Introduction
The glomerulus is a common structural organization
among vertebrates and kidney types (pronephros, mesonephros and
metanephros) (1–4). The glomerular filtration barrier,
which is responsible for the size and charge-selective properties
of the filter, comprises a fenestrated endothelium, composed of the
glomerular basement membrane and the podocyte, which are highly
specialized epithelial cells of the kidney. The podocyte is
composed of three subcellular compartments: a cell body, major
processes that extend outward from the cell body and more distally
located foot processes that are spanned by a slit diaphragm (SD),
which is an essential element of the filtration barrier (5). This basic cellular architecture of
podocytes is conserved in vertebrates (6,8).
Intensive research has focused on the molecular
basis of SD structure and function and has resulted in the
identification of a number of novel SD-associated proteins,
including NEPHRIN, NEPH1, CD2-associated protein and PODOCIN
(1-5,9-22).
These SD-specific proteins are crucial in the formation of the SD
and mutations in the NEPHRIN and PODOCIN genes cause
congenital nephrotic syndrome of the Finnish type and autosomal
recessive steroid-resistant nephrotic syndrome, respectively
(23,25). Numerous disease-associated
mutations have been reported in the NEPHRIN and
PODOCIN genes (25,26), however, the information on the
functional abnormalities induced by gene mutations in
NEPHRIN and PODOCIN remains limited.
Zebrafish homologues of Nephrin and Podocin are
predominantly expressed in the pronephric glomerulus, which is
similar to their expression in mammalian metanephric glomerulus and
play crucial roles in the formation and function of the SD in the
zebrafish pronephric glomerulus (3,9,27).
Three morpholino antisense oligos (MOs) used in zebrafish to target
Nephrin and Podocin resulted in failure to form normal podocyte
architecture, including regular foot processes and SD in zebrafish
larvae (9,22). However, updated zebrafish genomic
DNA sequences and Gene Tools LLC no longer support these previously
used MOs. In the present study, the role of Nephrin and Podocin in
the zebrafish pronephros glomerulus was analyzed using different
MOs and their evolutionary conservation with human homologues was
assessed.
Materials and methods
Fish maintenance
The animal experiments were performed in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health and
were approved by the Institutional Animal Care and Use Committee of
the University of Oklahoma Health Sciences Center (IACUC protocol
no. 12-033 to T.O.). The AB strain of zebrafish was maintained at
28.5°C under a 14 h light/10 h dark cycle. Embryos were maintained
at 28.5°C in 0.5X E2 egg medium.
Cloning of zebrafish nephrin and
podocin
Full-length zebrafish nephrin cDNA that was
subcloned into the pCR-BluntII-TOPO vector was a kind gift from Dr
Iain Drummond (9). Full-length
zebrafish podocin cDNA was obtained by performing RT-PCR on
the total RNA isolated from 4 days post-fertilization (dpf) embryos
using RNAqueous-4PCR kit (Life Technologies, Carlsbad, CA, USA) and
subsequently performing nested PCR. RT-PCR was performed using
SuperScript III One-Step RT-PCR System with Platinum Taq High
Fidelity (Life Technologies) and nested PCR using Phusion
High-Fidelity DNA Polymerase (ThermoScientific, Waltham, MA, USA).
The primer sets used were: RT-PCR, forward: 5′-ATC TGC ACT GGC CTC
CTG ATA-3′ and reverse: 5′-ATG CGA AGG AAA TCC GTC AAC-3′ and
nested PCR, forward: 5′-CAC CAG AGG ACA CTT CAC AAC A-3′ and
reverse 5′-CAG CCA ATA ATC AGT ACA GTC TTG AAA-3′. Podocin
cDNA was subcloned into pCR-BluntII-TOPO and verified by DNA
sequencing.
In situ hybridization
In situ hybridization was conducted as
previously described (1–3,9). In
brief, the pCR-BluntII-TOPO- nephrin and -podocin
digested with XhoI were used as a template for anti-sense
RNA probe. The probe was synthesized with an SP6 RNA polymerase
(New England BioLabs, Ipswich, MA, USA) and DIG-RNA labeling kit
(Roche Diagnostics, Mannheim, Germany). Embryos were fixed in 4%
paraformaldehyde (PFA), 0.1% Tween-20 in PBS for 2 h at room
temperature (RT), altered to 100% methanol and stored at −20°C.
Whole-mount in situ hybridization was performed as
previously described (28).
Following color development, the samples were dehydrated with a
graded series of methanol and embedded in JB-4 resin (Polysciences,
Inc., Warrington, PA, USA). Ten micron sections were sliced using
an RN2255 microtome (Leica Microsystems, Wetzlar, Germany) and
counter-stained with special eosin II (BBC Biochemical, Mount
Vernon, WA, USA). After mounting in Poly-Mount (Polysciences,
Warrington, PA, USA), the stained sections were photographed on a
Provis AX-70 microscope (Olympus, Tokyo, Japan) equipped with a
RETIGA EXi digital camera (QImaging, Surrey, Canada).
Antibodies
A polyclonal anti-zebrafish Nephrin antibody was
prepared as previously described (3,21).
Rabbit polyclonal anti-zebrafish Podocin antibody was raised in
rabbits using the amino-terminal peptide VKLQEPHKRKE (amino acids
43–53) coupled to KLH. The antiserum was affinity-purified against
the immunizing peptide (Covance, Denver, PA, USA).
Immunoblot analysis and
immunohistochemistry
Proteins were extracted from 1 to 5 dpf zebrafish.
Prior to protein extraction, the yolk ball was removed from 4 dpf
zebrafish larvae, as a large amount of yolk-derived proteins
occasionally affects SDS-PAGE and immunoblot analysis (29). Deyolked larvae were homogenized and
solubilized in the protein extraction buffer (1% NP-40, 150 mM
NaCl, 50 mM KI, 1 mM EDTA and 10% glycerol in 50 mM HEPES, pH 7.4).
The homogenate was centrifuged at 11,300 × g for 10 min and the
supernatant was collected as the lysate. Each lysate containing 50
μg of protein was subjected to SDS-PAGE (Mini-PROTEAN TGX Gel,
4–15% gradient; Bio-Rad Laboratories, Hercules, CA, USA) and
separated proteins were transferred onto a PVDF membrane (Immobilon
Transfer Membrane; Millipore, Billerica, MA, USA). Each membrane
was incubated with the primary antibody for 2 h at RT and then with
an anti-HRP-conjugated secondary antibody (working dilution
1:5,000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA)
for 1 h at RT. Dilutions of primary antibodies were 1:2,500 for
anti-Nephrin, 1:5,000 for anti-Podocin and 1:5,000 for
anti-α-tubulin.
Immunohistochemical detection of Nephrin
and Podocin
Immunohistochemical analysis was performed as
previously described (3). Larvae
were fixed with Dent’s fixative (20% DMSO in methanol) overnight at
4°C. Fixed samples were rehydrated with a graded series of methanol
and washed with PBS containing 0.5% Triton X-100 (PBSTx). For
antigen retrieval, the samples were heated in Antigen Retrieval
Reagent Universal (R&D Systems, Minneapolis, MN, USA) for 15
min at 95°C on a heat block. Subsequently, the samples were blocked
with the incubation solution (PBSTx containing 10% normal goat
serum and 1% DMSO) for 2 h at RT and incubated with the primary
antibody (working dilution 1:100) in the incubation solution for 12
h at 4°C. After washing with PBSTx, the samples were incubated with
Alexa-Fluor546-conjugated goat anti-rabbit IgG (H+L; Jackson
ImmunoResearch Laboratories) and diluted with the incubation
solution (1:1,000) for 2 h at RT. Stained samples were dehydrated
with a graded series of methanol, embedded in JB-4 resin
(Polysciences, Inc.) and sliced into 10-μm sections with a RN2255
microtome. The sections were photographed with an FV-1000 confocal
laser scanning microscope (Olympus).
Production of Flag-tagged zebrafish
podocin
cDNA corresponding to zebrafish podocin was
generated using RT-PCR, digested with BamHI and XbaI,
subcloned into pEV3S-Flag vector and verified by DNA sequencing.
This cDNA was used to generate the R150Q mutation, which was also
cloned into the pEV3S-Flag vector and verified by DNA sequencing.
Human embryonic kidney 293T cells, which were grown to ~25%
confluency in 60-mm dishes, were transiently transfected with 2 μg
Flag-Podocin and 7 μg pBluescript KS+ using the calcium
phosphate coprecipitation method (30). Following 12-h incubation, the
precipitate was removed by washing twice with 2 ml PBS and the
cells were incubated for an additional 36 h in DMEM supplemented
with 10% fetal bovine serum at 37°C. Thereafter, the cells were
lysed by boiling in Laemmli buffer and the insoluble material was
removed by centrifugation (31).
The resultant protein lysate was subjected to SDS-PAGE and the
separated proteins were transferred to a PVDF membrane (Immobilon;
Millipore) and processed for immunoblotting with the indicated
antibodies (32).
MOs and efficacy checking
MOs were designed for the splice blocking of
nephrin-exon 25 (nephrinMOex25: 5′-TGC ACC AAC ACG
ACT CAC CTC TGC TC-3′) and of podocin-exon 3
(podocinMOex3: 5′-TGT AGT CAC TTT TGC AGA CCT GGG CT-3′)
(Gene Tools LLC, Philomath, OR, USA). The two morpholinos target
the splice acceptor site. The morpholinos were diluted with the
injection solution containing 100 mM KCl and 10 mM HEPES (pH 7.6)
and were injected at a final concentration of 0.15 mM
(nephrinMOex25) and 0.25 mM (podocinMOex3) into one-
or two-cell stage embryos using a Nanoliter 2000 microinjector
(World Precision Instruments, Sarasota, FL, USA). The injected
volume was ~4.6 nl. Splice blocking was verified using RT-PCR and
nested PCR. Total RNA was isolated from five embryos with an
RNAqueous-4PCR kit (Life Technologies). RT-PCR was performed using
SuperScript III One-Step RT-PCR System with Platinum Taq High
Fidelity (Life Technologies) followed by nested PCR using Phusion
High-Fidelity DNA Polymerase (New England Biolabs). The primer sets
used were: forward, 5′-GGC AGG ATC TGC AAG CTA CAT-3 and 5′-CTC AGG
GCC TTC AGG GT GAG-3′ (RT-PCR for nephrin); 5′-CTC CTG AAC
CCA TTC ATC TGC-3′ and 5′-CTC ATA GAC GCT GCT GTC AGG-3′ (nested
PCR for nephrin); 5′-GAT GCT TCC TGC GGA GAT AGA-3′ and
5′-TTC CTG TCC AGC AAA ATG TCA-3′ (RT-PCR for podocin) and
5′-TCA TCT CTA GCA GCA CGG TTG-3′ and 5′-TCT GGA ATG CTA GCG AAG
GAG-3′ (nested PCR for podocin). Altered PCR products were
subcloned into pCR-BluntII-TOPO and sequenced.
Hematoxylin and eosin staining
Hematoxylin and eosin staining was conducted as
previously described (32). In
brief, larvae were fixed with histology fixative (1.5%
glutaraldehyde, 4% PFA, 3% sucrose in 0.1 M phosphate buffer, pH
7.3) overnight at 4°C, dehydrated with a graded series of methanol
and embedded in JB-4 resin. Four micron sections were sliced and
stained with Harris hematoxylin and special eosin II (BBC
Biochemical, Mount Vernon, WA, USA). The stained sections were
photographed with a Provis AX-70 microscope equipped with a RETIGA
EXi digital camera (QImaging).
Transmission electron microscopy
Transmission electron microscopy was conducted as
previously described (3). Larvae
were fixed with histology fixative overnight at 4°C. The samples
were immersed in 1% OsO4 in 0.1 M phosphate buffer for 1
h, dehydrated with a graded series of ethanol and then embedded in
Epon-Araldite resin (Electron Microscopy Sciences, Hatfield, PA,
USA). Ultrathin silver-gold sections were produced with an ultra
45° diamond knife (Diatome, Biel, Switzerland) and were transferred
to copper grids (50 mesh, Nisshin EM) with a carbon-coated Formvar
membrane. The sections were stained with uranyl acetate and lead
citrate and photographed on a H-7600 transmission electron
microscope (Hitachi High Technologies Inc., Schaumburg, IL, USA)
equipped with a Kodak 2K × 2K digital camera.
Co-immunoprecipitation assay
cDNA encoding the intracellular region of zebrafish
Nephrin (amino acids 1,065–1,242) was generated by performing PCR
on the total RNA from 2 dpf zebrafish larvae as a template,
subcloned into pCS3+-6Myc vector and verified by DNA
sequencing. Generation of Flag-Podocin vector was mentioned
earlier. Each 1 μg of indicated Flag-Podocin and 6Myc-Nephrin-C
vectors were transfected together with 7 μg pBluescript
KS+ into 293T cells essentially as previously described
(31). Following 36 h incubation,
the cells were lysed in 600 μl 50 mM Tris (pH 7.4), 50 mM NaF, 150
mM NaCl, 0.2 mM DTT, 0.5% NP-40, 1 mM PMSF, 10 μg/ml leupeptin, 2
μg/ml aprotinin, 1 μg/ml pepstatin A and 0.2 mM
Na3VO4. Cell lysates were processed as
described earlier utilizing the anti-Flag M2 monoclonal antibody
(Sigma-Aldrich, St. Louis, MO, USA) for the immunoprecipitation
(34). Immunoprecipitated
complexes were resolved by SDS-PAGE and co-precipitated proteins
were detected by anti-Myc immuno-blot analysis (35).
Synthesis and egg injection of capped
RNA
The human NEPHRIN and PODOCIN cDNAs
were kind gifts from Dr Lawrence Holzman. PCR was used to introduce
a non-sense mutation into human NEPHRIN cDNA causing R1109X
and a single base substitution into human PODOCIN cDNA
causing R138Q. The following primer sets were used for the
generation of mutated cDNA: for NEPHRIN mutant:
HNEPHRIN-forward: 5′-GTC AAA GCT TAT GGC CCT GGG GAC GAC GCT
CAG and -reverse: 5′-AAG GAT AGC GGC CGC CTA CAC CAG ATG TCC CCT
CAG CTC GAA G; HNE- PHRIN-1109X-forward: 5′-GGG TCG GAA GAG
GAC TAG GTC AGG AAC GAA TAT GAG GAG AGC C-3′ and -reverse: 5′-GGC
TCT CCT CAT ATT CGT TCC TGA CCT AGT CCT CTT CCG ACC CTG CC-3′; for
PODOCIN mutant: HPODOCIN-forward: 5′-GTC AAA GCT TAC
CAT GGA GAG GAG GGC GCG GAG CTC CTC CAG-3′ and -reverse: 5′-AAG GAT
AGC GGC CGC CTA TAA CAT GGG AGA GTC TTT CTT TTT AGG-3′,
HPODOCIN-138Q-forward: 5′-AGA GTA ATT ATA TTC CAG CTG GGA
CAT CTG CTT CCT GGA AGA G-3′ and -reverse: 5′-CAG ATG TCC CAG CTG
GAA TAT AAT TAC TCT TTC ATA CTC TTG TAC AAC C-3′. The PCR products
were subcloned into pCR-BluntII-TOPO and verified by DNA
sequencing. These vectors were digested with restriction enzymes
and used as templates for the synthesis of capped RNA. Capped RNA
was synthesized using the mMESSAGE mMACHINE kit (Life
Technologies). Synthesized capped RNA (~0.64 pg for human wild-type
and mutated NEPHRIN and ~32 pg for human wild-type and
mutated PODOCIN) was injected into one-cell stage embryos
along with morpholinos (0.15 mM nephrinMOex25 or 0.25 mM
podocinMOex3).
Results
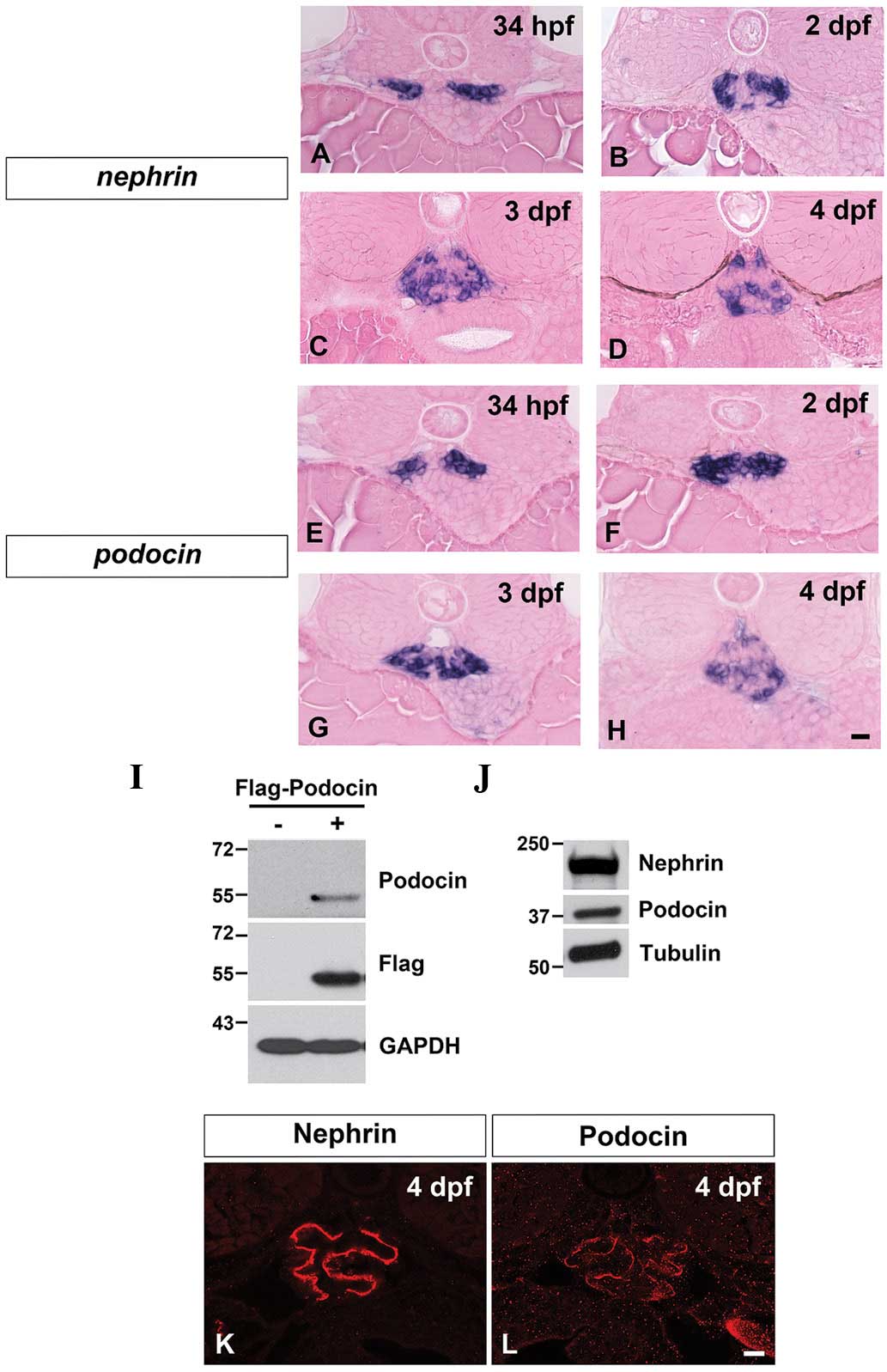
mRNA and protein expression
Throughout zebrafish pronephric development [34 h
post-fertilization (hpf) to 4 dpf], nephrin and
podocin mRNA expression was detected in the glomerular
primordia and compared with the glomerulus (Fig. 1A–H). The expression patterns of
nephrin and podocin markedly resembled that for
Wilms’ tumor 1a, which is predominantly expressed in the podocytes
within the pronephric glomerulus (1).
Antibodies specific to Nephrin and to Podocin were
developed to determine their expression pattern and localization.
The specificity of the anti-Nephrin antibody was previously
described (3). To examine the
specificity of the anti-Podocin antibody, the possibility of a
reaction with Flag-tagged zebrafish Podocin expressed in 293T cells
was investigated. Immunoblot analysis showed that the anti-Podocin
antibody was capable of detecting an ~55 kDa protein, corresponding
to the Flag-tagged Podocin (Fig.
1I). These anti-Nephrin and anti-Podocin antibodies could also
detect ~240 and ~40 kDa protein bands corresponding to endogenous
Nephrin and Podocin, respectively (Fig. 1J).
With these antibodies, Nephrin and Podocin were
immunohistochemically detected in 4 dpf larvae as two continuous
tortuous lines, which were likely to be situated along the
glomerular basement membrane (Fig. 1K
and L). A similar tortuous linear pattern for Nephrin
localization was also observed for the rodent metanephric
glomerulus (36,37).
Splice blocking of nephrin and podocin
pre-mRNA
To determine the function of Nephrin and Podocin in
the development of the zebrafish pronephric glomerulus, splice
blocking MOs were designed for Nephrin and Podocin to target the
splice acceptor sites of exon 25 for nephrin
(nephrinMOex25) and exon 3 for podocin
(podocinMOex3), respectively (Fig. 2A and B).
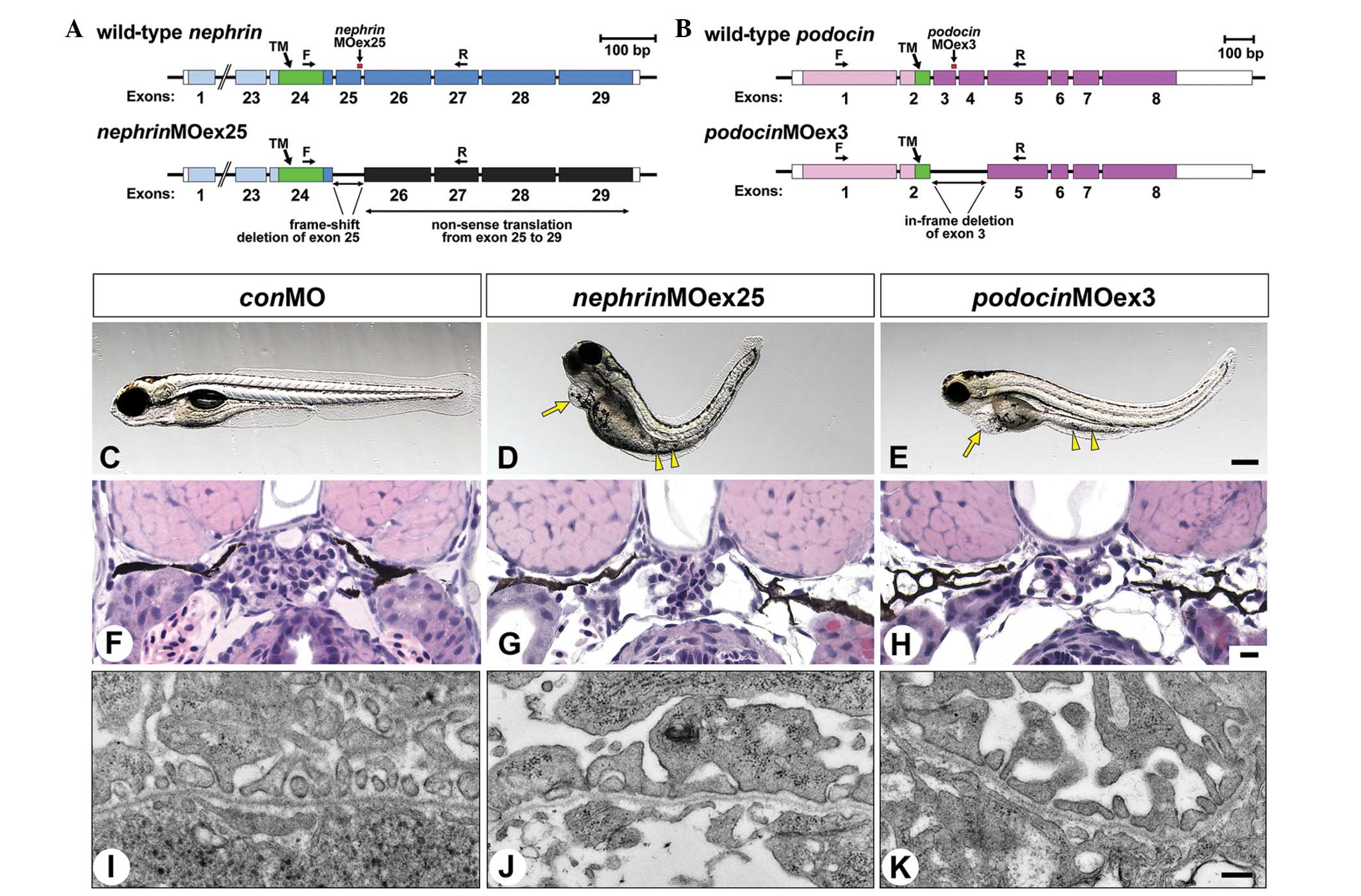 | Figure 2Gene knockdown using splice-blocking
morpholinos. (A) nephrinMOex25 targets the splice-acceptor
site of nephrin exon 25 and induces a frame-shift deletion.
(B) PodocinMOex3 targets the splice-acceptor site of podocin
exon 3 and induces an in-frame deletion of exons 3 and 4. (F) and
(R) show the sites of primer sets for the testing of the efficacy
of target modification (PCR data shown in Fig. 1A and B). (C–E) Lateral view of 4
dpf larvae. NephrinMOex25 and podocinMOex3 morphants
exhibit pericardial edema (arrow), edematous swelling of yolk sac
extension (arrowheads) and body-axis curvatures. (F–H) Glomerulus
in the 4 dpf larvae in HE-stained sections. The control larva
exhibits a well-developed glomerular capillary and mesangium. In
the morphants, glomerular development is markedly disrupted. (I–K)
Ultrastructure of glomerular capillary wall. The control larva
exhibits fine regular foot processes and slit diaphragm, present in
a ‘beads on a string’ pattern. In the nephrinMOex25
morphant, the regular foot processes are not observed at all,
however, in the podocinMOex3 morphant, foot processes with
slit diaphragm are formed. TM, transmembrane domain coding region.
Bar scales, 250 μm in (E); 10 μm in (H) and 50 nm in (K). dpf, days
post-fertilization. |
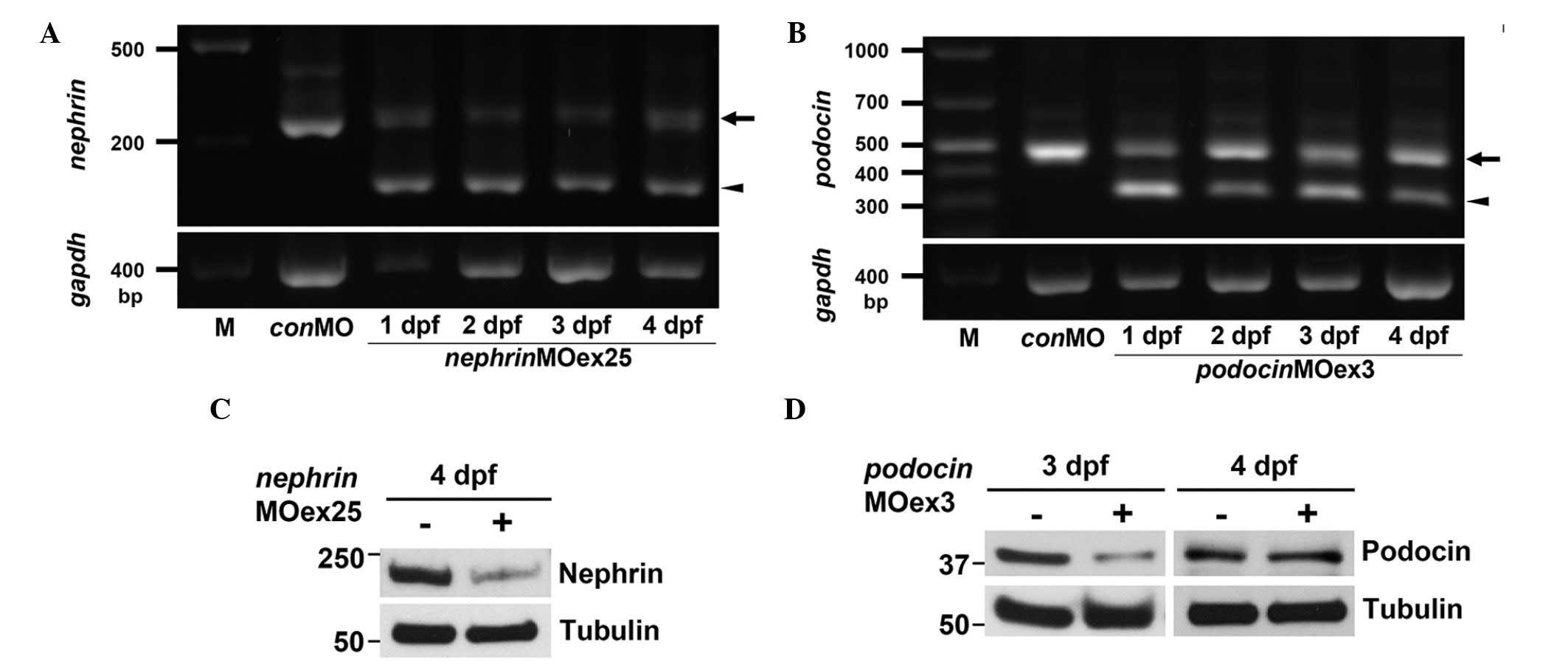
Injection of nephrinMOex25 resulted in
mis-spliced mRNA as detected by an altered RT-PCR product (Fig. 3A). This altered product was ~50 bp
smaller than the wild-type. Sequencing of the altered RT-PCR
product revealed a deletion of exon 25 that resulted in a frame
shift and non-sense translation of the subsequent exons, resulting
in an almost complete loss of the intracellular domain of the
Nephrin protein in nephrinMOex25 morphants (Fig. 2A). The efficacy for
nephrinMOex25 was consistently recognized from 1 to 4 dpf
(Fig. 3A) and the protein
expression of Nephrin was reduced at 4 dpf (Fig. 3C).
Administration of podocinMOex3 also resulted
in mis-spliced mRNA as detected by an altered RT-PCR product that
was ~150 bp smaller than the wild-type amplicon (Fig. 3B). Sequencing of the altered RT-PCR
product revealed an in-frame deletion of exons 3 and 4 (Fig. 2B). The truncated RT-PCR product was
recognized from 1 to 4 dpf, although a significant expression of
intact Podocin mRNA was also detected (Fig. 3B). The reduced expression of the
Podocin protein was observed in the 3 dpf morphants, however, the
expression was recovered at the same level as in the control at 4
dpf (Fig. 3D).
NephrinMOex25 and PodocinMOex3
morphants exhibited pericardial and yolk edema from 3 to 4 dpf
(Fig. 2C–E). In addition to edema,
the majority of the morphants showed body-axis curvature (Fig. 2C–E). In wild-type zebrafish, a pair
of pronephric glomerular primordia merged with glomerular
capillaries and mesangium at the midline to form a single
glomerulus (Fig. 2F) (1). The glomerulus was smaller in size in
the morphants compared with that in the wild-type larvae and
contained poorly developed glomerular capillaries and mesangium
(Fig. 2G and H).
In 4 dpf control larvae, regular foot processes with
SD covered a large area of the urinary surface of the glomerular
basement membrane (Fig. 2I). At
the same stage, nephrinMOex25 morphants were associated with
a great reduction in the area covered with regular foot processes.
Instead of the foot processes, irregularly shaped processes covered
the larger area of its urinary surface and the SD was not observed
between these irregularly shaped processes (Fig. 2J). In 4 dpf podocinMOex3
morphants, the foot processes with SD covered a large area of the
glomerular wall, however, the processes varied in shape and size
(Fig. 2K).
Rescue of the morphant phenotypes by
human intact mRNA
To investigate whether administration of human
intact mRNA rescues the morphant phenotypes, splice blocking
morpholino and human wild-type mRNA were simultaneously injected
into one-cell stage embryos. Human NEPHRIN and
PODOCIN mRNA were detected in the 4 dpf-injected larvae by
RT-PCR (Fig. 4B), indicating that
the injected mRNA remained until at least four days following
injection. nephrinMOex25 and podocinMOex3
morpholino-injected embryos exhibited pericardial edema, yolk edema
and body-axis curvature. Moreover, the glomerulus was smaller in
size compared with the wild-type larvae and contained poorly
developed glomerular capillaries and mesangium (Fig. 2F–H). All these features may be
rescued by ~89% by co-injection of human Nephrin and Podocin mRNAs
(Fig. 4D). In addition, rescued
morphants exhibited a straight body axis (Fig. 4E and F) and a well-developed
pronephric glomerulus at 4 dpf (Fig.
4G and H), although slight pericardial edema was observed in
the larvae injected with human PODOCIN mRNA and
podocinMOex3 (Fig. 4F).
These data therefore indicate that the functions of human and
zebrafish Nephrin and Podocin are interchangeable.
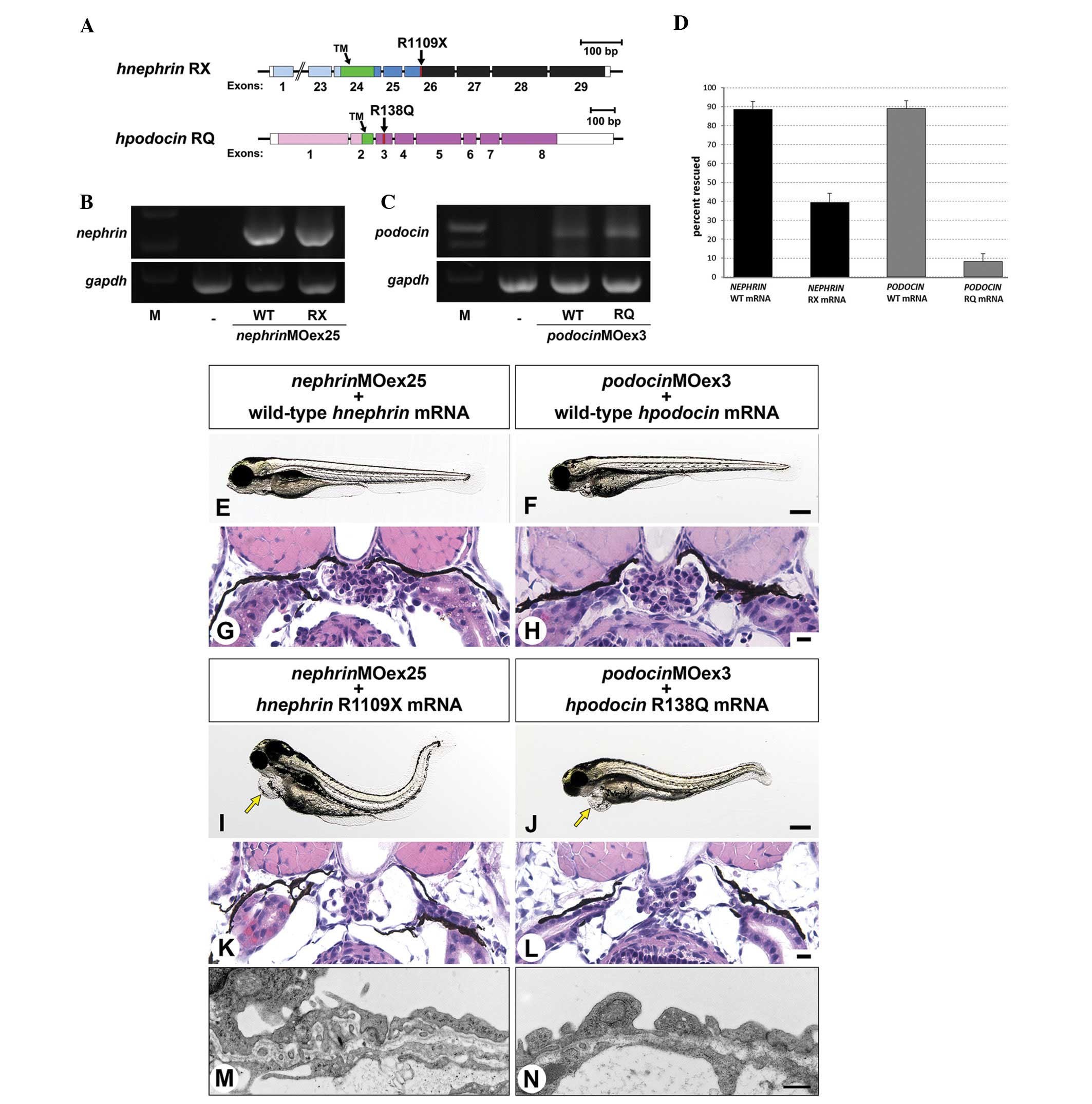 | Figure 4Human mRNA injection. (A) The
mutation sites introduced in the human Nephrin and Podocin mRNAs.
(B and C) Three types of injected human mRNA (wild-type,
NEPHRIN-R1109X and PODOCIN-R138Q) are detected in 2
dpf larvae by RT-PCR. (D) Wild-type mRNAs of human Nephrin and
Podocin may rescue ~89% of nephrinMOex25 and
podocinMOex3 morphants, respectively, however, the
percentage of rescued larvae is significantly reduced when mutated
mRNA is co-injected at 39 and 8.1%, respectively. The phenotype was
scored by pericardial edema, body-axis curvature, glomerulus
morphology and altered glomerular barrier. (E–H) Wild-type human
Nephrin and Podocin mRNA rescue the morphant phenotypes. (E and F)
The larvae exhibit a straight body-axis with no or slight
pericardial edema, (G and H) and a well-developed glomerulus. (I–N)
Mutated human NEPHRIN-R1109X and PODOCIN-R138Q mRNA
do not rescue the morphant phenotypes as frequently. (I and J)
Larvae injected with mutated mRNA exhibit pericardial edema and
body-axis curvature, (K and L) hypoplastic glomerulus (M and N) and
no regular foot processes. Bar scales, 250 μm in (F), (J); 10 μm in
(H), (L) and 100 nm in (N). dpf, days post-fertilization. |
The phenotype induced by a nephrinMOex25
or podocinMOex3 in zebrafish embryos may be rescued by wild-type
human NEPHRIN or PODOCIN but not by NEPHRIN-R1109X and
PODOCIN-R138Q mutant mRNA
A number of types of disease-causing mutations in
Nephrin and Podocin proteins have been reported in human patients,
including R1109X in Nephrin (25)
and R138Q in Podocin (26).
NEPHRIN-R1109X is a non-sense mutation leading to a large deletion
of the intracellular domain of Nephrin and it is one of the two
major disease-causing mutations in Finnish-type congenital
nephrotic syndrome (24) (Fig. 4A). PODOCIN-R138Q is a missense
mutation resulting in the substitution of arginine at position 138
for the uncharged amino acid glutamine and is also one of the most
common disease-causing mutations in steroid-resistant nephrotic
syndrome (23) (Fig. 4A).
To determine the effect of mutated mRNA on the
rescue of the morphant phenotypes, nephrinMOex25 and
podocinMOex3 were injected together with mutated mRNA
encoding human NEPHRIN-R1109X and PODOCIN-R138Q,
respectively, into one-cell stage embryos. The persistence of the
two mutated mRNAs was detected in 4 dpf larvae by RT-PCR (Fig. 4B and C), but the ratio of rescued
larvae was significantly lower for the mutated mRNAs compared with
the wild-type mRNA (Fig. 4D).
Non-rescued larvae exhibited pericardial edema, body-axis curvature
and hypoplastic glomerulus, as observed in the larvae injected with
the morpholino only (Fig. 4G, H, K and
L). The formation of regular foot processes was affected in the
larvae injected with NEPHRIN-R1109X and
nephrinMOex25, as with nephrinMOex25 alone (Fig. 4M). Notably, the larvae injected
with PODOCIN-R138Q and podocinMOex3 did not exhibit
any regular foot processes with SD (Fig. 4N). However, larvae injected with
podocinMOex3 formed foot processes to some extent (Fig. 2K), indicating that
PODOCIN-R138Q exacerbated the morphant phenotype observed
with podcinMOex3 alone. The phenotype was scored by
morphology and altered glomerular barrier and, in contrast to human
Nephrin and Podocin mRNAs, co-injection of and nephrinMOex25
and mutant NEPHRIN-R1109X or podocinMOex3 and
PODOCIN-R138Q only rescued 39 and 8.1%, respectively.
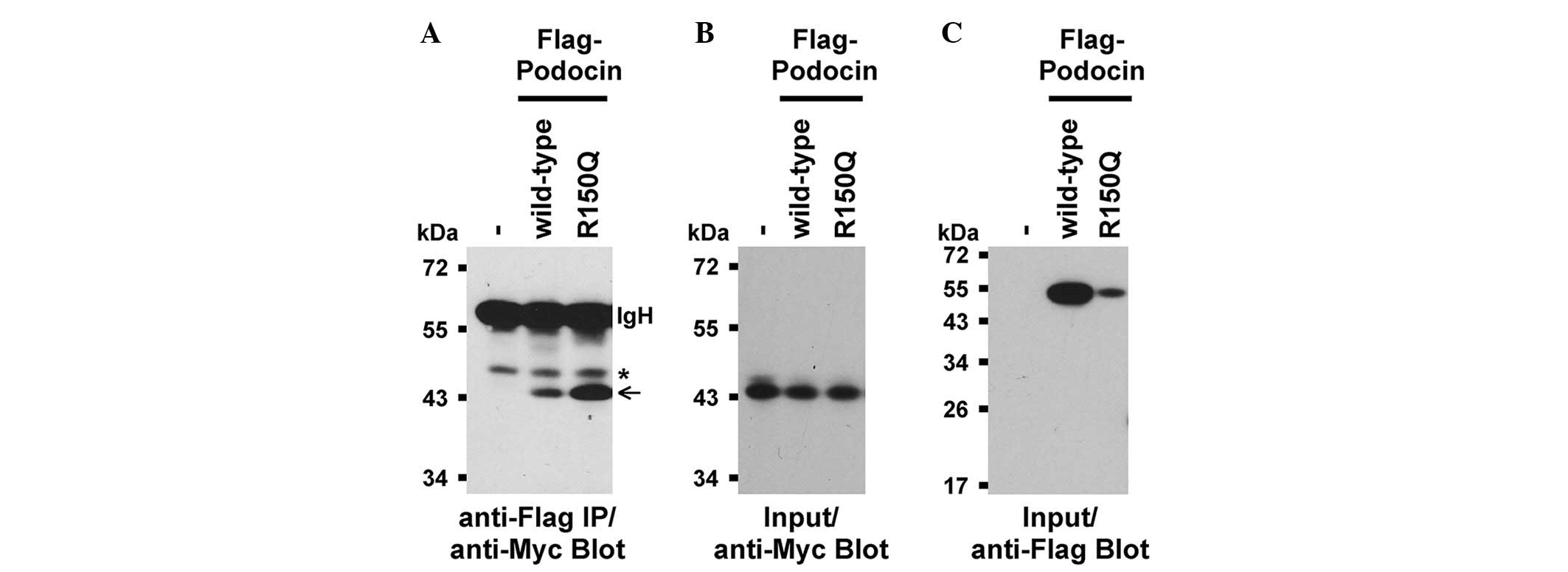
Protein interaction between Nephrin and
Podocin
To examine whether the protein properties of a
mutated PODOCIN were biochemically altered, the abilities of
Flag-tagged wild-type and mutated (R150Q) zebrafish full-length
Podocin to bind to the Myc-tagged C-terminal intracellular domain
of zebrafish Nephrin were compared. Notably, the R150Q zebrafish
Podocin mutation corresponded to the disease-causing R138Q mutation
in human Podocin. Consistent with previous studies for mammalian
Nephrin and Podocin (17,38), binding between wild-type zebrafish
Podocin and the intracellular region of Nephrin (Fig. 5) was observed. Binding between the
mutated Podocin-R150Q and Nephrin (Fig. 5) was also detected. However,
mutated Podocin interacted significantly more with Nephrin despite
the lower expression levels of mutated Podocin compared with
wild-type Podocin (Fig. 5). The
latter may suggest that the mutation of Podocin makes it more prone
to protein degradation.
Discussion
In the mammalian kidney, Nephrin and Podocin
localize at the SD between the podocyte foot processes and play
essential roles in the formation and maintenance of the SD. A
previous study of the localization of zebrafish Nephrin during
development revealed its predominant localization at the podocytes
within the pronephros, which is similar to its localization in the
mammalian metanephros (3).
Similarly, Podocin was previously identified to localize in two
tortuous line segments within the pronephros, which was similar to
that of Nephrin, indicating that Podocin also predominantly
localizes at the podocytes in the zebrafish pronephros.
NephrinMOex25 and PodocinMOex3 induced
the altered splicing of pre-mRNA to greatly attenuate the
expression of full-length protein products. Nephrin and Podocin
were already expressed in the primitive podocytes of glomerular
primordia, which had not yet formed foot processes and SD,
suggesting that Nephrin and Podocin contribute to the
differentiation of primitive podocytes. The attenuated expression
of these two proteins is thus likely to affect the appropriate
development and maturation of podocytes. In zebrafish and mammals,
podocytes are known to produce vascular endothelial growth factor,
which contributes to the migration of primitive capillary
endothelial cells and the maturation of glomerular capillaries
(27,39). Therefore, impairment of podocyte
differentiation may subsequently affect the formation and
maturation of other glomerular structures (so-called endocapillary
region). The aforementioned scenario may explain the underlying
mechanism for the glomerular hypoplasia in the morphants
examined.
Although identity in amino acid sequence is low in
Nephrin and Podocin homologues between zebrafish and humans, the
alignment of the functional domains within the proteins is highly
conserved (9,21). Notably, human wild-type Nephrin and
Podocin mRNAs may rescue the phenotype of the nephrinMOex25
and podocinMOex3 morphants, respectively. Thus, the current
data suggest that the protein function of Nephrin and Podocin is
highly conserved between zebrafish and humans.
Human Nephrin and Podocin mRNAs containing
disease-causative mutations exhibited significantly lower
efficiency in rescuing the morphant phenotypes than the wild-type
mRNAs. Moreover, the mRNA coding PODOCIN-R138Q mutant
prominently affected the formation of regular foot processes
interspaced with SD, although the injection of podocinMOex3
alone did not largely disturb the SD formation. These results
suggest that the morpholino and mutated mRNA synergistically
interfered with the formation of the foot processes interspaced
with SD. Therefore, this experimental system offers potential for
investigation of the spatiotemporal consequences of gene mutations
associated with the nephrotic syndrome in the early phase of
glomerular development. In addition, it may be useful to explore
the pathogenic mechanisms underlying human congenital nephrotic
syndromes.
Multi-protein complexes, including those containing
Nephrin and Podocin are formed at the SD and are believed to be
crucial in the establishment and maintenance of the SD. The current
study demonstrates that an interaction occurred between wild-type
Nephrin and Podocin in zebrafish. Nishibori et al (40) reported that the human
PODOCIN-R138Q mutant, which corresponds to the zebrafish
podocin-R150Q, is trapped in the endoplasmic reticulum and
the trapped Podocin is hypothesized to interfere with trafficking
of Nephrin (40). The present
pull-down assay indicated that the binding activity of zebrafish
podocin-R150Q to Nephrin was stronger compared with
wild-type Podocin. If the zebrafish Podocin-R150Q is also trapped
and accumulated in the endoplasmic reticulum, the mutated Podocin
most likely impaired Nephrin trafficking from the endoplasmic
reticulum to its proper destination in the SD near the foot
processes due to its abnormally strong ability to bind Nephrin.
This hypothesis is in agreement with the observation that the
formation of regular foot processes and SD was more detrimentally
affected in the larvae injected with human PODOCIN-R138Q
mRNA plus podocinMOex3 than with the morpholino alone.
The present study highlights the high evolutionary
conservation in the function of Podocin and Nephrin from zebrafish
to humans and establishes a system by which disease-causing
mutations in human PODOCIN and NEPHRIN may be
assessed for their biological consequences. This information is
likely to facilitate studies to gain an improved understanding of
the development of several kidney diseases, including nephrotic
syndrome.
Acknowledgements
The authors would like to thank Drs Iain Drummond
and Lawrence Holzman for their kind gifts of the plasmids; Dr
Koichiro Ichimura for his technical assistance in electron
microscopy; Drs Deborah Garrity, Kristina Wasson-Blader and
Hiroyuki Matsumoto for helpful criticism and comments on the
manuscript. T.O. acknowledges financial support from the University
of Oklahoma Health Sciences Center (OUHSC). T.O. was supported by
NIH grants R21-DK069604 and R01-DK078209. This study was supported
in part by the Diabetes Histology and Image Acquisition and
Analysis Core Facility at OUHSC (NIH, COBRE-1P20RR024215) and the
Imaging Core Facility at the Oklahoma Medical Research
Foundation.
References
|
1
|
Ichimura K, Bubenshchikova E, Powell R,
Fukuyo Y, Nakamura T, Tran U, Oda S, Tanaka M, Wessely O, Kurihara
H, Sakai T and Obara T: A comparative analysis of glomerulus
development in the pronephros of medaka and zebrafish. PloS One.
7:e452862012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ichimura K, Fukuyo Y, Nakamura T, Powell
R, Sakai T and Obara T: Structural disorganization of pronephric
glomerulus in zebrafish mpp5a/nagie oko mutant. Dev Dyn.
241:1922–1932. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ichimura K, Fukuyo Y, Nakamura T, Powell
R, Sakai T, Janknecht R and Obara T: Developmental localization of
nephrin in zebrafish and medaka pronephric glomerulus. J Histochem
Cytochem. 61:313–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roselli S, Gribouval O, Boute N, Sich M,
Benessy F, Attié T, Gubler MC and Antignac C: Podocin localizes in
the kidney to the slit diaphragm area. Am J Pathol. 160:131–139.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kriz W and Kaissling B: Structural
Organization of the Mammalian Kidney. Physiology and
pathophysiology. Seldin and Giebisch’s The Kidney. Alpern RJ and
Hebert SC: 4th edition. Academic Press; Waltham, MA: pp. 479–563.
2007
|
|
6
|
Takahashi-Iwanaga H: Comparative anatomy
of the podocyte: A scanning electron microscopic study. Microsc Res
Tech. 57:196–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ichimura K, Kurihara H and Sakai T: Actin
filament organization of foot processes in vertebrate glomerular
podocytes. Cell Tissue Res. 329:541–557. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ichimura K, Kurihara H and Sakai T:
Beta-cytoplasmic actin localization in vertebrate glomerular
podocytes. Arch Histol Cytol. 72:165–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kramer-Zucker AG, Wiessner S, Jensen AM
and Drummond IA: Organization of the pronephric filtration
apparatus in zebrafish requires Nephrin, Podocin and the FERM
domain protein Mosaic eyes. Dev Biol. 285:316–329. 2005. View Article : Google Scholar
|
|
10
|
Fukasawa H, Bornheimer S, Kudlicka K and
Farquhar MG: Slit diaphragms contain tight junction proteins. J Am
Soc Nephrol. 20:1491–1503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pavenstädt H, Kriz W and Kretzler M: Cell
biology of the glomerular podocyte. Physiol Rev. 83:253–307.
2003.
|
|
12
|
Donoviel DB, Freed DD, Vogel H, Potter DG,
Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery
CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP
and Powell DR: Proteinuria and perinatal lethality in mice lacking
NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol.
21:4829–4836. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neumann-Haefelin E, Kramer-Zucker A,
Slanchev K, Hartleben B, Noutsou F, Martin K, Wanner N, Ritter A,
Gödel M, Pagel P, Fu X, Müller A, Baumeister R, Walz G and Huber
TB: A model organism approach: defining the role of Neph proteins
as regulators of neuron and kidney morphogenesis. Hum Mol Genet.
19:2347–2359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dustin ML, Olszowy MW, Holdorf AD, Li J,
Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA,
Allen PM and Shaw AS: A novel adaptor protein orchestrates receptor
patterning and cytoskeletal polarity in T-cell contacts. Cell.
94:667–677. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shih NY, Li J, Karpitskii V, Nguyen A,
Dustin ML, Kanagawa O, Miner JH and Shaw AS: Congenital nephrotic
syndrome in mice lacking CD2-associated protein. Science.
286:312–315. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shih NY, Li J, Cotran R, Mundel P, Miner
JH and Shaw AS: CD2AP localizes to the slit diaphragm and binds to
nephrin via a novel C-terminal domain. Am J Pathol. 159:2303–2308.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schwarz K, Simons M, Reiser J, Saleem MA,
Faul C, Kriz W, Shaw AS, Holzman LB and Mundel P: Podocin, a
raft-associated component of the glomerular slit diaphragm,
interacts with CD2AP and nephrin. J Clin Invest. 108:1621–1629.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruotsalainen V, Ljungberg P, Wartiovaara
J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C and Tryggvason K:
Nephrin is specifically located at the slit diaphragm of glomerular
podocytes. Proc Natl Acad Sci USA. 96:7962–7967. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holthofer H, Ahola H, Solin ML, Wang S,
Palmen T, Luimula P, Miettinen A and Kerjaschki D: Nephrin
localizes at the podocyte filtration slit area and is
characteristically spliced in the human kidney. Am J Pathol.
155:1681–1687. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holzman LB, St John PL, Kovari IA, Verma
R, Holthofer H and Abrahamson DR: Nephrin localizes to the slit
pore of the glomerular epithelial cell. Kidney Int. 56:1481–1491.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ebarasi L, He L, Hultenby K, Takemoto M,
Betsholtz C, Tryggvason K and Majumdar A: A reverse genetic screen
in the zebrafish identifies crb2b as a regulator of the glomerular
filtration barrier. Dev Biol. 334:1–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hentschel DM, Mengel M, Boehme L, Liebsch
F, Albertin C, Bonventre JV, Haller H and Schiffer M: Rapid
screening of glomerular slit diaphragm integrity in larval
zebrafish. Am J Physiol Renal Physiol. 293:F1746–F1750. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boute N, Gribouval O, Roselli S, Benessy
F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P and Antigna
C: NPHS2, encoding the glomerular protein podocin, is mutated in
autosomal recessive steroid-resistant nephrotic syndrome. Nat
Genet. 24:349–354. 2000. View
Article : Google Scholar
|
|
24
|
Kestilä M, Lenkkeri U, Männikkö M,
Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T,
Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A
and Tryggvason K: Positionally cloned gene for a novel glomerular
protein - nephrin - is mutated in congenital nephrotic syndrome.
Mol Cell. 1:575–582. 1998.
|
|
25
|
Beltcheva O, Martin P, Lenkkeri U and
Tryggvason K: Mutation spectrum in the nephrin gene (NPHS1) in
congenital nephrotic syndrome. Hum Mutat. 17:368–373. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caridi G, Perfumo F and Ghiggeri GM: NPHS2
(Podocin) mutations in nephrotic syndrome. Clinical spectrum and
fine mechanisms. Pediatr Res. 57:54R–61R. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O’Brien LL, Grimaldi M, Kostun Z, Wingert
RA, Selleck R and Davidson AJ: Wt1a, Foxc1a, and the Notch mediator
Rbpj physically interact and regulate the formation of podocytes in
zebrafish. Dev Biol. 358:318–330. 2011.PubMed/NCBI
|
|
28
|
Thisse C and Thisse B: High-resolution in
situ hybridization to whole-mount zebrafish embryos. Nat Protoc.
3:59–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Link V, Shevchenko A and Heisenberg CP:
Proteomics of early zebrafish embryos. BMC Dev Biol. 6:12006.
View Article : Google Scholar
|
|
30
|
Wu J and Janknecht R: Regulation of the
ETS transcription factor ER81 by the 90-kDa ribosomal S6 kinase 1
and protein kinase A. J Biol Chem. 277:42669–42679. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shin S and Janknecht R: Diversity within
the JMJD2 histone demethylase family. Biochem Biophys Res Commun.
353:973–977. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papoutsopoulou S and Janknecht R:
Phosphorylation of ETS transcription factor ER81 in a complex with
its coactivators CREB-binding protein and p300. Mol Cell Biol.
20:7300–7310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bubenshchikova E, Ichimura K, Fukuyo Y,
Powell R, Hsu C, Morrical SO, Sedor JR, Sakai T and Obara T: Wtip
and Vangl2 are required for mitotic spindle orientation and cloaca
morphogenesis. Biol Open. 1:588–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Knebel J, De Haro L and Janknecht R:
Repression of transcription by TSGA/Jmjd1a, a novel interaction
partner of the ETS protein ER71. J Cell Biochem. 99:319–329. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim TD, Shin S and Janknecht R: Repression
of Smad3 activity by histone demethylase SMCX/JARID1C. Biochem
Biophys Res Commun. 366:563–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruotsalainen V, Patrakka J, Tissari P,
Reponen P, Hess M, Kestilä M, Holmberg C, Salonen R, Heikinheim M,
Wartiovaara J, Tryggvason K and Jalanko H: Role of nephrin in cell
junction formation in human nephrogenesis. Am J Pathol.
157:1905–1916. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kawachi H, Abrahamson DR, St John PL,
Goldstein DJ, Shia MA, Matsui K, Shimizu F and Salant DJ:
Developmental expression of the nephritogenic antigen of monoclonal
antibody 5-1-6. Am J Pathol. 147:823–833. 1995.PubMed/NCBI
|
|
38
|
Huber TB, Simons M, Hartleben B, Sernetz
L, Schmidts M, Gundlach E, Saleem MA, Walz G and Benzing T:
Molecular basis of the functional podocin-nephrin complex:
mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft
microdomains. Hum Mol Genet. 12:3397–3405. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kitamoto Y, Tokunaga H and Tomita K:
Vascular endothelial growth factor is an essential molecule for
mouse kidney development: glomerulogenesis and nephrogenesis. J
Clin Invest. 99:2351–2357. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nishibori Y, Liu L, Hosoyamada M, Endou H,
Kudo A, Takenaka H, Higashihara E, Bessho F, Takahashi S, Kershaw
D, Ruotsalainen V, Tryggvason K, Khoshnoodi J and Yan K:
Disease-causing missense mutations in NPHS2 gene alter normal
nephrin trafficking to the plasma membrane. Kidney Int.
66:1755–1765. 2004. View Article : Google Scholar : PubMed/NCBI
|