumor-bearing mice treated by cytoxan. Nanjing
Zhongyiyao Daxue XueBao. 4:254–256. 2008.(In Chinese).
|
|
4
|
Krutovskikh VA, Piccoli C and Yamasaki H:
Gap junction intercellular communication propagates cell death in
cancerous cells. Oncogene. 21:1989–1999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin JH, Weigel H, Cotrina ML, Liu S, Bueno
E, Hansen AJ, Hansen TW, Goldman S and Nedergaard M:
Gap-junction-mediated propagation and amplification of cell injury.
Nat Neurosci. 1:494–500. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Q, You T, Yuan D, Han X, Hong X, He
B, Wang L, Tong X, Tao L and Harris AL: Cisplatin and oxaliplatin
inhibit gap junctional communication by direct action and by
reduction of connexin expression, thereby counteracting cytotoxic
efficacy. J Pharmacol Exp Ther. 333:903–911. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu ML, Zhang CL, Yuan DD, Tong XH and Tao
L: Panax notoginseng saponins enhances the cytotoxicity of
cisplatin via increasing gap junction intercellular communication.
Biol Pharm Bull. 35:1230–1237. 2012. View Article : Google Scholar
|
|
8
|
Hong X, Wang Q, Yang Y, Zheng S, Tong X,
Zhang S, Tao L and Harris AL: Gap junctions propagate opposite
effects in normal and tumor testicular cells in response to
cisplatin. Cancer Lett. 317:165–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He B, Tong X, Wang L, Wang Q, Ye H, Liu B,
Hong X, Tao L and Harris AL: Tramadol and flurbiprofen depress the
cytotoxicity of cisplatin via their effects on gap junctions. Clin
Cancer Res. 15:5803–5810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koreen IV, Elsayed WA, Liu YJ and Harris
AL: Tetracycline-regulated expression enables purification and
functional analysis of recombinant connexin channels from mammalian
cells. Biochem J. 383:111–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldberg GS, Bechberger JF and Naus CC: A
pre-loading method of evaluating gap junctional communication by
fluorescent dye transfer. Biotechniques. 18:490–497.
1995.PubMed/NCBI
|
|
12
|
Jensen R and Glazer PM:
Cell-interdependent cisplatin killing by Ku/DNA-dependent protein
kinase signaling transduced through gap junctions. Proc Natl Acad
Sci USA. 101:6134–6139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erdlenbruch B, Nier M, Kern M, Hiddemann
W, Pekrun A and Lakomek M: Pharmacokinetics of cisplatin and
relation to nephrotoxicity in paediatric patients. Eur J Clin
Pharmacol. 57:393–402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tao L and Harris AL: 2-aminoethoxydiphenyl
borate directly inhibits channels composed of connexin26 and/or
connexin32. Mol Pharmacol. 71:570–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka M and Grossman HB: Connexin 26 gene
therapy of human bladder cancer: induction of growth suppression,
apoptosis, and synergy with cisplatin. Hum Gene Ther. 12:2225–2236.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mesnil M and Yamasaki H: Bystander effect
in herpes simplex virus-thymidine kinase/ganciclovir cancer gene
therapy: role of gap junctional intercellular communication. Cancer
Res. 60:3989–3999. 2000.
|
|
17
|
Mesnil M, Piccoli C, Tiraby G, Willecke K
and Yamasaki H: Bystander killing of cancer cells by herpes simplex
virus thymidine kinase gene is mediated by connexins. Proc Natl
Acad Sci USA. 93:1831–1835. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azzam EI, de Toledo SM and Little JB:
Oxidative metabolism, gap junctions and the ionizing
radiation-induced bystander effect. Oncogene. 22:7050–7057. 2003.
View Article : Google Scholar : PubMed/NCBI
|