Introduction
Angiogenesis, the formation of novel
microvasculature by capillary sprouting, occurs when hemangioblasts
(the common pre-cursor cells of embryonic erythrocytes and
endothelial cells) differentiate in the area vasculosa and the
primary capillary plexus forms in situ(1). The significance of angiogenesis in
tumor progression is well known. Vasoganglions supply blood and
nutrition aiding solid tumor growth, and determining whether
necrosis and apoptosis or invasion and metastasis of tumor cells
occurs. The growth and metastasis of malignant cell populations
also rely on the development of microvessels to grow and
metastasize (2). There have been
numerous studies concerning angiogenesis inhibitors. Sunitinib
exhibited broad and potent antitumor activity by targeting the
vascular endothelial growth factor receptor (3), with angiogenesis inhibitors,
Marimastat and TNP-470, exhibiting antitumor activity in
preclinical studies (4,5). However, these inhibitors each have
different limitations. There is an urgent requirement for a more
efficient system for screening angiogenesis and angiogenesis
inhibitors.
The allantois of the chick embryo appears at ~3.5
days of incubation (6). An
extremely rich vascular network develops in chorioallantoic
membrane (CAM) from day 4 to 14 (7). Thus, the chick embryo CAM may be used
to study the angiogenic and angiogenic inhibitory activity of drugs
and macromolecules (8).
Macromolecules and compounds, such as a variety of growth factors
(fibroblast growth factor-2, transforming growth factor-β and tumor
necrosis factor-α) and protein kinase c were demonstrated to induce
CAM angiogenesis (9–11). CAM is used to investigate tumor
angiogenesis and metastasis. However, as the chick’s immune system
is not completely developed at the early stages of chick embryo
formation (12), the CAM assay has
limitations including non-specific inflammatory reactions (13) and rearrangement of existing vessels
that follows contraction of the membrane (14). These limitations influence the
observations of vasoproliferative responses. Bouins and
paraformaldehyde fluid solution are often used to repair the CAM
for ≥3 h after the shell has separated from the membrane (15) as damage to the membrane and
morphological changes in CAM occur easily during these
operations.
The capillary plexus in the yolk sac vascular tree
develops into arteries and veins on days 3–5 of embryonic
development (16). Consequently,
the yolk sac membrane assay, which was essentially adapted from the
CAM assay, was exploited. Polyamines and spermidine were observed
to induce angiogenesis in the chick yolk sac membrane (17). The yolk sac membrane model was also
utilized for studying the effect of radiation on vascular density
in a rapidly growing tissue (18);
however, there are a few studies concerning the chicken yolk sac
membrane model for screening angiogenic and angiogenic inhibitors.
Methylcellulose disk or drugs containing disk-shaped support are
commonly used as drug containers and are placed on the yolk sac
membrane (19). However, accurate
results are not achieved when using this method as these containers
cannot limit the area on the yolk sac membrane that is influenced
by the drug.
In early vertebrate embryos, hematopoietic and
endothelial lineages are derived from blood islands (aggregates of
mesodermal cells) in the extra-embryonic yolk sac (20). Following the differentiation of
blood island cells into blood and endothelial lineages, endothelial
cells from individual blood islands anastomose to form the primary
vascular plexus. The chick embryo remains a powerful model for
studying developmental hematopoiesis and erythropoiesis (21). Although blood island formation is
essential to analyze the early developmental stage of the embryonic
blood vessels, to the best of our knowledge, the early embryonic
blood island assay has not previously been utilized for anticancer
drug screening.
FVB/N-Tg (MMTV-PyMT) 634Mul-transgenic mice, under
the regulation of the mouse mammary tumor virus (MMTV) long
terminal repeat, express the mouse polyomavirus middle-T antigen
(PyMT). These transgenic animals consistently develop multifocal
mammary tumors and exhibit a high incidence of pulmonary metastasis
(22). In MMTV-PyMT mice, four
distinctly identifiable stages of tumor progression from
premalignant to malignant stages occur in a single primary tumor
focus. Therefore, this model is a powerful tool for studying tumor
progression (23).
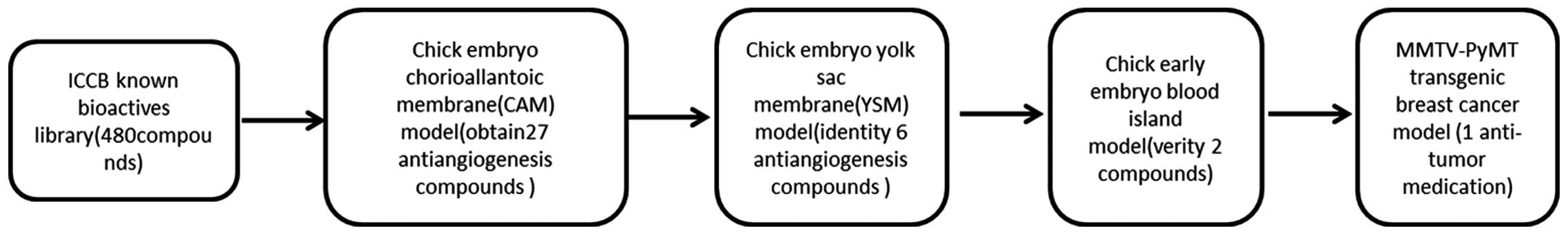
In the present study, using the chick embryo CAM
model, chick yolk sac membrane model, early chick embryo blood
island assay and the MMTV-PyMT tumor model as a four-step screening
system, potential angiostatic drugs were screened and the influence
of these drugs on tumor development and tumor progression was
investigated.
Materials and methods
Animals
Fertilized white leghorn chicken eggs were obtained
from the Avian Farm of South China Agriculture University
(Guangzhou, China), and were incubated under conditions of 50–60%
humidity and 37.5°C when the shell had been cleaned with 1%
geramine.
FVB/N-TgN (MMTV-PyMT) 634Mul-transgenic mice have
been described previously (22)
and were obtained from the Jackson Memorial Laboratory (Bar Harbou,
MA, USA). All animals were weaned and the tails were clipped at 4
weeks of age. The mice were genotyped by PCR using a primer pair
specific for the MMTV-PyMT transgene and distributed randomly into
the treatment and control groups (G0 treatment group, N=7; DMSO
control group, N=8). The mice were housed and cared for in
accordance with the 2011 Office of Animal Care and Use NIH
guidelines.
Reagent
Four hundred and eighty compounds for the CAM assay
were purchased from Enzo Life Science (Shenzhen, China; Lot
#3-P6574m) (Fig. 1). All 480
compounds were marked with a drug code (A0–A19; B0–B19; … X0–X19)
and dissolved in dimethylsulfoxide (DMSO; Amresco Inc., Solon, OH,
USA) to prepare a5 mg/ml stock solution. The G0 and G2 drugs to be
tested by the yolk sac membrane, blood island assay and MMTV-PyMT
mice assays were purchased from Sigma-Aldrich (St. Louis, MO, USA).
D1 was purchased from Enzo Life Science.
Chick embryo chorioallantoic membrane
assay
The CAM assay was conducted as described previously,
but with modifications (24). On
the 9th day, the shell area beyond the air chamber of the egg was
marked. Small windows (10×10 mm2) were opened in the
center of the marked area using a slow-speed dental molar which is
used in dental surgery to smooth the teeth (STRONG90; Yijia Health
Medical Devices Ltd., Gansu, China). The egg shells were then
opened with sterile forceps. The sample solution [50 μg/ml of
experimental compounds and the negative control 0.3 μl DMSO in 30
μl phosphate-buffered saline (pH 7.4)], were then placed directly
onto the CAM through the egg shell openings. The windows were
sealed with a medical proof fabric medical proof fabric (Shanghai
Medical Device Ltd., Shanghai, China; Lot #080901) and the eggs
were returned to the incubator at 37.8°C and 60% humidity. The egg
shells were then cut carefully along the axis of the median line
and the content was discarded after 48 h. The CAM vasculature
around the egg shell windows was observed and the treated area was
defined as the area on the CAM where the compounds or control DMSO
were attached. Digital images were captured at room temperature
with a Canon camera (Tokyo, Japan). The images were analyzed and
the number of every treatment condition (10 embryos per condition)
in which the vessels had become less dense around the treated area
was recorded as a positive number.
Chick embryo yolk sac membrane assay
On the 3rd day of incubation at 37.8°C and 60%
humidity, chicken embryos were transferred to a sterilized culture
dish. Eggs where the chicken embryo vessels faced upward were
selected. Two silica gel circles with red and black marker,
respectively, were placed in a symmetrical position of the yolk sac
membrane of the chicken embryos. The culture dish was covered
tightly prior to returning it to the incubator at 37.8°C and 60%
humidity. A total of 30 μl drug (50 μg/ml in gelatin) was
administered into the circles of the healthy embryos which had
well-developed chicken embryos vessels 3 h later. Images of the
vessels in the circles were captured by Image acquisition system
OPTPRO 2007 (Guangpujia Technology Ltd., Beijing, China) at 0, 12
and 24 h, respectively, following drug administration. The images
were quantitatively analyzed by Image-Pro Plus 6.0 (Media
Cyberneticss, Inc., Rockville MD, USA). The vessel growth rate in
the DMSO-treated silica gel circle was treated as one. A t-test was
conducted to show the difference between the vessel growth rate in
the control and experimental groups.
Early chick embryo blood island
assay
Half of the chicken embryos were exposed to the
medium containing drug at stages HH3-5 (25). The agar protein medium was
separated equally into two containing test compound or control
solvent. Graded concentrations (0.025, 0.05 and 0.1 mg/ml) of DMSO,
G0 and D1 were mixed into different sides of the agar protein
medium, then the early embryo was placed in the treated medium.
Following 28 h of incubation at 37.8°C and 60% humidity and 4%
paraformaldehyde fixing, a vascular endothelial (VE)-cadherin
plasmid (obtained from the laboratory of C.J. Weijer, University of
Dundee, Dundee, UK) was used for whole-mount in situ
hybridization. The techniques used for in situ hybridization
were essentially as described previously (26). The embryos were embedded with
glutin-sucrose and cut into serial sections. The results were
observed under an Olympus stereomicroscope (SZX16 Olympus, Tokyo,
Japan).
MMTV-PyMT mice treatment
Glipizide or DMSO (100 μg/20 g mouse weight) was
injected intraperitoneally into the 9-week-old MMTV-PyMT mice every
three days. Tumors were measured every four days and tumor volume
was calculated as: Length × width2 × 0.52. After 28
days, the mice were sacrificed and the tumors were collected for
weight analysis.
Immunohistochemistry
Tumor tissue sections were stained with goat
polyclonal antibodies against CD31 (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA; SC-1506) according to the manufacturer’s
instructions. Corresponding secondary antibody was purchased from
Sigma-Aldrich and used according to the manufacturer’s
instructions. The blood vessel density was determined by
quantifying the number of CD31-positive capillaries in 10 fields of
vision from each section of different areas of the tumor
(magnification, ×200).
Statistical analysis
To examine the independent and normal distribution,
the Student’s t-test was used to determine whether if certain
responses (immunohistochemical results, spontaneous tumor volumes
and tissue weights) were influenced by drug treatment. P<0.05
was considered to indicate a statistically significant
difference.
Results
Twenty-seven potential angiogenesis
inhibitors were obtained through high-throughput screening of 480
compounds utilizing the CAM assay
Ten embryos were used for each condition and the
positive number in each group was recorded. All 480 compounds were
marked with a drug code (A0–A19; B0–B19; … X0–X19). Twenty-seven
potential angiogenesis inhibitors were isolated from the 480
compounds obtained from the ICCB known bioactives library (Table I and Fig. 1).
 | Table IHigh-throughput screening of 480
compounds by utilizing the CAM assay. |
Table I
High-throughput screening of 480
compounds by utilizing the CAM assay.
| Positive no. | Compound code |
|---|
| 6 | A10, E10 |
| 5 | C9, G0, G6,
H4 |
| 4 | B2, C5, D1,
E7 |
| 3 | B5, B6, B10, C6, D3,
D4, D7, D8, E4, E11, G2, G4, H6, H8, H11, P1, P3 |
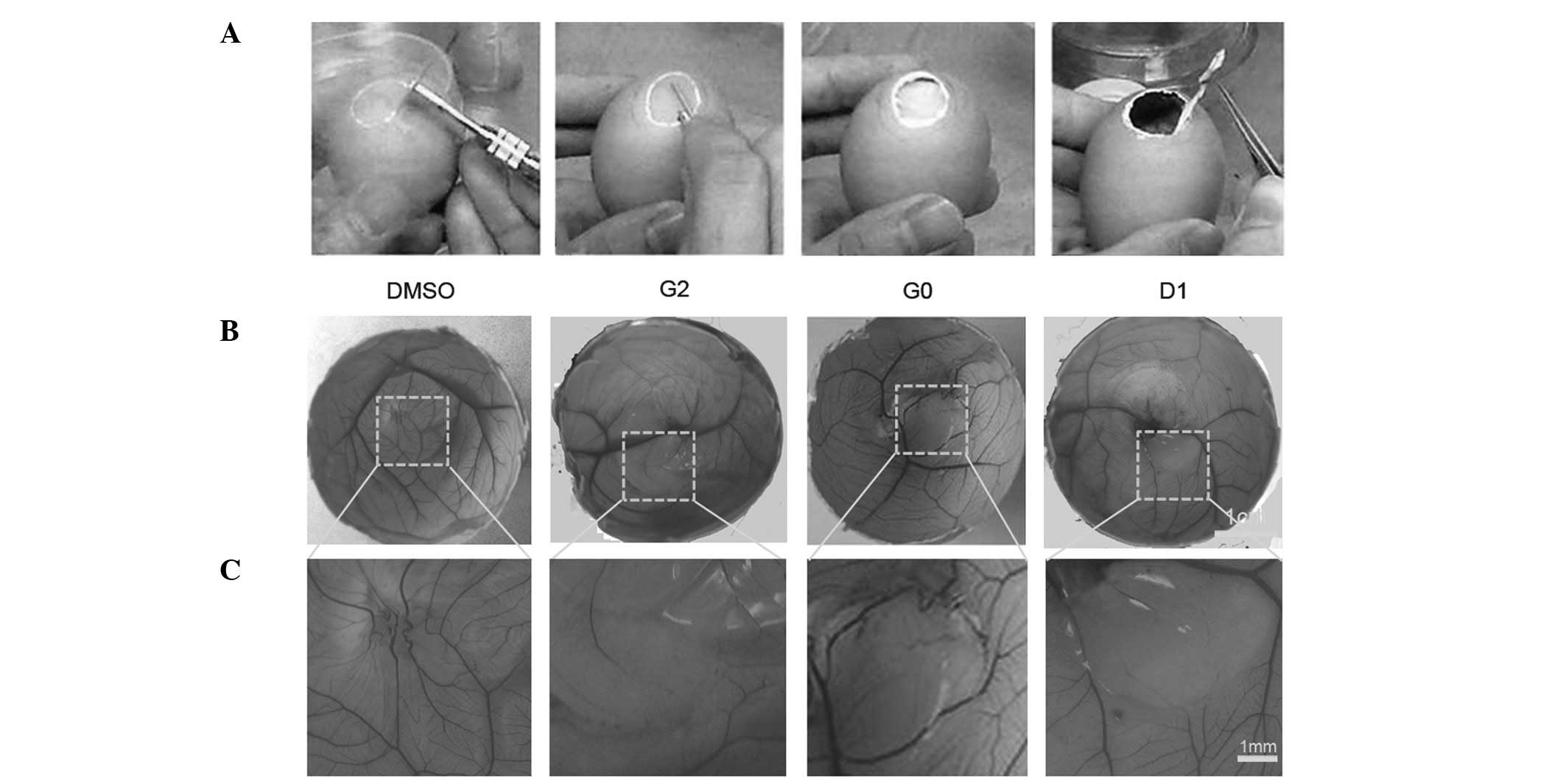
Drug G0, G2 and D1 induce the inhibition
of angiogenesis in the CAM assay
Twenty-seven potential angiogenesis inhibitors were
obtained from 480 bioactive compounds by high-throughput screening
based on the results of the CAM assay (Table I). In G0-treated CAM, the quantity
and density of vessels decreased in the drug-treated area in five
CAMS (n=10). The number of experimental CAMS treated with D1 and G2
which demonstrated angiogenic inhibition was 4 and 3, respectively
(n=10). No vascular reaction was detectable in CAMS treated with
DMSO alone (negative control) (Fig.
2). This showed that the application of G0, G2 and D1, but not
DMSO suppressed the formation of neovasculature in chick embryo
CAM.
G0, G2 and D1 induced the inhibition of
angiogenesis of the embryo yolk sac membrane
The percentage of available embryos was 60% of the
total when the chicken embryo contents were transferred to the
sterilized culture dish. Thirty percent of the total quantity of
the chicken embryos had well-developed vessels following 12 h of
drug treatment. The yolk sac membrane from these embryos was
observed, representative images were captured (Fig. 3B) and statistical analysis was
conducted (Fig. 3C–E). The vessel
growth rate following treatment with G2, G0 or D1 was notably
decreased after a 12-h incubation with these drugs (n=7, P=0.0295;
n=9, P=0.0013 and n=7, P=0.0301, respectively), but not
significantly decreased after 24 h incubation with G2 (n=7,
P=0.1073).
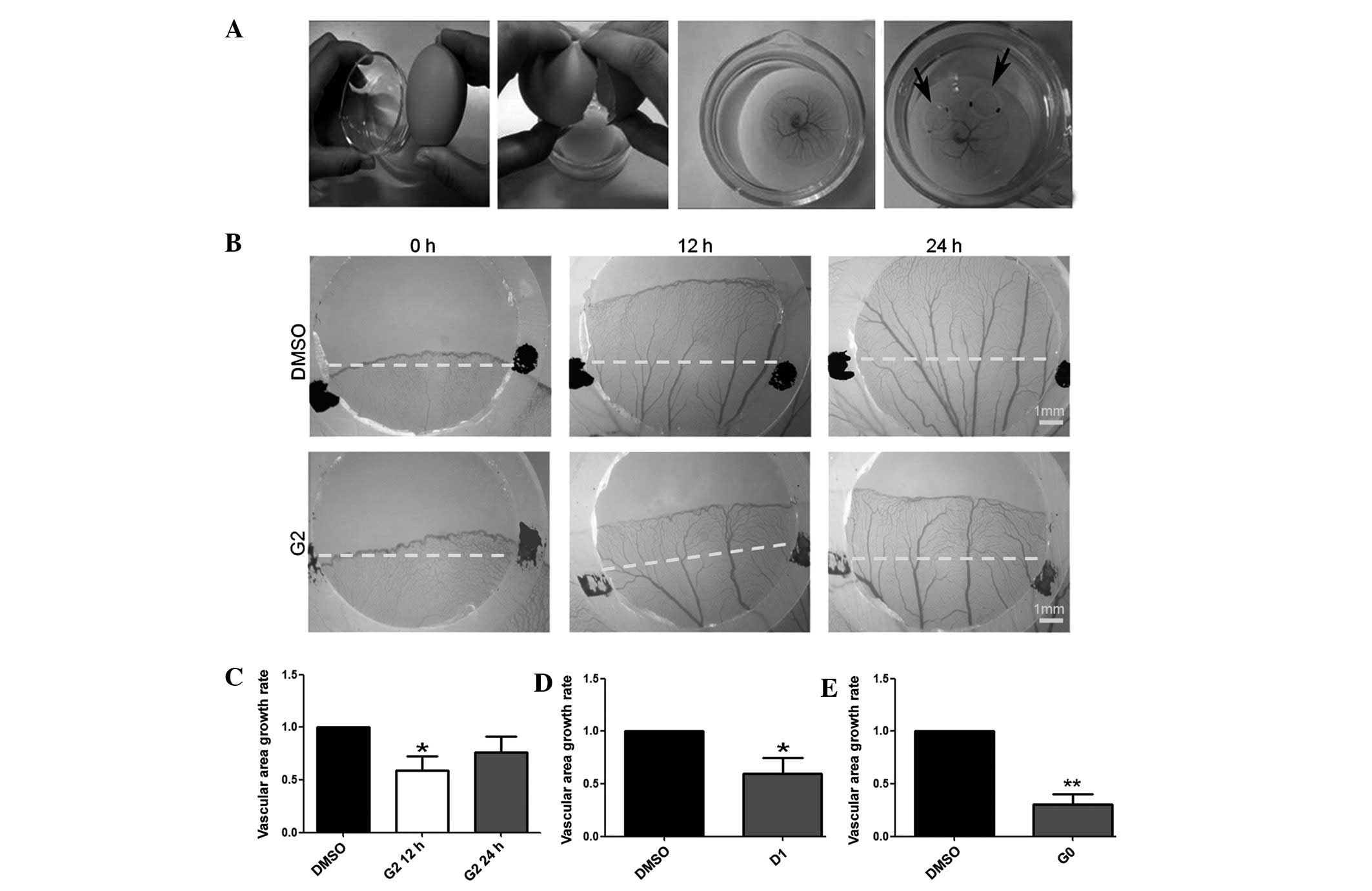 | Figure 3Compared with dimethylsulfoxide (DMSO)
treatment, G2, G0 and D1 treatment inhibited the angiogenesis of
the chicken embryo yolk sac membrane. (A) Yolk sac membrane model
protocol. (B) Digital images of silica gel circles of vessels on
chicken embryos yolk sac membrane captured by the Image acquisition
system OPTPRO 2007 (0, 12 and 24 h after treatment). The area
covered by black marked silica gel circles was treated with DMSO
and the red-marked area was treated with G2. (C) The vascular area
growth rate was analyzed according to the image captured by the
Stereo Microscope. Compared with DMSO treatment, G2 treatment
inhibited angiogenesis of the yolk sac membrane after 12 h
(*P=0.0295, n=7), but not 24 h after treatment
(P=0.1073, n=7). (D) Compared with DMSO treatment, D1 treatment
inhibited the angiogenesis of yolk sac membrane after 12 h
treatment (*P=0.0301, n=9). (E) After 12 h, G0 treatment
inhibited the angiogenesis of YSM compared with DMSO treatment
(**P=0.0013, n=7). *P<0.05 and
**P<0.001. |
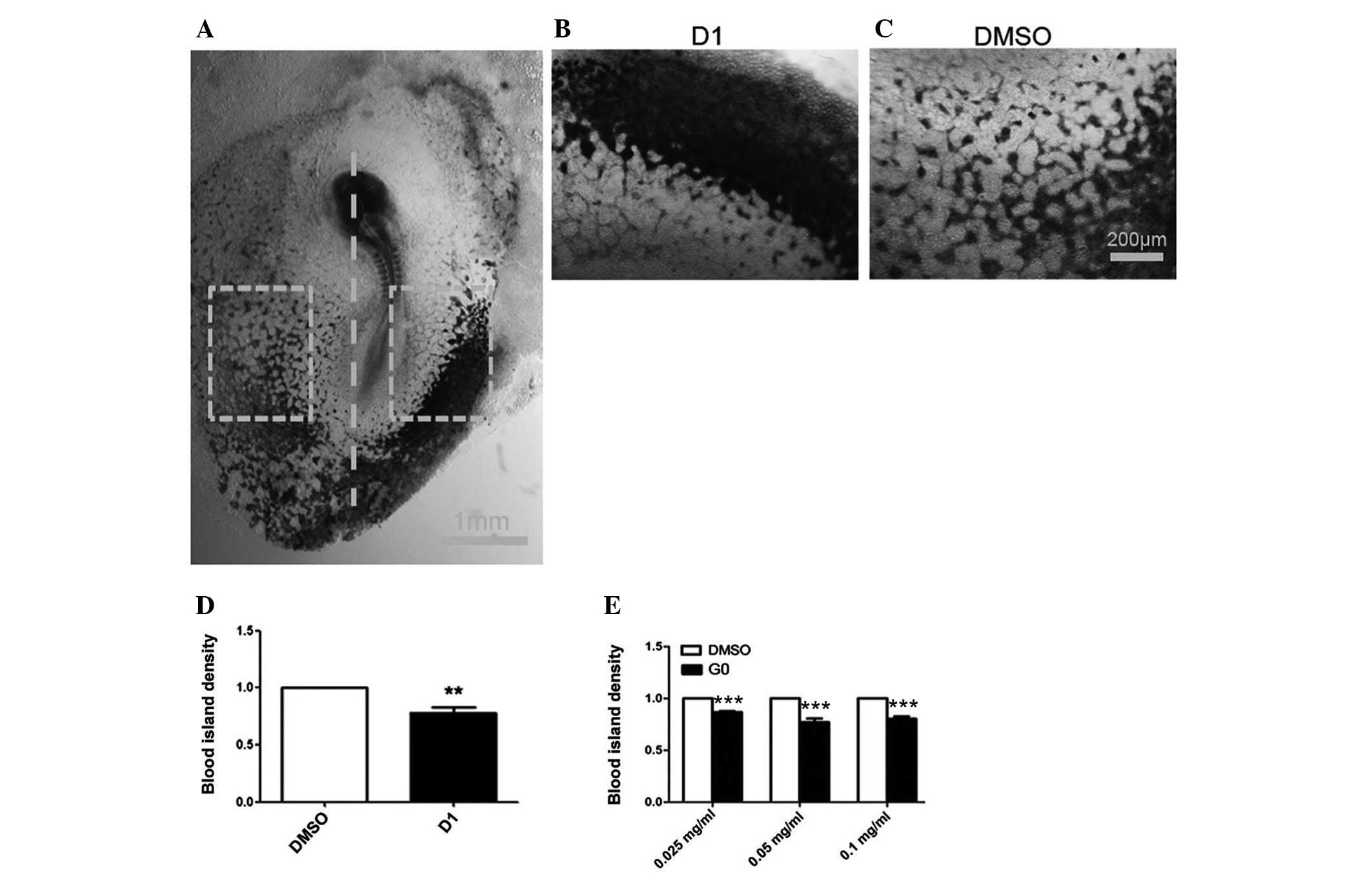
D1 and G0 inhibited the development of
the chicken embryo blood island in the early embryo model in
vivo
Following incubation, the blood islands were marked
with VE-cadherin. The blood island density of the area treated with
D1 (0.05 mg/ml concentration) compared with that treated with DMSO
was significantly decreased (P=0.0032, n=8) (Fig. 4D). The blood island density of the
area treated with three different concentrations of G0 (0.025, 0.05
and 0.1 mg/ml) was significantly decreased compared with the
DMSO-treated area (P≤0.0001, n=10; Fig. 4E).
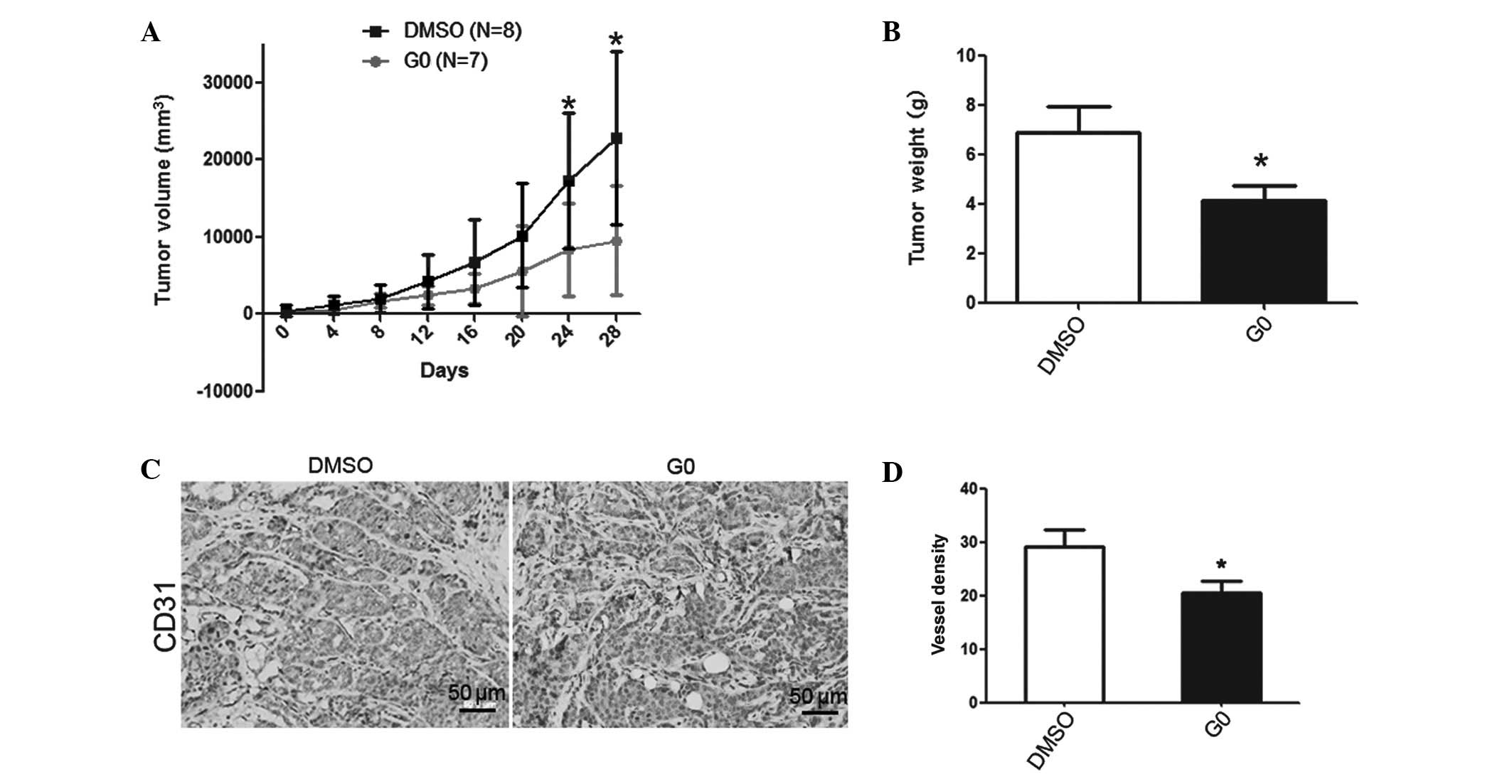
G0 alleviates the growth of spontaneous
breast cancer through the inhibitory function of tumor angiogenesis
in MMTV-PyMT mice
The G0-treated group exhibited a notable decrease in
tumor volume after 24 days treatment (Fig. 5A). All breast tumors from each
mouse were harvested after 28 days of treatment and the tumor
weight was measured. The tumor weight of the G0 group was observed
to be decreased (P=0.0312; DMSO group, n=14; G0 group, n=15;
Fig. 5B). Immunohistochemistry was
used to stain the breast tumor tissue with CD31 antibody to locate
the vessels (Fig. 5C). The
quantity of stained vessels in each unit area was counted. The
vessel density of the G0 group was also significantly reduced
compared with the DMSO control group (P=0.0357, n=7, Fig. 5D).
Discussion
In the CAM assay, compounds demonstrate an
angiogenic function in the form of increased vessel density around
the implant, with the vessels radially converging towards the
center similar to the spokes of a wheel (27). However, when the quantity and
density of vessels decreases and eventually disappears around the
implant, an inhibitory function of the tested compound is shown
(28). The instrument and device
required for the CAM assay are easily obtainable. In our assay, the
membrane was not detached from the shell or fixed as described in
other studies. Since the embryo inflammation and experimental time
were both reduced with this method, the procedure and acquisition
of results were simple and br. An air chamber was also selected to
add and locate the drugs for a reliable result. Using this method,
to initially screen drugs and macromolecules with angiogenic and
angiogenic inhibitory activity among compounds in the library is
feasible and effective, and renders the high-throughput screening
of 480 compounds possible.
The yolk sac membrane model is more sensitive to
chemical compounds and uses the same organism as the CAM model,
thus providing verification of the CAM results. In the present
study, silica gel circles were used to limit the area influenced by
drug treatment. Silica gel circles from the control and
experimental groups were placed symmetrically on the same chicken
embryo yolk sac membrane to avoid individual differences. In the
current study, a morphometric evaluation, utilizing a computerized
system, was undertaken to quantify the growth of blood vessels in
the chicken yolk sac area vasculosa. This provides greater
credibility to the test results.
Blood island formation is an important marker to
assess the development of the early embryo blood vessels. The early
embryo blood island assay demonstrates the inhibition of
angiogenesis. At present, to the best of our knowledge, there are
no studies regarding the function of the early embryo blood island
assay in verifying angiogenesis inhibitors, thus, this study is the
first to show it is valid.
Vascular angiogenesis is the most influential factor
for determining blood supply and nutrition for solid tumor growth.
Numerous angiogenesis inhibitory compounds and proteins are
potential anticancer drugs. Thus, MMTV-PyMT transgenic mice, which
spontaneously develops primary mammary tumor with four stages of
tumor progression were used to investigate the antitumor effect of
these compounds. The tumor volume, weight and microvessel density
were analyzed. Through the CD31 staining to label the vessel of the
tumor tissue, it is possible to determine whether the angiogenesis
inhibitory effect of the compound influences tumor growth and
progression.
Of 480 compounds, 27 inhibited angiogenesis in chick
embryo CAM models. Using the chick embryo yolk sac membrane model
and the blood island assay, three compounds were identified to
exhibit significant angiogenic inhibition. One of these drugs was
selected to verify the attenuation effect on the breast tumor
growth in MMTV-PyMT transgenic mice by inhibiting angiogenesis.
In conclusion, a four-step system was created for
screening angiogenesis inhibitors which combined the CAM, yolk sac
membrane and early embryo island assays to identify potential
angiogenic inhibitors and the spontaneous tumor genetically
engineered mouse to investigate the potential antitumor effect of
these drugs. Among 480 compounds from ICCB known bioactives
library, three angiogenesis inhibitors were identified and one of
these drugs was shown to exhibit antitumor activity by inhibiting
tumor angiogenesis, which demonstrated that this system is feasible
and effective. Thus, this study has demonstrated a four-step system
for identifying compounds that inhibit the angiogenesis of tumors
and may be developed as novel agents for the treatment of
carcinoma.
Acknowledgements
The authors would like to thank Professor Jian-Guo
Geng (University of Michigan) for providing the reagent and help
with the discussion and Jie Ye and Lu Han for their technical
assistance. This study was supported by the National Basic Research
Program of China (project no. 973, grant no. 2010CB529702) and the
Natural Science Foundation of China (grant no. 31271455 to Lijing
Wang).
References
|
1
|
Risau W, Sariola H, Zerwes HG, et al:
Vasculogenesis and angiogenesis in embryonic-stem-cell-derived
embryoid bodies. Development. 102:471–478. 1988.PubMed/NCBI
|
|
2
|
Weidner N, Folkman J, Pozza F, et al:
Tumor angiogenesis: a new significant and independent prognostic
indicator in early-stage breast carcinoma. J Natl Cancer Inst.
84:1875–1887. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mendel DB, Laird AD, Xin X, et al: In vivo
antitumor activity of SU11248, a novel tyrosine kinase inhibitor
targeting vascular endothelial growth factor and platelet-derived
growth factor receptors: determination of a
pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res.
9:327–337. 2003.
|
|
4
|
Toppmeyer DL, Gounder M, Much J, et al: A
phase I and pharmacologic study of the combination of marimastat
and paclitaxel in patients with advanced malignancy. Med Sci Monit.
9:PI99–PI104. 2003.PubMed/NCBI
|
|
5
|
Herbst RS: Targeted therapy using novel
agents in the treatment of non-small-cell lung cancer. Clin Lung
Cancer. 3(Suppl 1): S30–S38. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ribatti D, Nico B, Vacca A, Roncali L,
Burri PH and Djonov V: Chorioallantoic membrane capillary bed: a
useful target for studying angiogenesis and anti-angiogenesis in
vivo. Anat Rec. 264:317–324. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ausprunk DH, Knighton DR and Folkman J:
Differentiation of vascular endothelium in the chick
chorioallantois: a structural and autoradiographic study. Dev Biol.
38:237–248. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ribatti D, Vacca A, Roncali L and Dammacco
F: The chick embryo chorioallantoic membrane as a model for in vivo
research on angiogenesis. Int J Dev Biol. 40:1189–1197.
1996.PubMed/NCBI
|
|
9
|
Wilting J, Christ B and Bokeloh M: A
modified chorioallantoic membrane (CAM) assay for qualitative and
quantitative study of growth factors. Studies on the effects of
carriers, PBS, angiogenin, and bFGF. Anat Embryol (Berl).
183:259–271. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olivo M, Bhardwaj R, Schulze-Osthoff K,
Sorg C, Jacob HJ and Flamme I: A comparative study on the effects
of tumor necrosis factor-alpha (TNF-alpha), human angiogenic factor
(h-AF) and basic fibroblast growth factor (bFGF) on the
chorioallantoic membrane of the chick embryo. Anat Rec.
234:105–115. 1992. View Article : Google Scholar
|
|
11
|
Leibovich SJ, Polverini PJ, Shepard HM,
Wiseman DM, Shively V and Nuseir N: Macrophage-induced angiogenesis
is mediated by tumour necrosis factor-alpha. Nature. 329:630–632.
1987. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leene W, Duyzings MJ and van Steeg C:
Lymphoid stem cell identification in the developing thymus and
bursa of Fabricius of the chick. Z Zellforsch Mikrosk Anat.
136:521–533. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jakob W, Jentzsch KD, Mauersberger B and
Heder G: The chick embryo choriallantoic membrane as a bioassay for
angiogenesis factors: reactions induced by carrier materials. Exp
Pathol (Jena). 15:241–249. 1978.PubMed/NCBI
|
|
14
|
Knighton DR, Fiegel VD and Phillips GD:
The assay of angiogenesis. Prog Clin Biol Res. 365:291–299.
1991.
|
|
15
|
Ribatti D, Nico B, Vacca A and Presta M:
The gelatin sponge-chorioallantoic membrane assay. Nat Protoc.
1:85–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
le Noble F, Moyon D, Pardanaud L, et al:
Flow regulates arterial-venous differentiation in the chick embryo
yolk sac. Development. 131:361–375
|
|
17
|
Takigawa M, Nishida Y, Suzuki F, Kishi J,
Yamashita K and Hayakawa T: Induction of angiogenesis in chick
yolk-sac membrane by polyamines and its inhibition by tissue
inhibitors of metalloproteinases (TIMP and TIMP-2). Biochem Biophys
Res Commun. 171:1264–1271. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plasswilm L, Höper J, Cordes N and
Tannapfel A: Investigation of microvessel density after
irradiation. Radiat Res. 151:454–460. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dias PF, Siqueira JM Jr, Maraschin M,
Ferreira AG, Gagliardi AR and Ribeiro-do-Valle RM: A polysaccharide
isolated from the brown seaweed Sargassum stenophyllum
exerts antivasculogenic effects evidenced by modified
morphogenesis. Microvasc Res. 75:34–44. 2008.
|
|
20
|
Dieterlen-Lievre F: On the origin of
haemopoietic stem cells in the avian embryo: an experimental
approach. J Embryol Exp Morphol. 33:607–619. 1975.PubMed/NCBI
|
|
21
|
Sheng G: Primitive and definitive
erythropoiesis in the yolk sac: a bird’s eye view. Int J Dev Biol.
54:1033–1043. 2010.PubMed/NCBI
|
|
22
|
Guy CT, Cardiff RD and Muller WJ:
Induction of mammary tumors by expression of polyomavirus middle T
oncogene: a transgenic mouse model for metastatic disease. Mol Cell
Biol. 12:954–961. 1992.PubMed/NCBI
|
|
23
|
Lin EY, Jones JG, Li P, et al: Progression
to malignancy in the polyoma middle T oncoprotein mouse breast
cancer model provides a reliable model for human diseases. Am J
Pathol. 163:2113–2126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan T and Burggren W: Hypoxic incubation
creates differential morphological effects during specific
developmental critical windows in the embryo of the chicken
(Gallus gallus). Respir Physiol Neurobiol. 145:251–263.
2005. View Article : Google Scholar
|
|
25
|
Hamburger V and Hamilton HL: A series of
normal stages in the development of the chick embryo. 1951. Dev
Dyn. 195:231–272. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Breier G, Albrecht U, Sterrer S and Risau
W: Expression of vascular endothelial growth factor during
embryonic angiogenesis and endothelial cell differentiation.
Development. 114:521–532. 1992.PubMed/NCBI
|
|
27
|
Ribatti D, Urbinati C, Nico B, Rusnati M,
Roncali L and Presta M: Endogenous basic fibroblast growth factor
is implicated in the vascularization of the chick embryo
chorioallantoic membrane. Dev Biol. 170:39–49. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iurlaro M, Vacca A, Minischetti M, et al:
Antiangiogenesis by cyclosporine. Exp Hematol. 26:1215–1222.
1998.PubMed/NCBI
|