Introduction
Interleukin 10 (IL-10) is known to be a cytokine
synthesis inhibitory factor, which functions in inhibiting
immunocyte stimulation. A previous study has shown that IL-10
inhibits the synthesis and activity of Th1 and the expression of
major histocompatibility complex-II (MHC-II) by decreaseing antigen
presentation ability and the activtiy of natural killer cells
(1). On the basis of the rejection
capability of IL-10, numerous studies have been performed in order
to develop a novel immunosuppressive drug for clinical use. The
CD4+CD25+ regulatory T cell
(CD4+CD25+ Treg) is a T cell that exhibits an
immunosuppressive function (2,3) and
may be resistant to T cell differentiation, enhancement and
activity, and thus, is hypothesized to maintain the immunological
balance of organisms. Numerous autoimmune diseases may be induced
by the quantity decrease and the dysfunction of Tregs. Moreover,
Tregs are important in inflammatory diseases, neoplasms and organ
transplantation (4). Transforming
growth factor-β (TGF-β) exhibits various biological effects
including cell differentiation, proliferation and apoptosis, and
regulating the growth, development, injuries and regeneration of
extracellular materials in many physiological and pathological
processes. The current study used allograft rabbit skin
transplantation and cyclosporin A (CsA), as a positive control, to
observe the rejection time and survival time of grafted skin. The
alteration of CD4+CD25+ regulatory T cells
and the levels of IL-10 and TGF-β in the peripheral blood were also
investigated. The role of IL-10 in immunological rejection
mechanisms was also investigated. The findings may be useful for
future research and development of IL-10.
Materials and methods
Animals
A total of 36 New-Zealand white rabbits (n=36, male
or female) used for the study were aged between 3 and 4 months,
weighed between 2 and 2.5 kg, and were obtained from Zunyi Medical
College (Zhuhai, China). The study was approved by the Ethics
Committee of Zunyi Medical College (Zhuhai Campus, Zhuhai, China).
The rabbits were divided into two groups, donors (n=18) and
receptors (n=18), for skin transplant surgery. The groups were then
divided into six subgroups, with each subgroup containing three
rabbits, including normal saline (NS; 1 ml/d), recombinant human
interleukin-10 (rhIL-10) low-dose (5 μg/kg/d), rhIL-10 high-dose
(10 μg/kg/d), CsA low-dose (5 mg/kg/d), CsA high-dose (10 mg/kg/d)
and rhIL-10 (5 μg/kg/d) + CsA (5 mg/kg/d) groups. All rabbits
received intramuscular drug injection for ten days, beginning one
day prior to skin transplantation surgery.
Reagents and instruments
The rhIL-10 was expressed by the
SMD1168/pPICZaA-hIL-10 engineering strain, which was constructed by
the Immunology Department of Zunyi Medical College (Zunyi, China)
(5). Anti-rabbit CD4-fluorescein
isothiocyanate (FITC) and rabbit CD25 purified antibodies were
purchased from Antigenix America Inc. (Melville, NY, USA). Zenon
R-Phycoerythrin mouse IgG Labeling kit was purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). Erythrocyte lysis
buffer was purchased from BD Biosciences (San Jose, CA, USA).
Rabbit IL-10 and rabbit TGF-β detection kits were purchased from
Jingtian Biotechnology Company (Beijing, China). A FacsCalibur flow
cytometer was purchased from BD Biosciences. The ELx-800 microplate
reader was purchased from Bio-Tek (Winooski, VT, USA).
Skin allograft surgery
Prior to skin allograft surgery, the hair of rabbits
was removed from the backs with electric clippers and a neck strap
was marked for each animal. The rabbits were anesthetized with
intravenous injection of pentobarbital (3%, 0.04 mg/kg).
Medium-thickness skin (1.5×1.5 cm) was harvested from the donor and
immersed in a penicillin normal saline solution with excess fat
tissue removed and then transplanted on to the same area of the
receptor. The four corners were coaptated and then bandaged with
aseptic vaseline gauze (Sanwell Product Co. Ltd., Beijing
China).
Serum preparation
The peripheral blood was harvested from the ear
marginal vein. The blood was collected one day prior to surgery and
subsequently, one, four, seven, 14, 21 and 28 days following
surgery. Blood samples were allowed to stand at room temperature
for 30 min and centrifuged at 716 × g for 20 min to collect serum.
Serum IL-10 and TGF-β levels were detected by enzyme-linked
immunosorbent assay (ELISA) according to the manufacturer’s
instructions (Jingtian Biotechnology Company).
Flow cytometric analysis (FCM)
The proportion of CD4+CD25+
Tregs/CD4+ T cells was analyzed by FCM. Peripheral blood
cell preparations (50 μl) were stained with 5 μl anti-rabbit
CD4-FITC and 5 μl anti-rabbit CD25-PE, and incubated at room
temperature for 30 min in the dark. Erythrocyte lysis buffer (2 ml)
was mixed briefly with a vortex mixer (Mylab Corporation, Beijing,
China), incubated at room temperature for 10 min in the dark,
centrifuged at 179 × g for 10 min to collect the precipitate. The
precipitate was then washed with phosphate-buffered saline (PBS),
fixed with 2 ml 1% paraformaldehyde and measured with FCM.
Statistical analysis
All data underwent statistical analysis using the
Statistical Product and Service Solutions version 15.0 (SPSS, Inc.,
Chicago, IL, USA) and all values are expressed as the mean ± SD.
Differences between groups were assessed by the analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
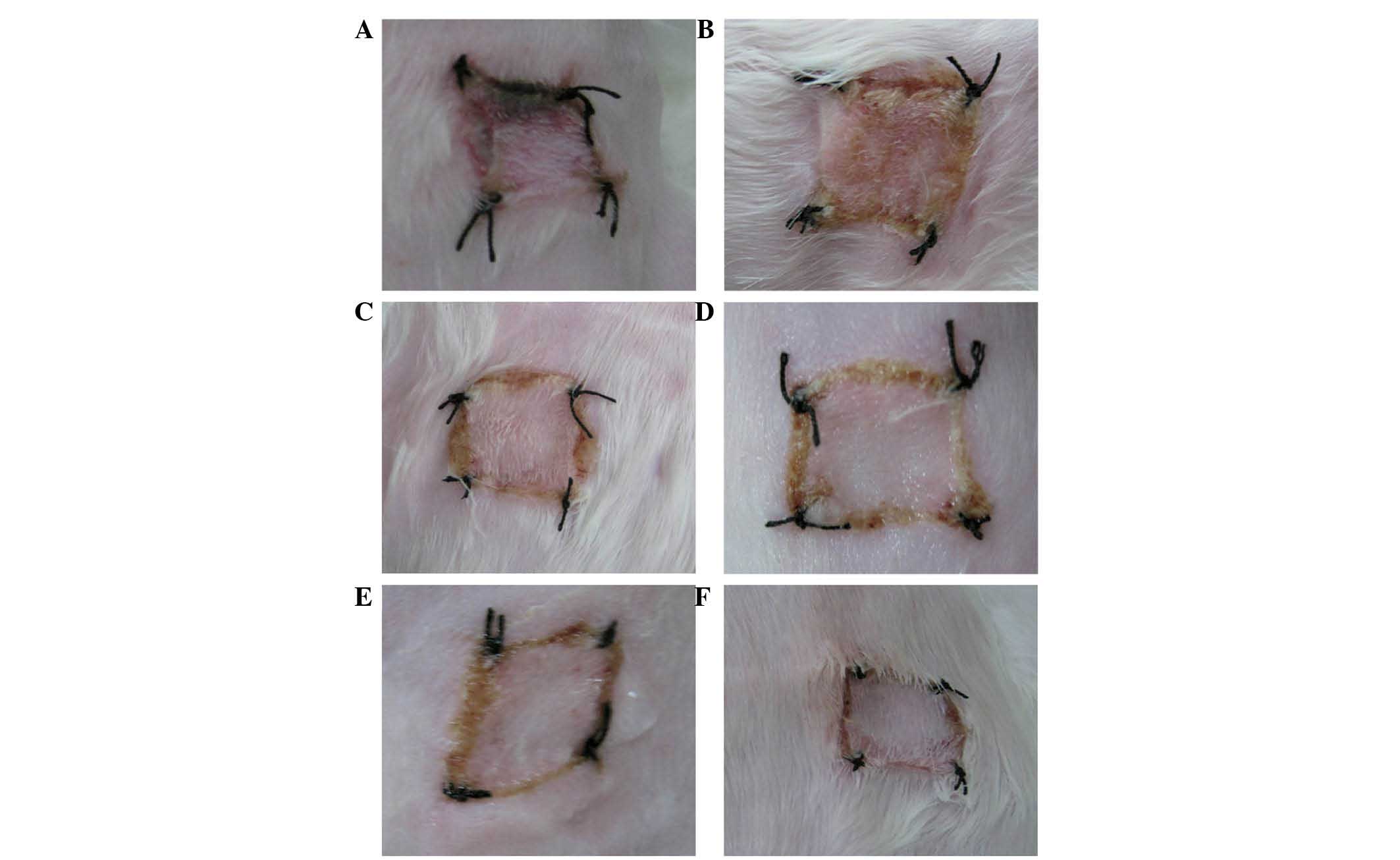
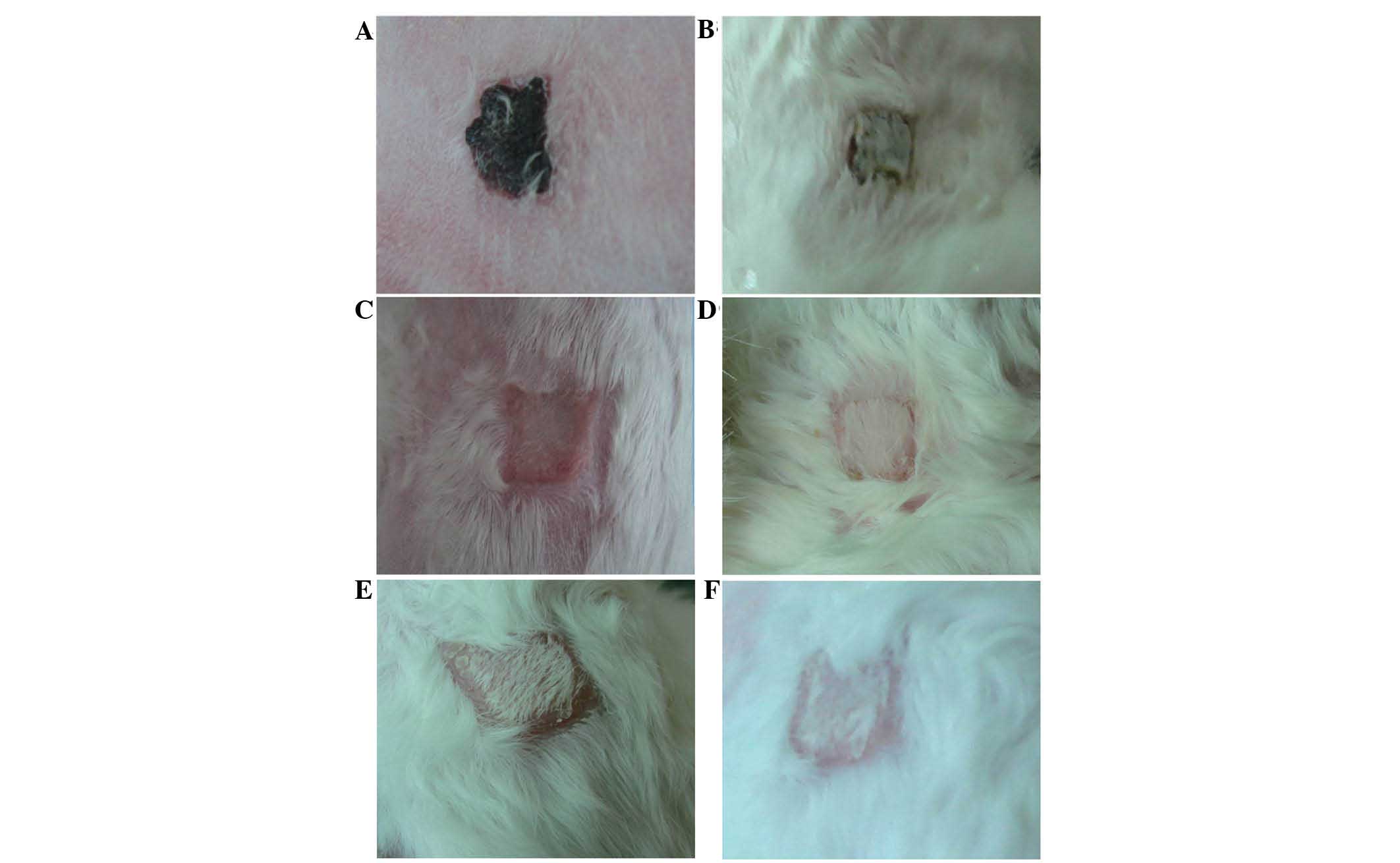
Evaluation of allograft survival and
rejection time
Studies to evaluate allograft survival and rejection
time comparing the rejection of the color of the skin graft were
preformed. When the color of the skin graft and the skin of the
receptor was similar, with no marked inflammation or hyperemia, the
skin graft remained in close proximity to the wound and exhibited
sufficient drying elasticity with a lack of secretions, which
indicated that there was no rejection phenomenon. When the color of
the grafted skin was dark, black or swelling was identified, this
indicated transplant rejection, and if 80% of the area of the
grafted skin appeared as such, it was considered to be necrotic
skin according to previous studies (6,7)
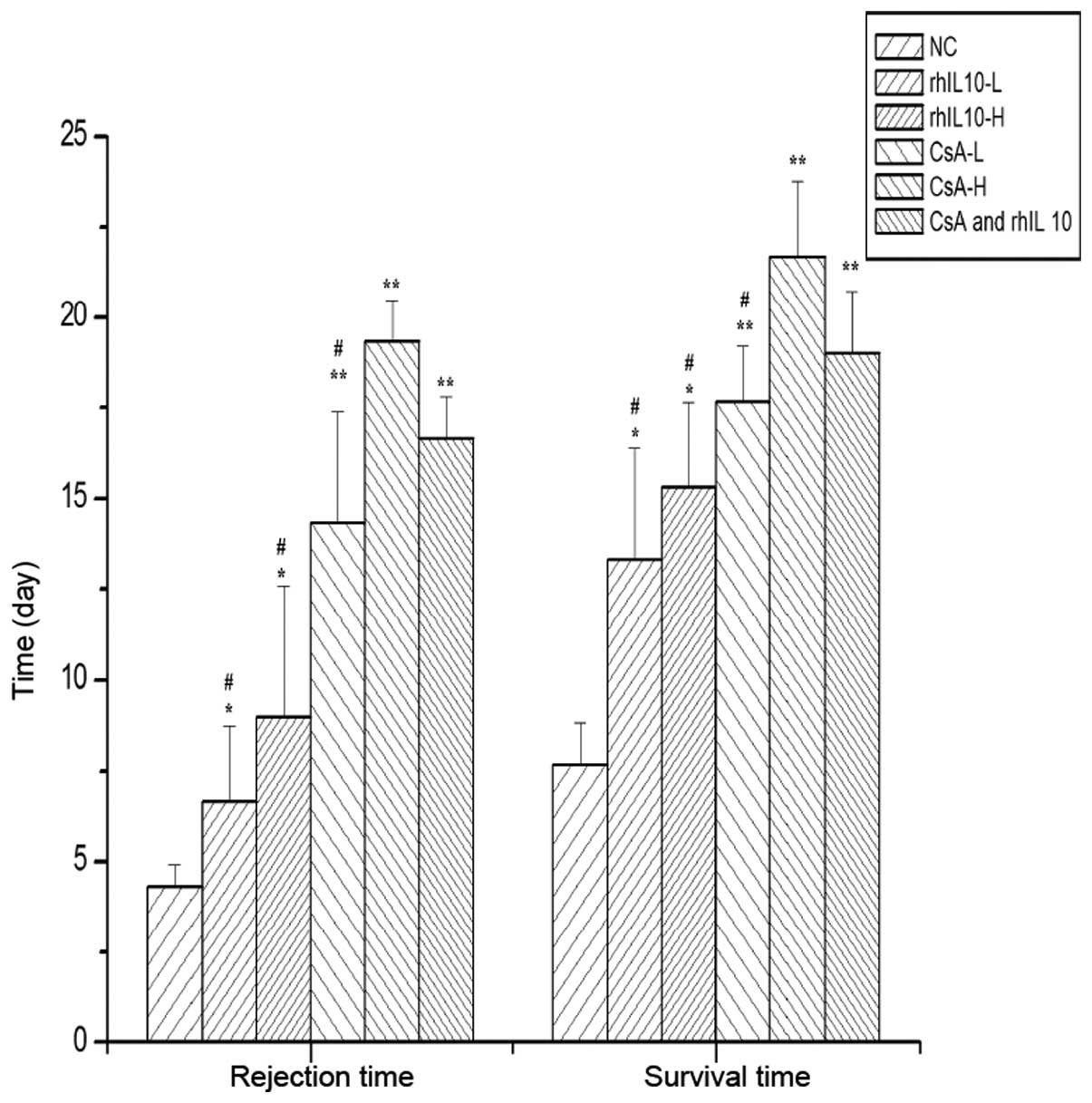
(Figs. 1 and 2). The allograft survival and rejection
time was then determined (Fig. 3).
The rejection phenomenon of the treatment groups were slower
compared with the control group and the survival time was
significantly longer than in the control group (P<0.05). The
survival time of the combined medication group was longer than the
rhIL-10 and CsA low dose group (P<0.05); however, the survival
time between the combined medication group and CsA high dose group
was similar (P>0.05).
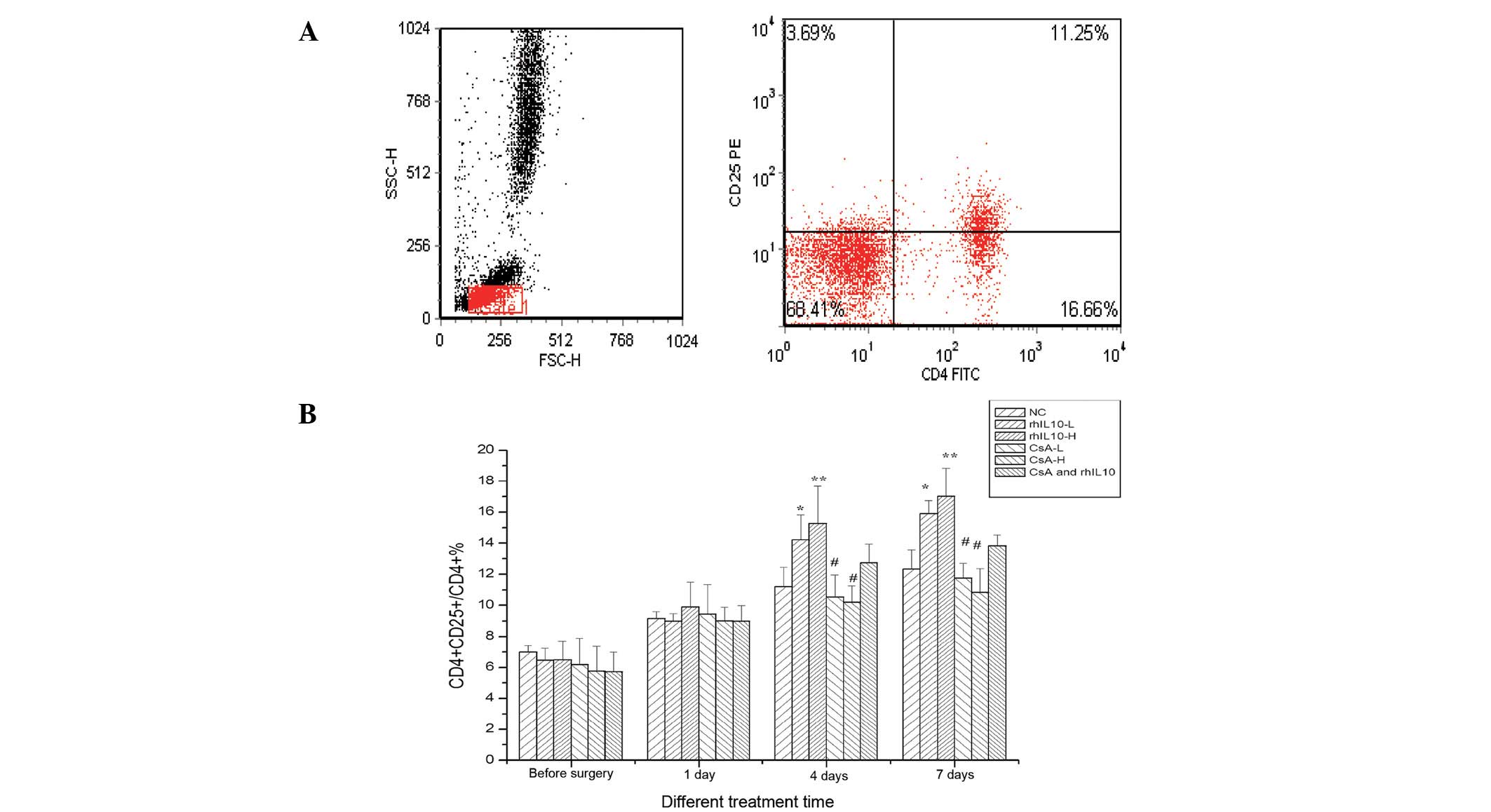
rhIL-10 significantly increases the
proportion of CD4+CD25+ Treg/CD4+
T cells (%)
CD4+CD25+ Treg cells, which
constitute ~5–10% of peripheral CD4+ T cells, are key in
the maintenance of immunological selftolerance and immune responses
(8). To determine whether the
rhIL-10 had an effect on the proportion of
CD4+CD25+ Treg/CD4+ T cells (%),
studies were performed to determine the proportion of
CD4+CD25+ Treg/CD4+ T cells (%) in
each group. As shown in Fig. 4,
post-transplantation, the levels of CD4+CD25+
Treg cells was significantly increased compared with
pre-transplantation samples (P<0.05). However, the lower and
higher dose groups of rhIL-10 four to seven days following surgery
were markedly higher compared with the control and the CsA groups
(P<0.05). The lower and higher dose groups of CsA were lower
compared with the control group (P<0.05). Thus, rhIL-10 may
result in the increased proportion of
CD4+CD25+ Treg/CD4+ T cells
(%).
Effects of rhIL-10 on IL-10 and TGF-β
levels
The effect of rhIL-10 on cytokine expression was
investigated. To assess changes in the expression, the levels of
IL-10 and TGF-β peripheral blood serum were detected by ELISA. The
results are shown in Fig. 5.
Post-transplantation, the levels of IL-10 were significantly higher
than those pre-transplantation (P<0.05). The lower and higher
dose groups of rhIL-10 following surgery between days four and
seven were markedly higher compared with the other groups
(P<0.05). The lower and higher dose groups of CsA following
surgery were significantly lower compared with the rhIL-10 and the
combined group. The levels of TGF-β were significantly higher
compared with those of preoperative samples (P<0.05). Notably
however, no difference was observed among treatment groups
following surgery on the 1st, 4th, 7th and 14th day (P>0.05).
Thus, rhIL-10 significantly increased the level of IL-10 but not
TGF-β.
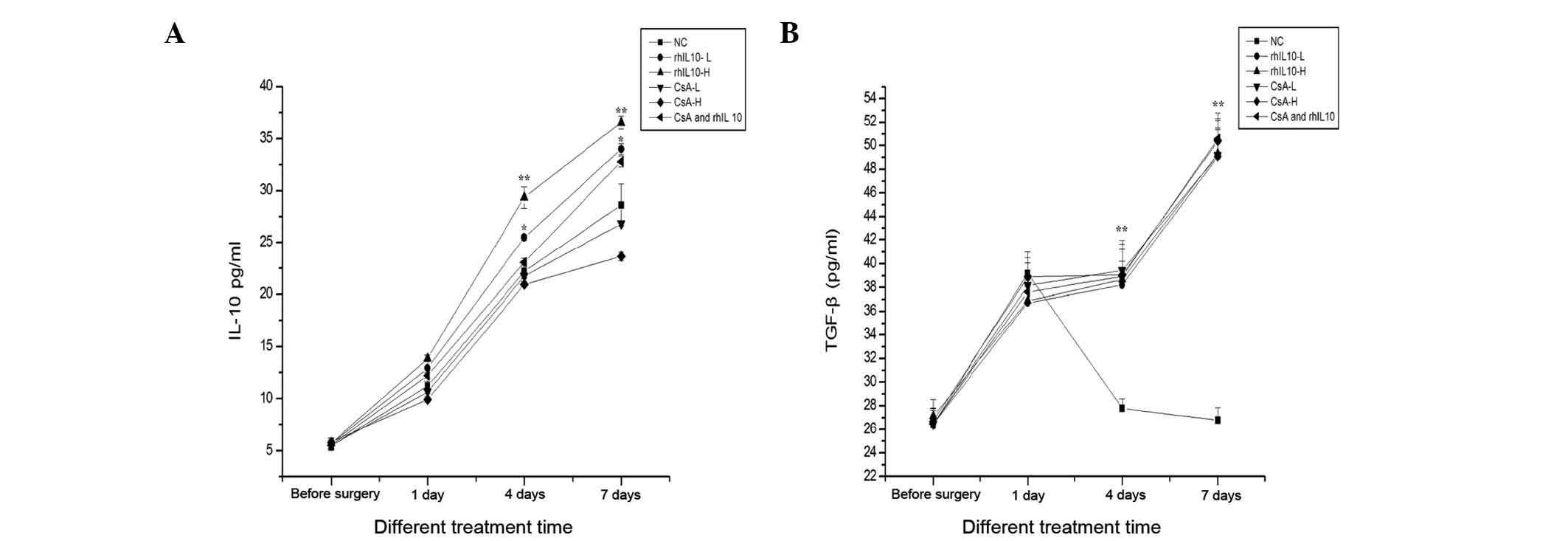 | Figure 5The effects of rhIL-10 on the levels
of IL-10 and TGF-β in peripheral blood at different time in each
group. The levels of (A) IL-10 and (B) TGF-β were detected by ELISA
compared prior to surgery and were significantly increased.
However, the rhIL-10-L and rhIL 10-H groups at day 4 and 7
post-transplantation were markedly higher compared with the NS,
CsA-L, CsA-H (*P<0.05, **P<0.01), while
the CsA-L, CsA-H was lower than NS (#P<0.05).
Compared with NS, the levels of TGF-β at day 4 and 7
post-transplantation was significantly higher
(**P<0.05). However, no significant difference in
each group at days 1, 4 and 7 post-transplantation, compared with
the NS was observed. rhIL-10, recombinant human interleukin-10;
CsA, cyclosporin A; NS, normal saline. |
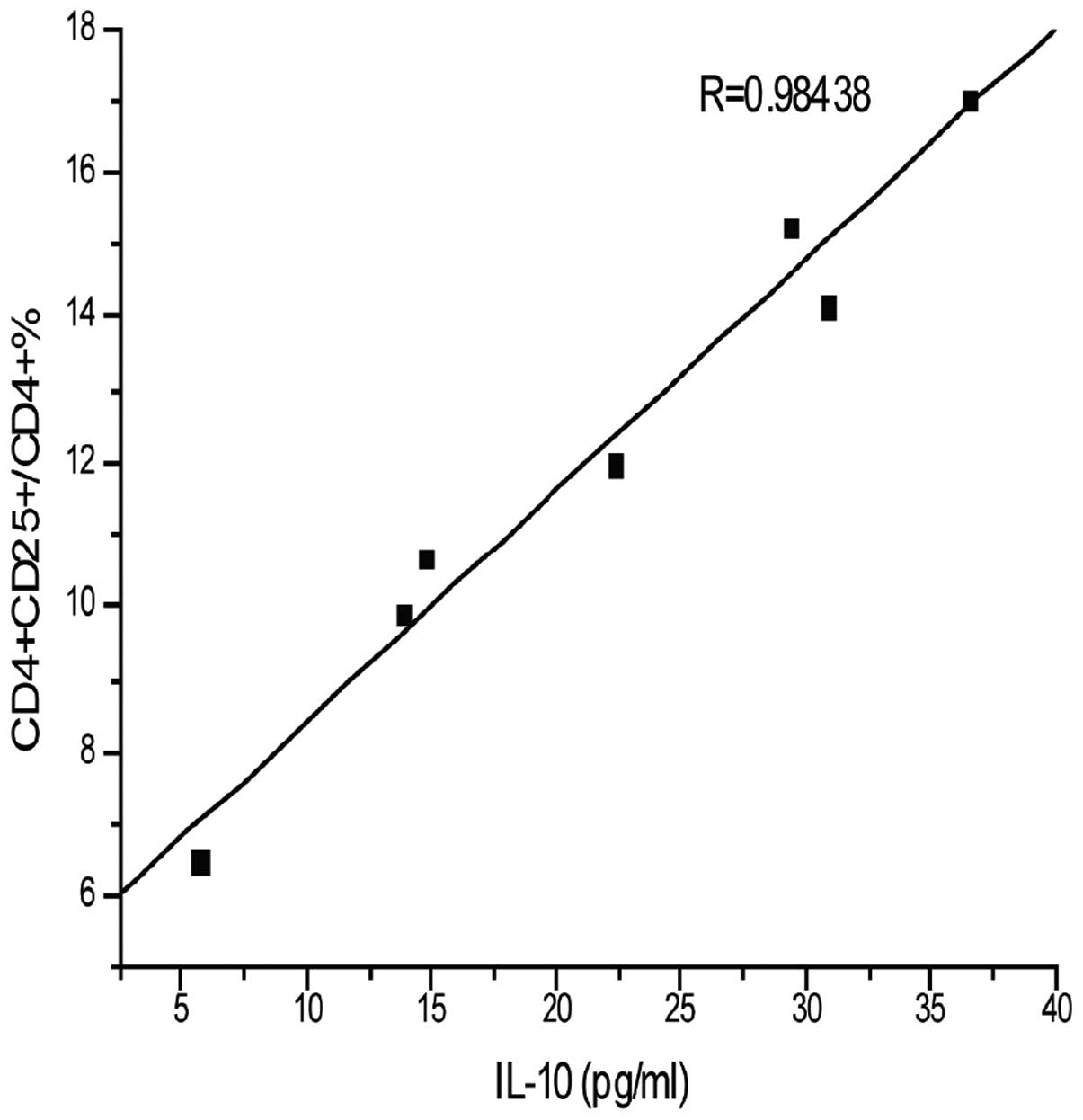
Correlation analysis of IL-10 and
CD4+CD25+ Treg/CD4+T cells
The aforementioned results were used to further
determine the correlation between IL-10 and
CD4+CD25+ Treg/CD4+ T cells (%).
As shown in Fig. 6, the levels of
IL-10 and CD4+CD25+ Treg/CD4+ T
cells (%) were significantly increased compared with
pre-transplation and the correlation analysis of IL-10 and
CD4+CD25+ Treg/CD4+ T cells (%)
were positively correlated. Thus, the IL-10 and
CD4+CD25+ Treg cells are interrelated and
interact in antirejection efforts. Therefore, intramuscular
injections of in vitro rhIL-10 post-transplantation led to
an increase in IL-10 and CD4+CD25+ Treg
cells, and this may be the mechanism by which IL-10 inhibits
allograft rejection.
Discussion
IL-10 is a cytokine synthesis inhibitory factor,
with a molecular weight of 17 kDa and contains 160 amino acid
residues and two intramolecular disulfide bonds. The human IL-10
gene is located on the long arm of the chromosome 1q31–32 region. A
number of human cells produce IL-10, including the specific T cell
subsets, Th2, Tr1, Tc2, macrophages, monocytes, mast cells, liver
cells and tumor cells (9). IL-10
is capable of inhibiting the cellular immune response and
inflammation through the following channels. By decreasing the
level of MHC-II, costimulatory molecules, including CD86 and
adhesion molecules on the cell surface, IL-10 inhibits the
antigen-presenting ability of monocytes and macrophages. Inhibition
of IL-2, produced by mononuclear cells, is the basic method in T
cell proliferation in the responses of specific cellular immunity.
Inhibition of monocytes and macrophages produces inflammatory
mediators, resulting in the reduction of IL-1, IL-8, IL-6, IFN-γ,
TNF-β, granulocyte colony stimulating factor and
granulocyte-macrophage colony stimulating factor when released
(10). Previous studies have shown
that local transfection of plasmid IL-10 in tissue and organs
inhibits T cell activation and proliferation, induces T cell
apoptosis, and reduces immunological rejection (11,12).
In a lung transplantation model of ischemia-reperfusion injury, the
receptor with transfected plasmid IL-10, exhibited lower IL-2,
IFN-γ and TNF-α levels compared with the control group. Its
pulmonary edema and function were improved in the control group
(11). The present study provided
evidence that IL-10 exhibits a protective effect in lung
transplantation. The results indicated that the application of
IL-10 in rabbit skin grafts may postpone the graft rejection and
extend the survival time. The survival time of the combined group
was longer than lower-dose IL-10 and low-dose CsA groups,
suggesting that IL-10 has a synergistic effect with CsA. The IL-10
in rabbits increased following use of rhIL-10. Thus, rhIL-10, as a
foreign antigen, is hypothesized to activate T cells, macrophages
and Tregs, enhancing the function of IL-10 secretion, resulting in
significantly increased postoperative levels of IL-10. IL-10
through the effects of autocrine, paracrine and endocrine systems
inhibits the immune responses.
The CD4+CD25+ Tregs comprise
~5–10% of CD4+ T cells in normal human or mouse
peripheral blood and are an important group of immune cells, which
have inhibitory functions. CD4+CD25+ may
negatively regulate the activation and proliferation of T cells.
They are important in inducing and maintaining peripheral tolerance
(8). Tregs may be divided into
natural Tregs, which originate in the thymic medulla and induce
regulatory T cells and adaptive Tregs, which arise from
CD4+ T cells when infection, transplantation or cancer
occurs. IL-10 and TGF-β may promote effector T cells into Tregs.
Clinical studies confirmed that CD4+CD25+
Tregs are associated with transplantation tolerance. In lung
transplant receptors, the number of Tregs in symptoms of chronic
rejection is lower compared with patients without clinical symptoms
and healthy controls. Following kidney transplantation, the number
of Tregs in the long-term survival in patients is significantly
increased. Tregs inhibit the donor CD4+ and
CD8+ T cells, leading to T cell loss of function and may
reduce the expression of the molecule at the antigen presenting
cell surface, which is required for T cell activation; thus,
reducing graft versus host disease (GVHD) (13). The Treg cells could be induced by
antigen and IL-10, and in turn IL-10 secretion by Treg cells
inhibited the activation and proliferation of T cells (14). The generation of Tregs also
inhibits the antigen-specific immune response and positive lower
immune responses. T cells have no response and Tregs are increased
by IL-10 to maintain the antigen specificity of peripheral T cell
tolerance (15). A previous study
showed that an increase occurred when IL-10 was added to Tregs
in vitro and the inhibition of Tregs following amplification
was similar to that of natural Tregs (16). The aforementioned studies suggest
that the inhibition of IL-10 immune function is closely associated
with Tregs. In the current study, Tregs were at a lower level in
the peripheral blood prior to skin graft and the levels were
significantly increased following transplantation of skin.
Allograft skin, as a foreign antigen, stimulates the immune
system, resulting in lymphocyte activation and proliferation and a
further increase in the Treg levels, the Tregs were present in
higher levels and exhibited a longer survival time. The Treg levels
in the IL-10 group were higher compared with the CsA and control
groups following surgery and the levels in the CsA group were lower
than in the control group. Thus, this indicates that rhIL-10 is
conducive to Treg proliferation and CsA inhibits Tregs. T cell
activation may be affected by CsA through blocking the
transcription of IL-2. IL-2, as a signal transmitter, is important
for the maintenance of Tregs. By blocking the transcription of
IL-2, the activation and function of Tregs may be altered.
Therefore, CsA not only reduced Treg proliferation, but also
inhibited its function continuously. However, in the current study,
IL-10 inhibited the rejection reaction in the grafts but not Tregs,
compared with increased normal levels of Tregs, indicating a
mechanism by which IL-10 inhibits allograft rejection.
TGF-β may reduce the immune response in a number of
component elements; thus, it may exhibit potential
immunosuppressant actions to be used for preventing graft
rejection. It was reported that in organ transplant receptors in
vivo, the expression of TGF-β is associated with graft versus
host disease (GVHD) and following transplantation, the patients
with highly expressed TGF-β, exhibited significantly reduced GVHD
(17). The membrane-type TGF-β is
activated when the foreign antigen activates Tregs, thus, the
levels of TGF-β in the peripheral blood increase and inhibit the
immune response (18). In the
current study, the increased levels of TGF-β may be associated with
increasing Tregs. The level of TGF-β did not alter the role of
IL-10 and CsA and thus, it may be hypothesized that the mechanism
of intramuscular injection of IL-10 to the inhibited immune
response is not associated with TGF-β.
Rejection reaction is problematic for clinicians and
immunologists and constantly causes difficulty in organ
transplantation application. Although rejection has been relieved
by tissue typing and immunosuppressant application e.g., CsA, the
issue of rejection has not been entirely overcome. It is urgent to
develop novel effective anti-rejection drugs. A number of studies
hypothesize that IL-10 may become a novel immunosuppressant for
clinical application due to its immunosuppressive activity. The
current results show that IL-10 may inhibit rabbit skin allograft
rejection and its mechanism may be associated with Tregs and
IL-10.
Acknowledgements
This study was funded by grants from the Guizhou
Province Talents Fund (grant no. 200607) and the Natural Science
Foundation of cultivation project of the Department of Education
(grant no. 2008032).
Abbreviations:
|
rhIL-10
|
recombinant human interleukin-10
|
|
FCM
|
flow cytometry
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
CsA
|
cyclosporin A
|
References
|
1
|
Moore KW, de Waal Malefyt R, Coffman RL
and O’Garra A: Interleukin-10 and the interleukin-10 receptor. Annu
Rev Immunol. 19:683–765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pacholczyk R, Kraj P and Ignatowicz L:
Peptide specificity of thymic selection of
CD4+CD25+T cells. J Immunol. 168:613–620.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feuerer M, Hill JA, Mathis D and Benoist
C: Foxp3+regulatory T cells: differentiation,
specification, subphenotypes. Nat Immunol. 10:689–695. 2009.
|
|
4
|
Feng JK, Sun WB, Chen FC, et al: Plasmid
construction and eukaryotic expression of recombinant human
Interleukin-10 and preliminary study in anti-graft rejection of
Skin. Biotech Bull. 3:168–172. 2010.(In Chinese).
|
|
5
|
Zhang X, Koldzic DN, Izikson L, et al:
IL-10 is involved in the suppression of experimental autoimmune
encephalomyelitis by CD25+CD4+regulatory T
cells. Int Immunol. 16:249–256. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YB, Xiao C and Gao JH: Whole ear
allograft transplantation by immunologic induction of
donor-specific tolerance by bone marrow transplantation. Chin J
Pract Aesthetic Plast Surg. 2:104–108. 2004.(In Chinese).
|
|
7
|
Liu XL, Li YX, Wang YF, et al: The test of
autograft of skin in rabbit. Anim Husbandry Vet Med. 37:34–36.
2005.(In Chinese).
|
|
8
|
Roncarolo MG and Battaglia M: Regulatory
T-cell immunotherapy for tolerance to self antigens and
alloantigens in humans. Nat Rev Immunol. 7:585–598. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herold KC: Achieving antigen-specific
immune regulation. J Clin Invest. 113:346–349. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou X, Schmidtke P, Zepp F and Meyer CU:
Boosting interleukin-10 production: therapeutic effects and
mechanisms. Curr Drug Targets Immune Endocr Metabol Disord.
5:465–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daddi N, Suda T, Dovidio F, et al:
Recipient intramuscular cotransfection of naked plasmid
transforming growth factor betal and interleukin 10 ameliorates
lung graft ischemia-reperfusion injury. J Thorac Cardiovasc Surg.
124:259–269. 2002. View Article : Google Scholar
|
|
12
|
Suda T, Daddi N, Tagawa T, et al:
Recipient intramuscular cotransfection of transforming growth
factor beta1 and interleukin 10 ameliorates acute lung graft
rejection. J Thorac Cardiovasc Surg. 129:926–931. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng YF, Laouar Y, Li MO, et al: TGF-beta
regulates in vivo expansion of Foxp3-expressing
CD4+CD25+regulatory T cells responsible for
protection against diabetes. Proc Natl Acad Sci USA. 101:4572–4577.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bellinghausen I, Brand U, Steinbrink K, et
al: Inhibition of human allergic T-cell responses by IL-10-treated
dendritic cells: differences from hydrocortisone-treated dendritic
cells. J Allergy Clin Immunol. 108:242–249. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wakkach A, Fournier N, Brun V, et al:
Characterization of dendritic cells that induce tolerance and T
regulatory 1 cell differentiation in vivo. Immunity. 18:605–617.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeng DZ, Wang ZQ, Zheng JS, et al: Effects
of IL-2 and IL-10 on T regulatory cell proliferation in vitro. Acta
Acad Med Mil Tertiae. 29:290–292. 2007.(In Chinese).
|
|
17
|
Hattori H, Matsuzaki A, Suminoe A, et al:
Polymorphisms of transforming growth factor-beta1 and transforming
growth factor-beta1 type II receptor genes are associated with
acute graft-versus-host-disease in children with HLA matched
sibling bone marrow transplantation. Bone Marrow Transplant.
30:665–671. 2002. View Article : Google Scholar
|
|
18
|
Sempowski GD, Cross SJ, Heinly CS, et al:
CD7 and CD28 are required for murine
CD4+CD25+regulatory T cell homeostasis and
prevention of thyroiditis. J Immunol. 172:787–794. 2004. View Article : Google Scholar : PubMed/NCBI
|