Introduction
Monophosphoryl lipid A (MPL) is a lipopolysaccharide
(LPS)-derived Toll-like receptor 4 (TLR4) agonist that exhibits
unique immunomodulatory properties at doses that are nonpyrogenic.
In addition, it is a chemically detoxified lipid A moiety derived
from Salmonella minnesota R595 LPS (1). Clinically, MPL is a component of
several vaccine formulations. It has been demonstrated that MPL
induces a strong phagocytic and a low inflammatory response via
TLR4 (2).
Myeloid-derived suppressor cells (MDSCs) and
dendritic cells (DCs) are derived from the same myeloid precursors;
however, they exhibit different roles in the immune response. DCs
demonstrated the capacity to initiate an innate and adaptive
response (3,4), while MDSCs have been observed to
suppress immune responses via arginase, inducible nitric oxide
synthase (5,6), reactive oxygen species (7–14)
and Foxp3+ regulatory cells (15,16).
The mechanisms that are involved in the differentiation of myeloid
precursor cells into MDSCs instead of DCs have not been fully
elucidated. It has been demonstrated that the development of DCs
from bone marrow (BM) precursor cells in vitro is impaired
in the presence of LPS (17).
Another study showed that a combination of LPS and IFN-γ inhibited
DC development; however, enhanced the suppressive functions of
MDSCs, including NO release and T cell suppression (18). In addition, other conserved
structural patterns of microbial components, such as
double-stranded RNA showed the capacity to regulate MDSC versus DC
development from the same myeloid precursor (19).
As MPL is an active immunomodulator, in the present
study, the involvement of MPL was investigated in the
differentiation of myeloid precursor cells into MDSCs versus DCs.
It was demonstrated in vivo and in vitro, that
sustained stimulation with MPL inhibited the expansion of DCs and
induced the development and expansion of MDSCs.
Materials and methods
Mice and treatments
Approximately 50 male and female wild-type C57BL/6
mice (age, 5–6 weeks) were purchased from the Chinese Academy of
Sciences (Shanghai, China). DO11.10 OVA323–339-specific
TCR-transgenic mice with a C57BL/6 background were obtained from
The Jackson Laboratory (Bar Harbor, ME, USA). All mice were housed
in a specific pathogen-free facility for all experiments. All
animal procedures were undertaken in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (NIH Publication No. 85-23, National Academy Press,
Washington, DC, revised 1996), with the approval of the Laboratory
Animal Center and Ethics Committee of the Second Military Medical
University (Shanghai, China).
Reagents
Recombinant mouse granulocyte macrophage
colony-stimulating factor (GM-CSF), interleukin (IL)-4, and an
enzyme-linked immunosorbent assay (ELISA) kit for murine IL-12,
IL-6, tumor necrosis factor (TNF)-α, IL-10 and transforming growth
factor (TGF)-β were purchased from R&D Systems (Minneapolis,
MN, USA). Fluorescein-conjugated mAbs to CD4, CD11b, CD80, CD86,
Ia, CD40, CD11c, CCR7 and isotype control were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Fluorescein-conjugated mAbs to Gr1 were obtained from eBioscience
(San Diego, CA, USA). LPS, 7-Aminoactinomycin D (7-AAD) and bovine
serum albumin (BSA) were purchased from Sigma-Aldrich (Carlsbad,
CA, USA).
Preparation of MPL from LPS of the
gram-negative bacteria Salmonella minnesota R595
The MPL was prepared by eliminating the core
oligosaccharide, hydrolyzing 1-phosphate from the reducing end
glucosamine and removing the acyl chain from the 3′ position of the
disaccharide (2,20,21).
Preparation of DCs from mouse BM and DC
pre-treatment
BM mononuclear cells were prepared from mouse femur
BM suspensions by depletion of red blood cells. The cells were then
cultured at a density of 2×106 cells/ml in 6-well plates
in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS),
10 ng/ml recombinant mouse GM-CSF and 1 ng/ml recombinant mouse
IL-4. Nonadherent cells were gently washed out on day 4 of culture.
On day 5, the dendritic proliferating clusters were collected and
purified by anti-CD11c microbeads as immature dendritic cells
(imDCs). ImDCs were stimulated with LPS (100 ng/ml) for another 2
days and then collected as mature DCs (mDCs). These cells were
cultured in 24-well plates using 1×106 cell/well. All
groups were cultured with granulocyte macrophage colony-stimulating
factor and IL-4 throughout. MPL (25 μg/ml) was administered to the
long stimulation group on day 0 and to the short stimulation group
on day 5. On day 6, all cells were collected separately for further
analysis (22).
Analysis of DC phagocytic ability
The cells of all groups were incubated at 37°C for 4
h with fluorescein isothiocyanate (FITC)-conjugated OVA at a final
concentration of 100 μg/ml in RPMI-1640 medium containing 10% FCS,
were washed twice with ice-cold phosphate-buffered saline (PBS; pH
7.2), containing 0.1% NaN3 and 0.5% BSA, and were
resuspended in chilled PBS for immediate flow cytometry. Cells were
incubated with OVA-FITC at 4°C.
Assay for cytokines and NO
Cytokines in the supernatant of the DC system were
assayed with ELISA kits. NO production was assayed by the
measurement of the nitrite concentration with the Griess assay.
Assays for Ag-specific CD4+
T-cell response
For the assay of the Ag-specific CD4+
T-cell proliferation, splenic CD4+ T cells from DO11.10
OVA323–339-specific TCR-transgenic mice were positively
selected with anti-CD4-coated microbeads (Miltenyi Biotec, Bergisch
Gladbach, Germany) by magnetic-activated cell sorting. The cells
were cocultured with DCs treated as indicated in the presence of
OVA323–339 peptide at a ratio of 1:10 (DC:T) in
round-bottom 96-well plates (1×105 T cells/200μl/well)
for 5 days. The proliferation of the T cells was analyzed by double
staining with anti-CD4+ and 7-AAD−, and cells
were counted by a fluorescence activated cell sorter (FACS).
Assay to determine the percentage of
MDSCs and DCs in vivo
Six of the wild-type C57BL/6 mice were administered
with MPL via the tail vein once (short stimulation) or once daily
for 5 days (long stimulation). In total, there were 12 mice with 6
in the control group. The administration dosage was dependent upon
the mouse body weight (0.2 mg/kg or 2 mg/kg). Following the
preparation of single-cell suspensions from the mouse spleen, cells
were stained with Ab-CD11c+ conjugated FITC,
Ab-CD11b+ conjugated R-Phycoerythrin (PE) or
Ab-Gr1+ conjugated FITC as indicated in the
manufacturer’s instructions.
Statistical analysis
Comparisons between experimental groups and relevant
controls were performed by Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
BM precursor cells exhibit a distinct
phenotype and cytokine profile following long stimulation with
MPL
Mouse BM-DCs were produced by standard protocol
using GM-CSF and IL-4 (23–26).
In vitro, the process of differentiation from myeloid
precursor cells to DCs required 5 or 6 days. On day 0, in the long
stimulation group, myeloid precursor cells were isolated from mouse
BM, washed and co-cultured with GM-CSF, IL-4 and MPL (20 μg/ml) for
5 days. In the short stimulation group, myeloid precursor cells
were treated with GM-CSF and IL-4 only until day 5. On day 5, cells
in the short stimulation group cells were treated once with MPL (20
μg/ml). On day 6, cells from the two groups and the control group
were harvested for further analysis. It was demonstrated that cells
from the long stimulation group exhibited a lower expression of
CD40, CD80, CD86 and Ia compared with that in the short stimulation
group. Notably, CD11c expression in the cells of the long
stimulation group was significantly decreased, which suggested that
the development of DCs was blocked. Chemokine (C-C motif) receptor
7 (CCR7) was demonstrated to be responsible for directing the
migration of DCs, in addition to the control of the
cytoarchitecture, the rate of endocytosis, DC survival, migratory
speed and DC maturation (27). In
the present study, the expression of CCR7 was significantly
decreased (Fig. 1A and B).
Compared with the cytokine profile of the short stimulation and
control groups, the cells in the long stimulation group secreted
lower levels of IL-6, IL-12 and TNF-α and higher levels of IL-10
and NO (Fig. 1C). The cytokine
profile of the long stimulation group suggested that the cells may
exhibit an immunosuppressive capacity.
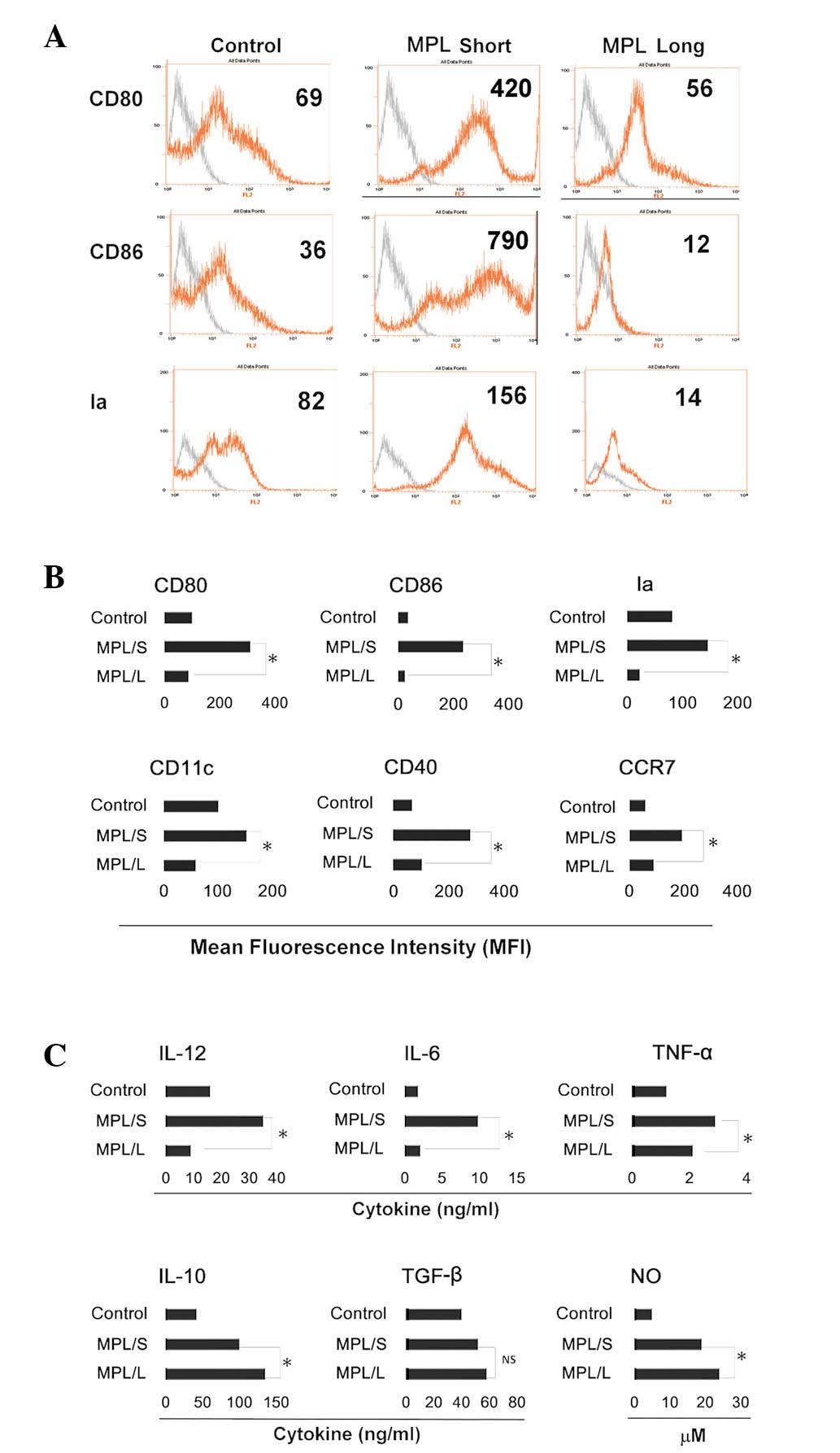 | Figure 1Phenotype and cytokine profiles of all
groups. Myeloid precursor cells were generated from C57BL/6 mouse
femur bone marrow suspensions by the depletion of red blood cells.
These cells were cultured in 24-well plates using 1×106
cell/well. All groups were cultured with granulocyte macrophage
colony-stimulating factor and IL-4 throughout. MPL was administered
to the long stimulation group on day 0 and to the short stimulation
group on day 5. On day 6, all cells were collected separately for
further analysis. (A) FACS charts of CD80, CD86 and Ia. Fold line
in grey represents the blank control (cells without staining).
Numbers in each chart represent the mean fluorescence intensity.
(B) The expression of CD80, CD86, Ia, CD11c, CD40 and CCR7 was
detected by FACS. Histograms represent the mean fluorescence
intensity. Results are presented as the mean ± SD of cells from
triplicate wells. (C) NO expression and the cytokine profile of
cells from different groups for 24 h. On day 6, cells of different
groups were collected and washed in phosphate-buffered saline 3
times and placed in the wells for another 24 h. IL-12, IL-6, tumor
necrosis factor-α, IL-10 and TGF-β levels were assayed by an
enzyme-linked immunosorbent assay and NO levels were assayed by a
Griess assay. Results are presented as the mean ± SD of triplicate
wells. *P<0.05 compared with the short stimulation
group. NS, no significance; IL, interleukin; MPL, monophosphoryl
lipid A; FACS, fluorescence-activated cell sorting; NO, nitric
oxide; TGF, transforming growth factor. |
Cells from the long stimulation group
show an enhanced phagocytic ability and the ability to suppress T
cell proliferation
The phenotype and cytokine profiles of cells from
the MPL long stimulation group suggested that these cells may
exhibit an immunosuppressive function. Thus, the cells were stained
with Gr1+CD11b+ and analyzed by FACS. This
demonstrated that double-positive Gr1+CD11b+
cells existed in the population (Fig.
2A and B). Therefore, it was hypothesized that these cells may
be MDSCs. Subsequent to this, the phagocytic function of the
different groups was analyzed, which demonstrated that cells from
the long stimulation group exhibited a significantly increased
phagocytic ability (Fig. 2C). In
addition, the ability of DCs to stimulate antigen-specific T cell
responses was investigated. It was observed that cells from the
long stimulation group exhibited a reduced ability to induce the
proliferation of OVA-specific CD4+ T cells. Notably,
cells from the long stimulation group were added to the mDCs/CD4 T
cell coculture system and it was demonstrated that the T cell
proliferation in vitro was partly suppressed (Fig. 2D).
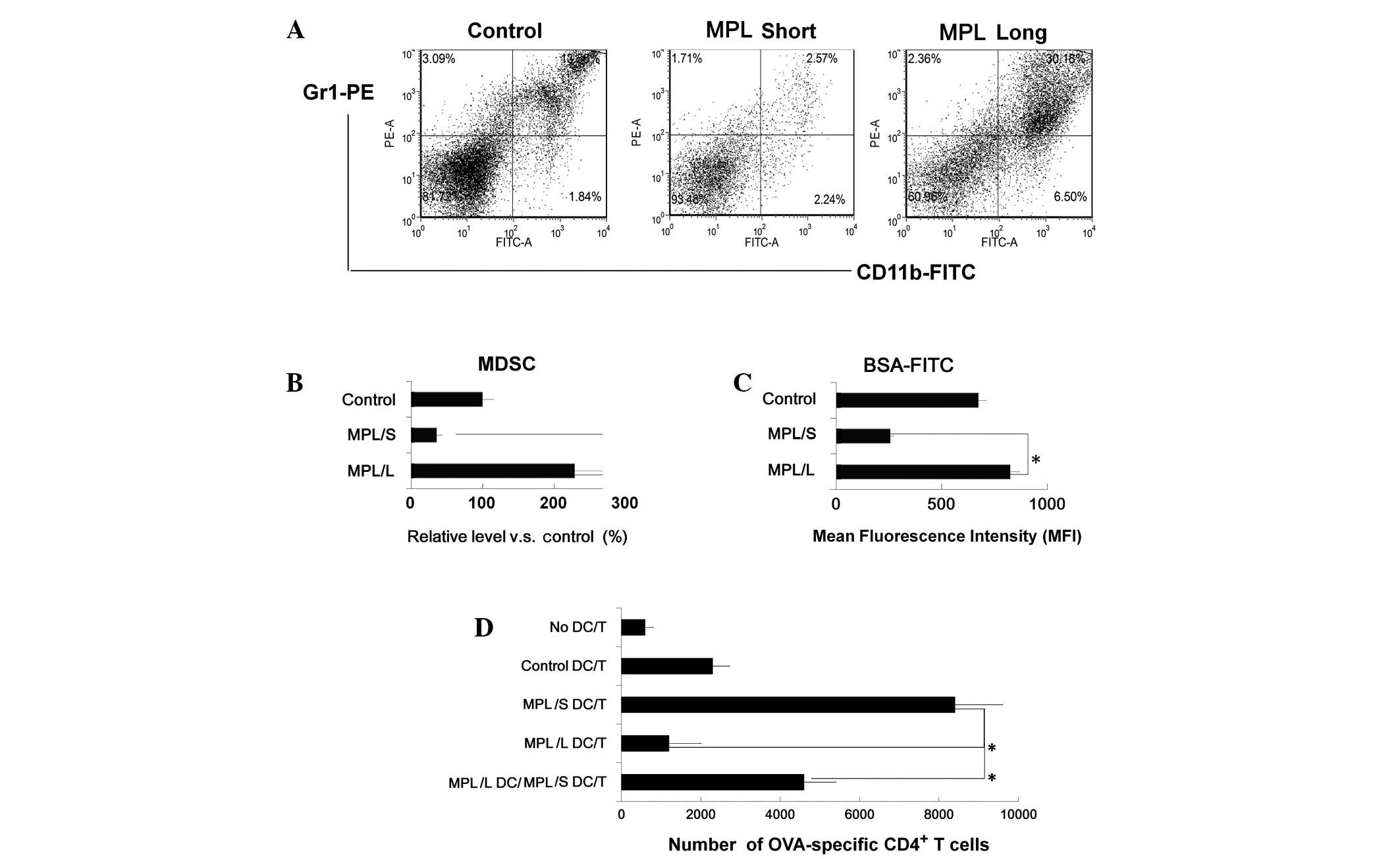 | Figure 2Gr1+CD11b+
cells in the long stimulation group showed an enhanced phagocytic
ability and inhibited CD4+ T cell proliferation. (A) The
phenotype of cells in the long stimulation group were analyzed by
flow cytometry, the percentage of Gr1+CD11b+
cells was higher than in the other groups. (B) The percentage of
Gr1+CD11b+ cells in all groups (the
percentage in control group was referred as 100%). Phagocytic
ability was assessed for OVA-FITC phagocytosis by flow cytometry.
Numbers in the histograms indicate the geometric mean fluorescence
of test samples, cells (1×106 cell/well) were incubated
with OVA-FITC at 4°C for 12 h, washed with phosphate-buffered
saline 3 times and analyzed by a fluorescence-activated cell
sorter. (D) CD4+ T cells from DO11.10
OVA323–339 specific (TCR-transgenic C57BL/6) F1 hybrid
mice were cocultured with cells from all groups, 5 days later, the
total number of viable CD4+ T cells (CD4+
7AAD−) cells in each well was measured by flow
cytometry. Results are presented as the mean ± SD of three
independent analyses. *P<0.05 compared with the short
stimulation group. FITC, fluorescein isothiocyanate; 7-AAD,
7-aminoactinomycin D; MDSC, myeloid derived suppressor cell; MPL,
monophosphoryl lipid A; DC, dendritic cell; BSA, bovine serum
albumin. |
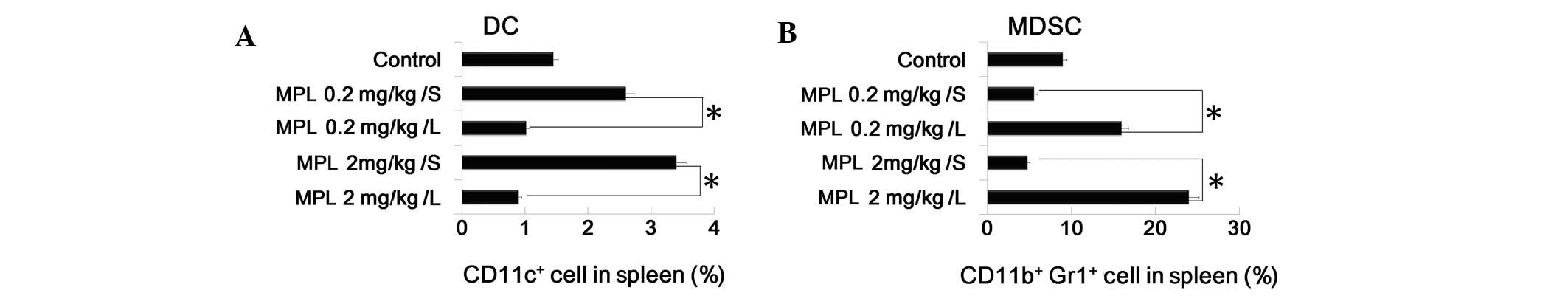
MPL expands MDSC population and
suppresses DC population in vivo
To investigate the effect of MPL in vivo, the
MPL short and long stimulation model was conducted. Mice were
administered with MPL via the tail vein once daily. In the short
stimulation group, mice received MPL treatment on day 0, then 24 h
later, on day 1, mice were euthanized. In the long stimulation
group, mice received MPL treatment every 24 h and were euthanized
on day 5. The spleens were isolated from the two groups and the
control to analyze the DC and MDSC population by FACS. It was
demonstrated that MPL upregulated the percentage of DCs
(CD11c+ cells) and downregulated the percentage of MDSCs
(CD11b+Gr1+ cells) in the spleen. This data
is consistent with the previous results (Fig. 3A and B).
Discussion
The results of the present study suggested that the
TLR4 agonist (MPL) in the long stimulation group, disturbed the
development of DCs and induced myeloid precursor cells to
differentiate into CD11b+Gr1+ cells
(considered to be MDSCs) with an immunosuppressive function.
Previous studies have demonstrated that factors that induce MDSC
expansion include cyclooxygenase-2, prostaglandins (28–30),
stem-cell factor (28), M-CSF,
IL-6 (31), GM-CSF and vascular
endothelial growth factor (32).
In addition to expansion, the suppressive activity of MDSCs
requires factors to induce their activation, which include IFN-γ
(33,34), ligands for Toll-like receptors,
IL-13 (35), IL-4 and TGF-β
(35). In this study, IL-4, GM-CSF
and MPL were investigated and were suggested to be involved in the
differentiation of myeloid precursors into MDSCs.
MPL is a detoxified lipid A moiety derived from
Salmonella minnesota R595 LPS. It is at least 100-fold less
pyrogenic than LPS, yet maintains a number of the immunomodulatory
properties of LPS (36). Previous
studies have demonstrated that LPS and poly (I:C) may suppress the
immune response (18,19). The results indicated that MPL
derived from LPS may also impair DC development.
The cells from the long stimulation group exhibited
a CD11b+Gr1+ phenotype and were able to
suppress T cell proliferation. This suggests that these cells
exhibit an immunosuppressive function in the immune response.
However, these cells also showed enhanced phagocytic ability. A
previous study demonstrated that stimulation of microglia and
monocytes with MPL induced increased rates of phagocytosis of
amyloid-β (Aβ) and that this mechanism may reduce the accumulation
of Aβ and improve spatial memory in APPswe/PS1 mice (2). Therefore, this similar phenomenon
suggests that the enhanced phagocytic ability of the
CD11b+Gr1+ cells may have important roles in
this process.
As BM precursor cells can be triggered to
differentiate by conserved structural patterns of pathogens, it was
questioned whether DCs only have one fate. However, Abdi et
al(37)demonstrated that
LPS-activated DCs lose their responsiveness to LPS, yet remain
capable of producing inflammatory cytokines in response to signals
from activated T cells, CD40-ligand and soluble T cell-derived
signals. Furthermore, these DCs retained sufficient plasticity to
respond differentially to the interaction with Th0, Th1, Th2 and
Th17 T cells (37). Thus, it
appears that the MPL stimulated cells are not rested or exhausted,
and suppress Th1 proliferation. However, the result of interaction
with Th0, Th2 and Th17 cells and the understanding of whether these
cells are equivalent to classical MDSCs remains to be
elucidated.
In conclusion, CD11b+Gr1+
cells were generated in vivo and in vitro, and these
cells showed an ability to suppress CD4+ T cell
proliferation and enhance phagocytic ability; however, further
studies are required to fully determine this effect.
Acknowledgements
The authors would like to thank Dr Pu You and Dr
Bing Yu from the Department of Cell Biology (Second Military
Medical University, 800 Xiangyin Road, Shanghai 200433, P.R. China)
for help with the discussion.
References
|
1
|
Casella CR and Mitchell TC: Putting
endotoxin to work for us: monophosphoryl lipid A as a safe and
effective vaccine adjuvant. Cell Mol Life Sci. 65:3231–3240. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Michaud JP, Hallé M, Lampron A, et al:
Toll-like receptor 4 stimulation with the detoxified ligand
monophosphoryl lipid A improves Alzheimer’s disease-related
pathology. Proc Natl Acad Sci USA. 110:1941–1946. 2013.PubMed/NCBI
|
|
3
|
Steinman RM: The dendritic cell system and
its role in immunogenicity. Annu Rev Immunol. 9:271–296. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar
|
|
5
|
Bronte V and Zanovello P: Regulation of
immune responses by L-arginine metabolism. Nat Rev Immunol.
5:641–654. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodríguez PC and Ochoa AC: Arginine
regulation by myeloid derived suppressor cells and tolerance in
cancer: mechanisms and therapeutic perspectives. Immunol Rev.
222:180–191. 2008.PubMed/NCBI
|
|
7
|
Youn JI, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kusmartsev S, Nefedova Y, Yoder D and
Gabrilovich DI: Antigen-specific inhibition of CD8+ T
cell response by immature myeloid cells in cancer is mediated by
reactive oxygen species. J Immunol. 172:989–999. 2004.PubMed/NCBI
|
|
9
|
Schmielau J and Finn OJ: Activated
granulocytes and granulocyte-derived hydrogen peroxide are the
underlying mechanism of suppression of t-cell function in advanced
cancer patients. Cancer Res. 61:4756–4760. 2001.
|
|
10
|
Kusmartsev S, Nagaraj S and Gabrilovich
DI: Tumor-associated CD8+ T cell tolerance induced by
bone marrow-derived immature myeloid cells. J Immunol.
175:4583–4592. 2005.PubMed/NCBI
|
|
11
|
Szuster-Ciesielska A, Hryciuk-Umer E,
Stepulak A, Kupisz K and Kandefer-Szerszeń M: Reactive oxygen
species production by blood neutrophils of patients with laryngeal
carcinoma and antioxidative enzyme activity in their blood. Acta
Oncol. 43:252–258. 2004. View Article : Google Scholar
|
|
12
|
Waris G and Ahsan H: Reactive oxygen
species: role in the development of cancer and various chronic
conditions. J Carcinog. 5:142006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mantovani G, Macciò A, Madeddu C, et al:
Antioxidant agents are effective in inducing lymphocyte progression
through cell cycle in advanced cancer patients: assessment of the
most important laboratory indexes of cachexia and oxidative stress.
J Mol Med (Berl). 81:664–673. 2003. View Article : Google Scholar
|
|
14
|
Agostinelli E and Seiler N:
Non-irradiation-derived reactive oxygen species (ROS) and cancer:
therapeutic implications. Amino Acids. 31:341–355. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang R, Cai Z, Zhang Y, Yutzy WH, Roby KF
and Roden RB: CD80 in immune suppression by mouse ovarian
carcinoma-associated Gr-1+CD11b+ myeloid
cells. Cancer Res. 66:6807–6815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang B, Pan PY, Li Q, et al:
Gr-1+CD115+ immature myeloid suppressor cells
mediate the development of tumor-induced T regulatory cells and
T-cell anergy in tumor-bearing host. Cancer Res. 66:1123–1131.
2006.
|
|
17
|
Lutz MB, Kukutsch NA, Menges M, Rössner S
and Schuler G: Culture of bone marrow cells in GM-CSF plus high
doses of lipopolysaccharide generates exclusively immature
dendritic cells which induce alloantigen-specific CD4 T cell anergy
in vitro. Eur J Immunol. 30:1048–1052. 2000. View Article : Google Scholar
|
|
18
|
Greifenberg V, Ribechini E, Rössner S and
Lutz MB: Myeloid-derived suppressor cell activation by combined LPS
and IFN-gamma treatment impairs DC development. Eur J Immunol.
39:2865–2876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu C, Zhang C, Lu H, et al: Poly(I:C)
induce bone marrow precursor cells into myeloid-derived suppressor
cells. Mol Cell Biochem. 358:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baldridge JR and Crane RT: Monophosphoryl
lipid A (MPL) formulations for the next generation of vaccines.
Methods. 19:103–107. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ulrich JT and Myers KR: Monophosphoryl
lipid A as an adjuvant. Past experiences and new directions. Pharm
Biotechnol. 6:495–524. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsujimoto H, Efron PA, Matsumoto T, et al:
Maturation of murine bone marrow-derived dendritic cells with
poly(I:C) produces altered TLR-9 expression and response to CpG
DNA. Immunol Lett. 107:155–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M, Tang H, Guo Z, et al: Splenic
stroma drives mature dendritic cells to differentiate into
regulatory dendritic cells. Nat Immunol. 5:1124–1133. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang H, Guo Z, Zhang M, Wang J, Chen G and
Cao X: Endothelial stroma programs hematopoietic stem cells to
differentiate into regulatory dendritic cells through IL-10. Blood.
108:1189–1197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia S, Guo Z, Xu X, Yi H, Wang Q and Cao
X: Hepatic microenvironment programs hematopoietic progenitor
differentiation into regulatory dendritic cells, maintaining liver
tolerance. Blood. 112:3175–3185. 2008. View Article : Google Scholar
|
|
26
|
Li Q, Guo Z, Xu X, Xia S and Cao X:
Pulmonary stromal cells induce the generation of regulatory DC
attenuating T-cell-mediated lung inflammation. Eur J Immunol.
38:2751–2761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sánchez-Sánchez N, Riol-Blanco L and
Rodríguez-Fernández JL: The multiple personalities of the chemokine
receptor CCR7 in dendritic cells. J Immunol. 176:5153–5159.
2006.PubMed/NCBI
|
|
28
|
Pan PY, Wang GX, Yin B, et al: Reversion
of immune tolerance in advanced malignancy: modulation of
myeloid-derived suppressor cell development by blockade of
stem-cell factor function. Blood. 111:219–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sinha P, Clements VK, Fulton AM and
Ostrand-Rosenberg S: Prostaglandin E2 promotes tumor progression by
inducing myeloid-derived suppressor cells. Cancer Res.
67:4507–4513. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Serafini P, Carbley R, Noonan KA, Tan G,
Bronte V and Borrello I: High-dose granulocyte-macrophage
colony-stimulating factor-producing vaccines impair the immune
response through the recruitment of myeloid suppressor cells.
Cancer Res. 64:6337–6343. 2004. View Article : Google Scholar
|
|
31
|
Bunt SK, Yang L, Sinha P, Clements VK,
Leips J and Ostrand-Rosenberg S: Reduced inflammation in the tumor
microenvironment delays the accumulation of myeloid-derived
suppressor cells and limits tumor progression. Cancer Res.
67:10019–10026. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gabrilovich D, Ishida T, Oyama T, et al:
Vascular endothelial growth factor inhibits the development of
dendritic cells and dramatically affects the differentiation of
multiple hematopoietic lineages in vivo. Blood. 92:4150–4166.
1998.
|
|
33
|
Kusmartsev S and Gabrilovich DI: STAT1
signaling regulates tumor-associated macrophage-mediated T cell
deletion. J Immunol. 174:4880–4891. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Movahedi K, Guilliams M, Van den Bossche
J, et al: Identification of discrete tumor-induced myeloid-derived
suppressor cell subpopulations with distinct T cell-suppressive
activity. Blood. 111:4233–4244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Terabe M, Matsui S, Park JM, et al:
Transforming growth factor-beta production and myeloid cells are an
effector mechanism through which CD1d-restricted T cells block
cytotoxic T lymphocyte-mediated tumor immunosurveillance:
abrogation prevents tumor recurrence. J Exp Med. 198:1741–1752.
2003. View Article : Google Scholar
|
|
36
|
Cluff CW: Monophosphoryl lipid A (MPL) as
an adjuvant for anti-cancer vaccines: clinical results. Adv Exp Med
Biol. 667:111–123. 2010. View Article : Google Scholar
|
|
37
|
Abdi K, Singh NJ and Matzinger P:
Lipopolysaccharide-activated dendritic cells: ‘exhausted’ or alert
and waiting? J Immunol. 188:5981–5989. 2012.
|