Introduction
Tendons are connective tissues that join muscle to
bone. Tendon injuries are painful and widely distributed clinical
problems in society. While the healing of such disorders leads to
costly medical expenses, the original biological properties of the
tissue do not return to normal (1–3).
Although there have been advances in the development and
maintenance of tendon and ligament tissue, and on cellular and gene
therapy approaches for repair, tissue engineering remains far from
being capable of producing the ideal bioscaffold to replace, repair
or regenerate injured ligaments (4,5).
However, mesenchymal stem cells (MSCs) have been considered as a
source of cells for use in ligament regeneration.
MSCs are a pluripotent cell population capable of
differentiating into a variety of cell types, and were originally
isolated from the bone marrow (BM) (6). MSCs have been considered to be
promising tools for regeneration and cancer therapy in several
preclinical and clinical trials due to their plasticity (7–9).
Several studies support the hypothesis that MSCs differentiate into
multiple cell lineages, including osteoblasts, chondrocytes,
adipocytes, smooth muscle cells, skeletal and cardiac myocytes,
endothelial cells and neurons (10,11).
Growth of MSCs throughout life involves proliferation of epithelial
cells and their subsequent differentiation into multiple cell
lineages. An increasing number of studies have demonstrated the
involvement of fibroblast growth factor 2 (FGF2) in regulating cell
growth and differentiation; however, its effect on MSCs appears
complex and remains unclear.
FGF2 is a member of the FGF family, which is
synthesized as a 155 amino acid precursor and is subsequently
processed into a mature form consisting of 146 amino acid residues
(12). FGF2 mediates cellular
signal transduction through binding to fibroblast growth factor
tyrosine kinase receptors (FGFRs) and downstream signaling
molecules, such as phosphatidylinositide 3-kinase (PI3K)-Akt and
Ras-Raf-mitogen activated protein kinase (MAPK) (13). However, FGF2 exhibits bidirectional
effects on growth and differentiation of MSCs. FGF2 has been shown
to stimulate growth and preserve the differentiation potential of
MSCs during long-term culture expansion in vitro by inducing
cell motility, the expression of vimentin (VIM) and α-smooth muscle
actin (SMA) (14–19). FGF2 has also been demonstrated to
stimulate chondrogenic and adipogenic differentiation of human
(20,21) and rat (22) MSCs, respectively. By contrast,
recent studies have shown that the inhibition of mouse MSC
differentiation by FGF2 is strongly correlated with the
upregulation of Twist2 and Spry4, and the suppression of
extracellular signal-regulated kinase (ERK)1/2 activation (23). Thus, a number of intracellular
signaling cascades may be involved, in particular the MAPK pathway,
in which sequential phosphorylation of a series of protein kinases
ultimately activates ERK to control a variety of downstream
responses, including gene transcription. To investigate the
molecular mechanisms underlying the involvement of FGF2 in MSCs is
currently a predominant aim in biomedical research.
In the present study, it was hypothesized that the
differentiation induced by FGF2 may be mediated by alterations in
the levels of mRNA and proteins with known specific extracellular
matrix proteins and cytoskeletal elements. The effects of the
overexpression of FGF2 on collagen I, collagen III, scleraxis,
fibronectin and α-SMA expression were determined in vitro,
and the involvement of the MAPK signaling pathway was investigated
in MSC differentiation.
Materials and methods
Isolation and expansion of human
MSCs
Human MSCs were isolated from the iliac crest
obtained from four human donors due to vertebral fractures. The
donor population was reasonably homogenous (age, 25–47 years, equal
numbers of males and females). All procedures were approved by the
ethics committee of the Second Militarty Medical University,
Shanghai, China, and informed consent was obtained from all donors.
MSCs were isolated with a density gradient and resuspended in
complete culture medium. Human MSCs were cultured in Dulbecco’s
modified Eagle’s medium-high glucose containing 10% fetal calf
serum, 50 μM 2-ME (Sigma-Aldrich), penicillin/streptomycin (100
U/ml/100 μg/ml, HyClone) at 37ºC in a humid atmosphere with 5%
CO2. MSCs were cultured as adherent cells, non-adherent
cells were removed by medium change after three days. The medium
was replaced three days following plating and then every three days
subsequent to that. Cells from each donor were cultured separately.
Cells were passaged at 70–80% confluence and passages 3–8 were used
for experiments.
RNA isolation and qPCR
Total RNA was extracted from monolayer cells by
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA). RNA
quality was assessed by the Blue Pippin automated electrophoresis
system (Sage Science, Inc., Beverly, MA, USA) using the Experion
RNA StdSens Analysis kit (Bio-Rad, Hercules, CA, USA). cDNA was
prepared using the PrimeScript II First Strand cDNA Synthesis kit
(Takara Bio Inc., Shiga, Japan) according to the manufacturer’s
instructions, and 1 μl 5X diluted cDNA was used for further gene
amplification. qPCR was performed by FastStart Universal SYBR-Green
Master (Rox; Roche Dianostics, Mannheim, Germany). PCR was
performed in a Light Cycler real-time PCR machine (Bio-Rad) with
the following thermal profile: 2 min at 95ºC followed by 40 cycles
of denaturation at 95ºC for 30 sec, annealing at 58ºC for 30 sec,
extension at 72ºC for 1 min and a final extension at 72ºC for 3
min. The primers used were as follows: Forward:
5′-GGTGATGGTGGGAATGGG-3′, and reverse: 5′-GCAGGGTGGGATGCTCTT-3′ for
α-SMA; forward: 5′-TTCCTGCGCCTGATGTCC-3′ and reverse
5′-GGTTCAGTTTGGGTTGCTTGT-3′ for collagen I; forward:
5′-TGAAAGGACACAGAGGCTTCG-3′ and reverse: 5′-GCACCATTCTTACCAGGCTC-3′
for collagen III; forward: 5′-TTCCTGCGCCTGATGTCC-3′ and reverse:
5′-GGTTCAGTTTGGGTTGCTTGT-3′ for FGF2; forward:
5′-TCAGAAGAGCGAGCCCCT-3′ and reverse: 5′-GGGGTCTTTTGAACTGTGGA-3′
for fibronectin; forward: 5′-CCAGGCAAAGCAGGAGTC-3′ and reverse:
5′-GGGTATCAACCAGAGGGAGT-3′ for vimentin; forward:
5′-CATCTCGCACCTGGGCAA-3′ and reverse: 5′-CTGTTTGGGCTGGGTGTTC-3′ for
scleraxis; and forward: 5′-GGGAAACTGTGGCGTGAT-3′ and reverse
5′-GTGGTCGTTGAGGGCAAT-3′ for glyceraldehyde 3-phosphate
dehydrogenase (GAPDH). All primers were synthesized by Takara. Data
was analyzed using Bio-Rad iQ5 software. Expression of endothelial
genes was calculated relative to GAPDH levels by the comparative
ΔCT method.
Western blot analysis
The cells were harvested and lysed in RIPA buffer on
ice. The cell lysates were heated at 100ºC for 5 min and
centrifuged at 16,000 × g for 10 min. The supernatant was collected
and the protein concentration was determined by the Pierce
Bicinchoninic Acid Protein Assay kit (Thermo Scientific, Waltham,
MA, USA). Equivalent quantities of protein (40 μg) from each sample
were loaded and run on sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gels (Bio-Rad) and transferred onto nitrocellulose
membranes (Bio-Rad). Subsequent to blocking the membranes with 2%
bovine serum albumin in Tris-buffered saline with 0.1% Tween 20 at
room temperature for 2 h, the membranes were incubated with primary
antibodies in a 1:1,000 dilution at 4ºC overnight, washed with
phosphate-buffered saline with Tween 20 (PBST), and incubated with
horseradish peroxidase-conjugated secondary antibodies for 1 h at
room temperature. Antibodies used were as follows: Anti-FGF2
(catalog no. ab106245; Abcam, Cambridge, UK), phospho-p42/44MAPK
(Thr-202/Tyr-204; catalog no. 9101; Cell Signaling Technology,
Inc., Danvers, MA, USA), p42/44MAPK (catalog no. 9102; Cell
Signaling Technology, Inc.), collagen I (catalog no. ab21285;
Abcam), collagen III (catalog no. ab7778, Abcam), scleraxis
(catalog no. sc-87425; Santa Cruz Biotechnology Inc., Santa Cruz,
CA, USA), fibronectin (catalog no. AB2033; Millipore, Billerica,
MA, USA) and α-smooth muscle actin (catalog no. PA5-19465; Thermo
Scientific Pierce, Rockford, IL, USA). Subsequent to washing with
PBST, the immunoblots were visualized by chemiluminescence using an
enhanced chemiluminescent western blot analysis substrate
(Millipore). GAPDH was used to ensure equal protein loading.
Construction plasmids and recombinant
adenoviruses
Adenovirus was generated using the pAdTrack/pAdEasy
system. Briefly, full-length FGF2 cDNA was subcloned into pAdTrack
as described previously (24). The
pAdTrack plasmid was cotransfected with pAdEasy into DH5α cells to
generate recombinant plasmids. Recombinants (10 μg) were linearized
with PacI restriction enzyme (New England Biolabs, Ipswich,
MA, USA) and transfected into 293A packaging cells (from laboratory
stock). Recombinant adenoviruses were prepared, purified and
titered as described previously (25). MSCs were infected with vehicle
adenovirus or Ad-FGF2 at 2×107 multiplicity of infection
(MOI)/ml for 24 h. Adenoviral infection efficiency was assessed by
western blot analysis. The cDNAs encoding full open reading frames
of murine Spry1 and Spry4 were amplified and subcloned into
pcDNA3.1(+) (Invitrogen Life Technologies) with the BamHI
and EcoRI sites. The corresponding segments were amplified
using PCR with the following primers: Forward:
5′-AAAGGATCCATGGATTCC CCAAGTCAGC-3′ and reverse: 5′-AAAGAATTCTCATGA
CAGTTTGCCCTGAG-3′ for Spry1; and forward: 5′-AAA
GGATCCATGGAGCCCCCGGTTCCA-3′ and reverse,
5′-AAAGAATTCTCAGAAAGGCTTGTCAGACCTGC-3′ for Spry4.
For transient co-transfection of Spry1 and Spry4,
MSC cells were seeded onto 60 mm dishes and transfected with 4
μg/dish each plasmid or a control vector using
LipofectamineTM 2000 reagent (Invitrogen Life
Technologies) according to the manufacturer’s instructions.
Cell proliferation assay
A cell proliferation assay was performed using
tetrazolium compound based CellTiter 96® Aqueous One
Solution Cell Proliferation (MTS) assay (Promega Corporation,
Madison, WI, USA). Cells of each MSC clone were seeded into wells
of a 96-well plate at a density of 4×103. After 24, 48,
and 72 h of culture, an MTS assay was performed according to the
manufacturer’s instructions. Each experiment was performed in
triplicate and repeated 3 times (n=3).
Statistical analysis
Results are expressed as the mean ± SD. Significance
was analyzed with a two-tailed unpaired t-test using GraphPad Prism
5 Demo software for Windows (GraphPad Software, San Diego, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of FGF2 in MSCs by adenoviral
vectors
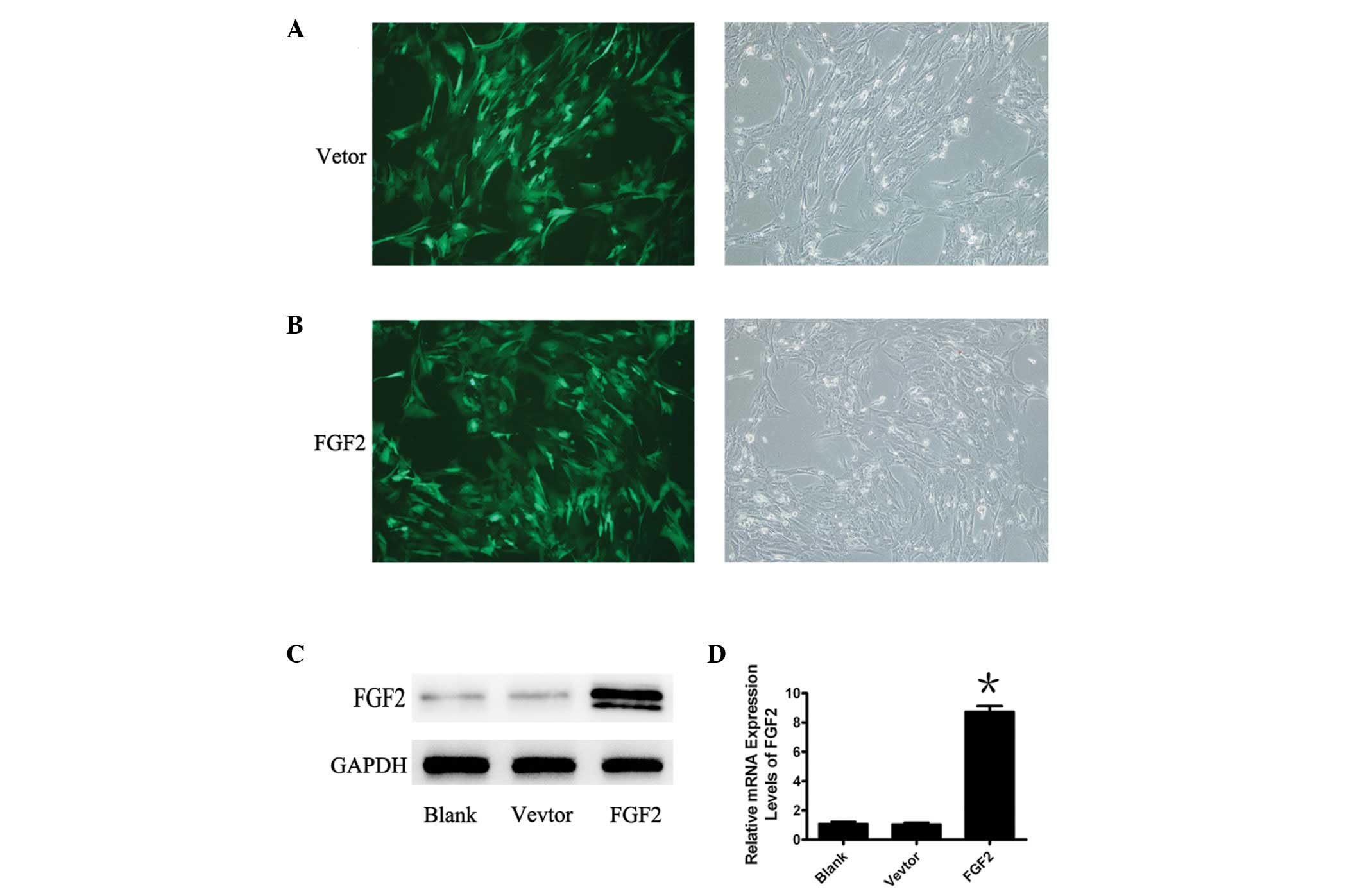
To gain an understanding of the involvement of FGF2
in MSCs, MSCs were infected with a replication deficient Ad5 vector
encoding FGF2 and GFP, or the control adenoviral vector, Ad-GFP.
Green fluorescence identifies adenovirus-infected cells following
infection with FGF2 or GFP (Fig. 1A
and B). A high infection efficiency was observed when the MOI
was 40 and the expression of mRNA and protein of FGF2 were verified
by qPCR and western blot analysis (Fig. 1C and D). The mRNA and protein
expression of FGF2 were markedly increased following treatment of
MSCs with Ad-FGF2, whereas treatment with the negative control did
not result in any change in FGF2 expression.
Overexpression of FGF2 induces tendon
marker expression
To demonstrate the therapeutic potential of FGF2 in
tendon injury, the expression of several important genes involved
in the differentiation of MSCs were analyzed on days 4, 7 or 14.
The mRNA of FGF2 was significantly increased in FGF2-treated MSCs
compared with the control on days 4 and 7 (Fig. 2A). As expected, exposing MSCs to
the combination of adenoviral vectors encoding FGF2 resulted in the
upregulation of the mRNA of molecular markers, including collagen
I, collagen III, fibronectin, scleraxis, α-SMA, and VMT on days 4,
7 and 14 (Fig. 2B–G). To confirm
the change of the corresponding genes, their expression was
detected by western blot analysis (Fig. 2H). These characteristics indicated
that FGF2 induced MSCs to differentiate into tenocytes.
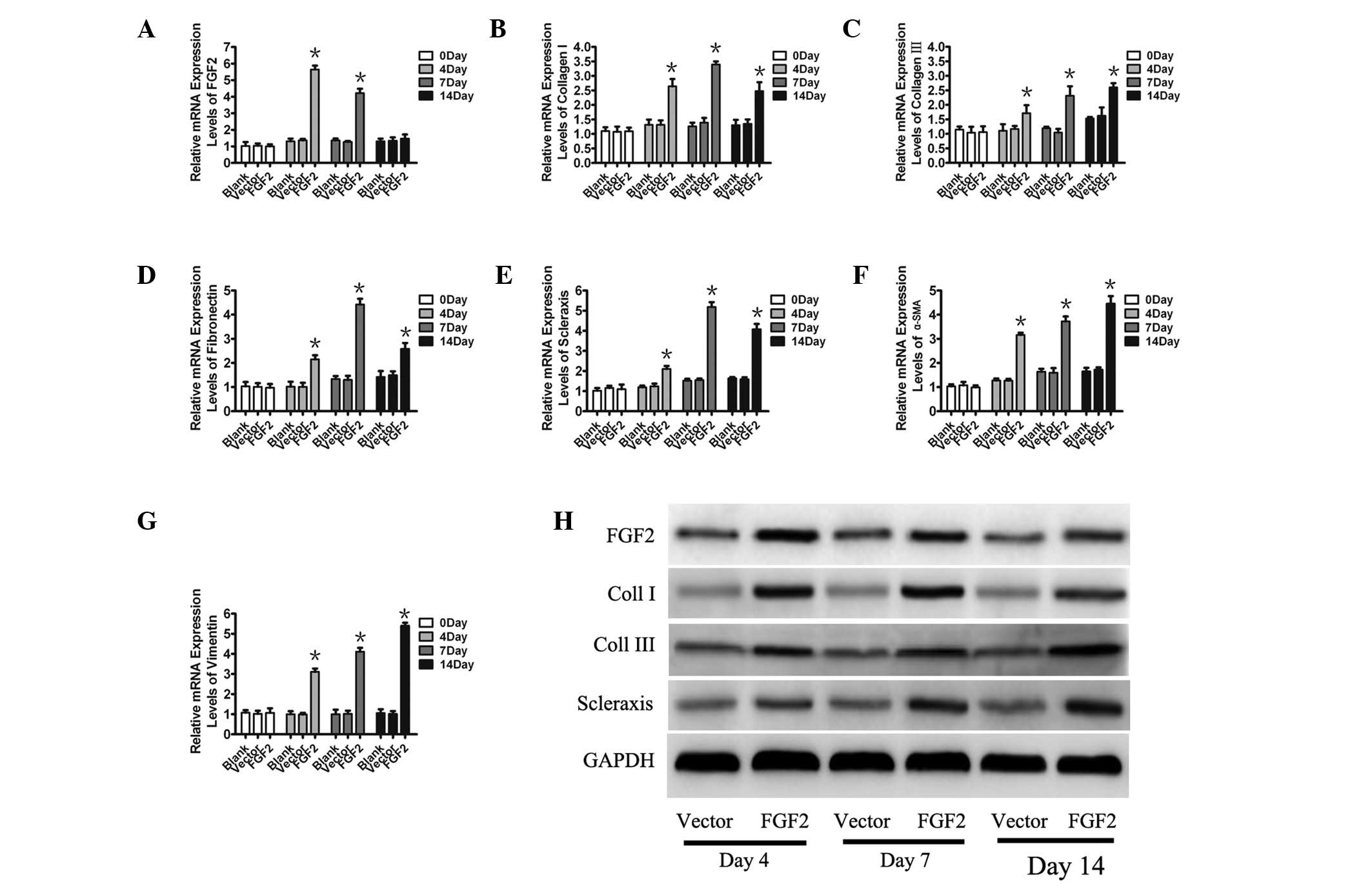 | Figure 2Relative mRNA expressions of key
cytokines and growth factors involved in the differentiation of
mesenchymal stem cells. (A–G) Relative mRNA expression of FGF2,
Coll I, Coll III, fibronectin, scleraxis, α-SMA and vimentin was
measured by qPCR and corrected with GAPDH levels. Data are
expressed as the mean ± SE; *P<0.05 vs control. (H)
The detection of FGF2, Coll I, Coll III and scleraxis was confirmed
by western blot analysis on days 4, 7 and 14. FGF2, fibroblast
growth factor 2; SMA, smooth muscle actin; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; Coll, collagen. |
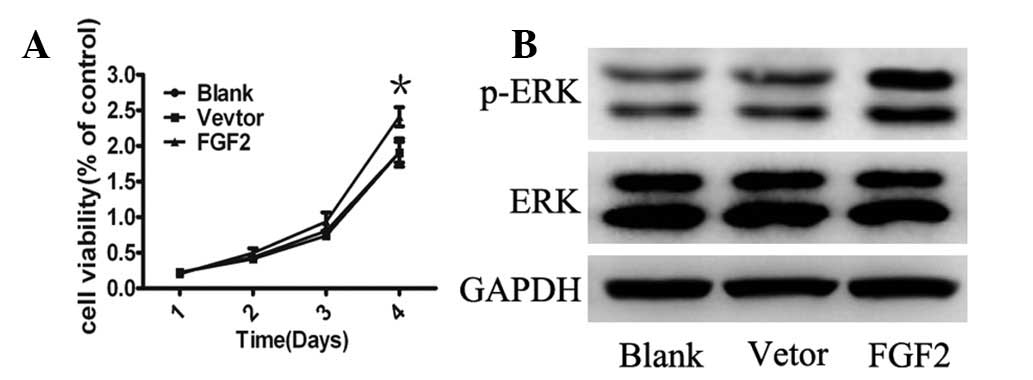
FGF2 promotes cell growth and activation
of MAPK signaling
To gain further insight into the biological effects
of FGF2 on MSCs, cell growth was analyzed and it was identified
that ectopic expression of FGF2 resulted in enhanced proliferation
compared with control cells (Fig.
3A). To investigate the signaling pathways regulating the
effects of FGF2, the MAPK pathway, a key cellular signal activated
in pluripotent cells was analyzed (26). Consistent with these findings, ERK
phosphorylation was upregulated in MSCs infected by Ad-FGF2
(Fig. 3B). These data suggest that
the overexpression of FGF2 in MSCs resulted in cell proliferation,
which appeared to be via the MAPK pathway.
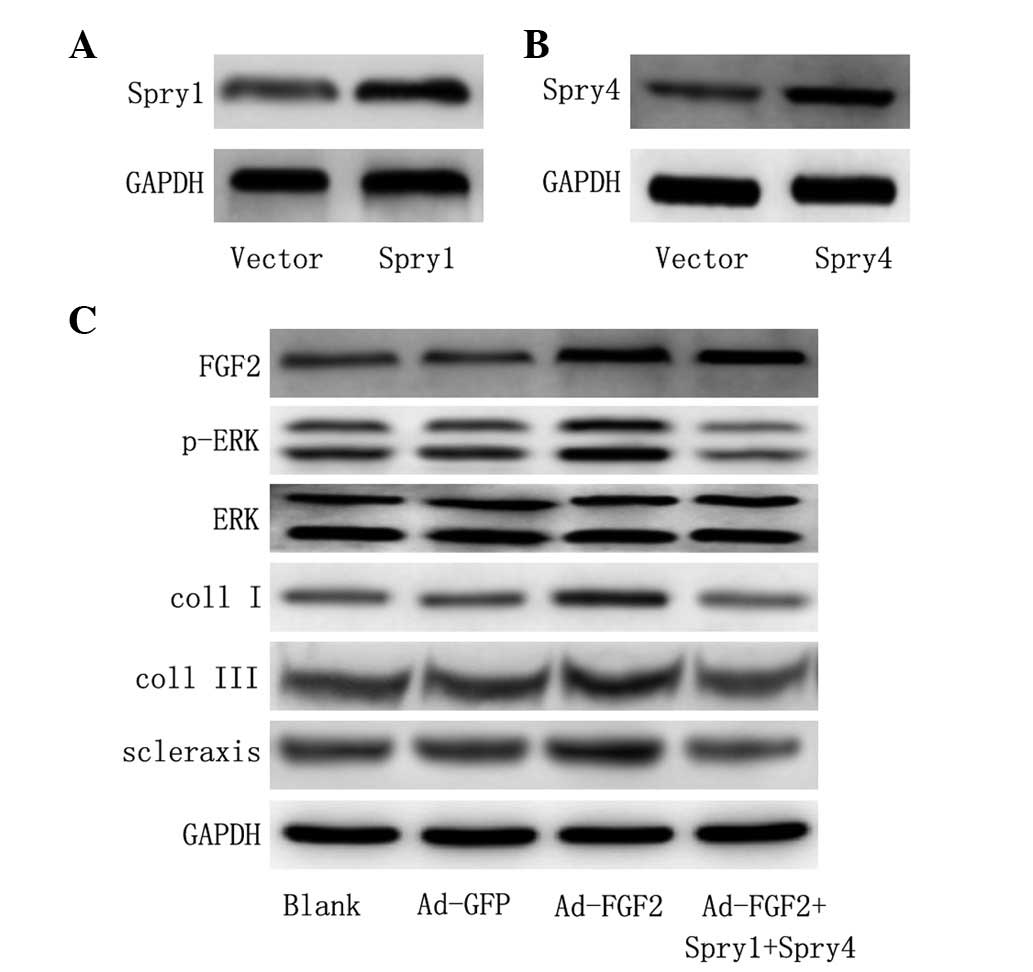
Downstream signaling from FGF2 is ERK1/2
dependent
To further validate whether the activation of ERK
was required in MSCs for differentiation into tenocytes, Spry1 and
Spry4 were co-transfected, which have been shown to be efficient in
the suppression of FGF2 induced ERK activation (27). The protein expression of Spry1 and
Spry4 was significantly increased compared with control vector
following ectopic expression of Spry1 and Spry4 (Fig. 4A and B). In addition,
overexpression of Spry1 and Spry4 inhibited the activation of ERK
(Fig. 4C). Furthermore, the
expression of Spry1 and Spry4 protein prevented the induction of
collagen I, collagen III and scleraxis expression relative to that
in untransfected cells (Fig. 4C).
Overall, data suggest that the activation of MAPK is involved in
the differentiation of MSCs by FGF2.
Discussion
BM MSCs have recently received widespread attention
due to their capacities for multilineage differentiation and
proliferation (6). To the best of
our knowledge, the present study showed for the first time, that
MAPK was important in the differentiation of MSCs induced by FGF2.
The results indicated that FGF2 is able to promote the
differentiation of MSCs into tendons by activating the MAPK
pathway, which regulates the expression of molecular biomarkers of
tenocytes.
To understand the molecular basis by which FGF2
determines the differentiation of MSCs, MSCs were maintained to
high passage in vitro and retained their proliferative and
multipotent abilities. The MSCs were obtained from human donors and
cultured without the loss of endothelial potential normally
observed in cells expanded up to such a level. Previous studies
have shown that FGF2 exhibited bidirectional effects on the growth
and differentiation of MSCs (14,18,20,23).
Expression of FGF2, which is an important tenocyte cytokine induced
tendon matrix protein type I collagen, type III collagen and
scleraxis. This result is consistent with studies showing that FGF2
is important in stimulating cell proliferation and differentiation
(4).
To further investigate the downstream signaling
which is required in MSC differentiation, it was hypothesized that
ERK signaling is essential for tendon differentiation. Data
demonstrated that phosphorylation of ERK1/2 is strongly upregulated
in MSCs following overexpression of FGF2 and promoted cell growth.
Recent studies by Ozaki et al(27) demonstrated that co-expression of
Sprouty1 and Sprouty4 efficiently suppressed the FGF2-induced ERK
activation. Sprouty1 and Sprouty4 were then co-transfected, which
efficiently inhibited the ERK activation induced by FGF2, while the
increase of collagen I, collagen III and scleraxis induced by FGF2
was inhibited. These results provided key insights in the
involvement of MAPK signaling in FGF2-induced differentiation.
In conclusion, the results indicated that
overexpression of FGF2 by adenovirus stimulates proliferation and
expression of extracellular matrix proteins, which are required for
the tissue engineering of tendons. In addition, it appears to
differentiate MSCs into a more fibroblast-like cell type. It was
also observed that FGF2 stimulated the activation of the
phosphorylation of ERK1/2 leading to the upregulation of essential
extracellular matrix molecules and cytoskeletal elements for
ligaments and tendons. It may be useful to determine whether FGF
signaling in MSCs is the same in vivo, as this would suggest
potential applications in tissue engineering and provides a tool
for various clinical studies.
Acknowledgements
This research was supported by National High
Technology Research and Development Program of China
(2008AA02Z418), National Natural Science Foundation of China
(81201378 and 81171753), Natural Science Foundation of Shanghai
Science and Technology Committee (12ZR1439000) and the Young
Researcher Programme of Shanghai Health Bureau (2011Y127). The
funders had no role in study design, data collection and analysis,
decision to publish or preparation of the manuscript.
References
|
1
|
Rees JD, Wilson AM and Wolman RL: Current
concepts in the management of tendon disorders. Rheumatology
(Oxford). 45:508–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Järvinen TA, Kannus P, Maffulli N and Khan
KM: Achilles tendon disorders: etiology and epidemiology. Foot
Ankle Clin. 10:255–266. 2005.
|
|
3
|
Wang JH, Iosifidis MI and Fu FH:
Biomechanical basis for tendinopathy. Clin Orthop Relat Res.
443:320–332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoffmann A and Gross G: Tendon and
ligament engineering: from cell biology to in vivo application.
Regen Med. 1:563–574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuo CK, Marturano JE and Tuan RS: Novel
strategies in tendon and ligament tissue engineering: Advanced
biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil
Ther Technol. 2:202010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruder SP, Fink DJ and Caplan AI:
Mesenchymal stem cells in bone development, bone repair, and
skeletal regeneration therapy. J Cell Biochem. 56:283–294. 1994.
View Article : Google Scholar
|
|
7
|
Jorgensen C, Gordeladze J and Noel D:
Tissue engineering through autologous mesenchymal stem cells. Curr
Opin Biotechnol. 15:406–410. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-beta delivery
into tumors. Cancer Res. 62:3603–3608. 2002.PubMed/NCBI
|
|
9
|
Khakoo AY, Pati S, Anderson SA, et al:
Human mesenchymal stem cells exert potent antitumorigenic effects
in a model of Kaposi’s sarcoma. J Exp Med. 203:1235–1247.
2006.PubMed/NCBI
|
|
10
|
Wu X, Chen S, Orlando SA, et al: p85alpha
regulates osteoblast differentiation by cross-talking with the MAPK
pathway. J Biol Chem. 286:13512–13521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giuliani N, Bataille R, Mancini C,
Lazzaretti M and Barillé S: Myeloma cells induce imbalance in the
osteoprotegerin/osteoprotegerin ligand system in the human bone
marrow environment. Blood. 98:3527–3533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Powers CJ, McLeskey SW and Wellstein A:
Fibroblast growth factors, their receptors and signaling. Endocr
Relat Cancer. 7:165–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu PJ, Ferrari G, Galloway AC, Mignatti P
and Pintucci G: Basic fibroblast growth factor (FGF-2): the high
molecular weight forms come of age. J Cell Biochem. 100:1100–1108.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van den Bos C, Mosca JD, Winkles J,
Kerrigan L, Burgess WH and Marshak DR: Human mesenchymal stem cells
respond to fibroblast growth factors. Hum Cell. 10:45–50.
1997.PubMed/NCBI
|
|
15
|
Tsutsumi S, Shimazu A, Miyazaki K, et al:
Retention of multilineage differentiation potential of mesenchymal
cells during proliferation in response to FGF. Biochem Biophys Res
Commun. 288:413–419. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bianchi G, Banfi A, Mastrogiacomo M, et
al: Ex vivo enrichment of mesenchymal cell progenitors by
fibroblast growth factor 2. Exp Cell Res. 287:98–105. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baddoo M, Hill K, Wilkinson R, et al:
Characterization of mesenchymal stem cells isolated from murine
bone marrow by negative selection. J Cell Biochem. 89:1235–1249.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi SC, Kim SJ, Choi JH, Park CY, Shim WJ
and Lim DS: Fibroblast growth factor-2 and -4 promote the
proliferation of bone marrow mesenchymal stem cells by the
activation of the PI3K-Akt and ERK1/2 signaling pathways. Stem
Cells Dev. 17:725–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Masola V, Gambaro G, Tibaldi E, et al:
Heparanase and syndecan-1 interplay orchestrates fibroblast growth
factor-2-induced epithelial-mesenchymal transition in renal tubular
cells. J Biol Chem. 287:1478–1488. 2012. View Article : Google Scholar
|
|
20
|
Solchaga LA, Penick K, Porter JD, Goldberg
VM, Caplan AI and Welter JF: FGF-2 enhances the mitotic and
chondrogenic potentials of human adult bone marrow-derived
mesenchymal stem cells. J Cell Physiol. 203:398–409. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Varas L, Ohlsson LB, Honeth G, et al:
Alpha10 integrin expression is up-regulated on fibroblast growth
factor-2-treated mesenchymal stem cells with improved chondrogenic
differentiation potential. Stem Cells Dev. 16:965–978. 2007.
View Article : Google Scholar
|
|
22
|
Neubauer M, Fischbach C, Bauer-Kreisel P,
et al: Basic fibroblast growth factor enhances PPARgamma
ligand-induced adipogenesis of mesenchymal stem cells. FEBS Lett.
577:277–283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lai WT, Krishnappa V and Phinney DG:
Fibroblast growth factor 2 (Fgf2) inhibits differentiation of
mesenchymal stem cells by inducing Twist2 and Spry4, blocking
extracellular regulated kinase activation, and altering Fgf
receptor expression levels. Stem Cells. 29:1102–1111. 2011.
View Article : Google Scholar
|
|
24
|
Frederick JP, Liberati NT, Waddell DS, Shi
Y and Wang XF: Transforming growth factor beta-mediated
transcriptional repression of c-myc is dependent on direct binding
of Smad3 to a novel repressive Smad binding element. Mol Cell Biol.
24:2546–2559. 2004. View Article : Google Scholar
|
|
25
|
Wang L, Blouin V, Brument N, Bello-Roufai
M and Francois A: Production and purification of recombinant
adeno-associated vectors. Methods Mol Biol. 807:361–404. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lanner F and Rossant J: The role of
FGF/Erk signaling in pluripotent cells. Development. 137:3351–3360.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ozaki K, Miyazaki S, Tanimura S and Kohno
M: Efficient suppression of FGF-2-induced ERK activation by the
cooperative interaction among mammalian Sprouty isoforms. J Cell
Sci. 118:5861–5871. 2005. View Article : Google Scholar : PubMed/NCBI
|