Introduction
Gastric cancer (GC) remains the most common type of
cancer and the second major cause of mortality from cancer in the
world (1,2). Despite the significant decline in GC
cases over the last decades, the mortality rates of GC in Asia and
other developing countries are considerably higher than in
developed countries (3,4), which is largely due to a lack of
timely diagnosis and effective treatment. Drug-assisted surgical
treatment has been widely used in clinics, but the side-effects of
chemotherapy drugs and the resistance to chemotherapy are
underlying problems in its treatment (5). Therefore, the requirement for novel
agents to enhance the effects of chemotherapeutic drugs and reduce
their resistance remains critical.
Chinese herbal antitumor agents, including
assolanine (6), an extract from
Rhodiola rosea rhizomes (7), Gleditsioside E from Gleditsia
sinensis (8) and saponins from
Gleditsia sinensis (9),
exert significant effects against a variety of tumors and have been
confirmed to be effective antitumor agents. Saffron is a derived
plant product from the dried stigma of the Crocus sativus
flower (family, Iridaceae), which has been used for several hundred
years in the treatment of cardiovascular diseases, inflammatory
diseases and several other tumors (10,11).
Extracts of saffron contain multiple biologically active compounds,
including crocin, crocetin, saffron bitter element, saffron
aldehyde, vitamins and flavonoids. Crocetin, a major active
ingredient of saffron extract, has been revealed to possess potent
antiproliferative and antioxidative characteristics. Moreover, the
antitumor activity of crocetin has been observed to enhance the
apoptosis of several types of cancer cells in vitro and
inhibit the growth of tumors in vivo, including human liver
(12), colorectal (13), pancreatic (14) and breast (15) cancer cells.
However, the effect of crocetin on human gastric
cells and its mechanism have never been investigated to date.
BGC-823 human GC cells have been widely used in antitumor drug
research (16,17). Therefore, the present study was
designed to investigate the effects of crocetin on the
proliferation of BGC-823 cells and its mechanism in
vitro.
Materials and methods
Materials
The following reagents were used: Crocetin (MP
Biomedicals, Solon, OH, USA;
C20H24O4; formula weight, 328.4;
purity, >98%), docetaxel (Jiangsu Hengrui Medicine Co., Ltd.,
Nanjing, China), trypsin (Gibco, Carlsbad, CA, USA), fetal bovine
serum (Gibco), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT; MP Biomedicals, Santa Ana, CA, USA) and TRIzol
(Invitrogen Life Technologies, Carlsbad, CA, USA). The Rh123 and
Hoechst 33258 dyes were purchased from Sigma (St. Louis, MO, USA),
and an inverted microscope obtained from Olympus (Tokyo, Japan) was
used. Cleaved caspase-3 and cytochrome c antibodies were
purchased from Cell Signaling Biotechnology (Beverly, MA, USA).
Horseradish peroxidase-conjugated anti-rabbit antibody was shipped
from Boster (Wuhan, China).
Crocetin preparation
Crocetin was diluted in phosphate-buffered saline
(PBS), filter sterilized (GE Healthcare, Greenwich, CT, USA) and
stored at 4°C or −20°C (for a maximum of 2 months) in the dark.
Cell line and cultures
The GC BGC-823 cell line was obtained from the
Experimental Animal Center of Sun Yat-sen University (Guangzhou,
China). The GC cell line was cultured in RPMI-1640 medium
supplemented with 10% fetal-bovine serum, 100 units/ml penicillin
and 100 mg/ml streptomycin. The cell culture dish was placed in a
humidified atmosphere with 5% CO2 at 37°C. The medium
was changed every 2–3 days and the cells were trypsinized,
harvested and seeded in a novel plate once the cells had reached
80–90% confluence.
Cell viability assays
The effect of crocetin on cell proliferation was
determined by the MTT uptake method. The cells
(1×105/ml/well) were incubated with varying
concentrations of crocetin in a 96-well plate and then incubated
for 24, 48 or 72 h at 37°C. MTT solution (10 μl) was added to each
well and incubated for 4 h at 37°C. Subsequently, the medium was
replaced by 150 μl dimethylsulfoxide (DMSO) per well to dissolve
the formazan crystals. The absorbance value of each well was
determined by the enzyme-linked immunosorbent assay (wavelength,
570 nm).
Experimental groups and morphological
analysis
The following groups were used: Group A, the blank
control group; group B, the 200-μmol crocetin group; group C, the
docetaxel 5-μmol group (18); and
group D, the 0.5% DMSO group. The four groups were supplemented
with 2×106/ml BGC-823 cells for 48 h, and changes in
cell morphology were observed by inverted fluorescence microscopy
(Leica DMI4000B, Leica, Mannheim, Germany).
Hoechst 33258 staining
DNA staining by Hoechst 33258 dyes were used to
evaluate the chromosomal condensation and morphological changes.
The BGC-823 cells were plated in 6-well plates. Following
treatment, the BGC-823 cells were fixed with 4% paraformaldehyde
for 20 min. Hoechst 33258 (5 mg/ml in PBS; pH 7.4) was added to the
cells for 10 min, followed by washing with PBS three times. The
nuclear morphology was observed using a fluorescence microscope
(Leica DMI4000B). The intensity of the mean fluorescence was
analyzed using ImageJ 1.41o software (NIH, Bethesda, Maryland,
USA).
Measurement of the mitochondrial membrane
potential (MMP)
The MMP was measured using Rh123, a cationic
fluorescent dye, as previously described (19). In the four groups, Rh123 was added
to the cultures to a final concentration of 2 μM for 30 min at
37°C. Following incubation, the cells were rinsed with PBS and
images were captured by Confocal Laser Scanning Microscopy (LSM
710; Zeiss, Oberkochen, Germany) immediately afterwards. The
intensity of the mean fluorescence was analyzed using ImageJ 1.41o
software (NIH).
Western blotting
To confirm the apoptosis mechanisms induced by
crocetin in the BGC-823 cells, the processing of cytochrome
c and cleaved caspase-3 was analyzed by western blotting.
The cells were treated as aforementioned and then washed three
times with cold PBS (pH 7.4) and lysed in ice-cold lysis buffer [1X
PBS, 1% NP40, 0.1% SDS, 5 mM EDTA, 0.5% sodium deoxycholate and 1%
phenylmethanesulfonyl fluoride (PMSF)]. The lysate was centrifuged
at 12,000 × g for 5 min at 4°C, then the supernatant was collected
and the protein concentration was quantified using a bicinchoninic
acid protein assay kit (Bioworld, Dublin, OH, USA). Equal
quantities of protein were separated on 15% SDS-polyacrylamide gels
(SDS-PAGE). The proteins were transferred to polyvinylidene
difluoride (PVDF) membranes, which were blocked with 5% skimmed
milk in Tris-buffered saline with Tween-20 (TBST) for 1 h at room
temperature. The membranes were incubated overnight with different
primary antibodies for cytochrome c (1:1,000) and cleaved
caspase-3 (1:1,000) at 4°C. Following three washes with TBST, the
blots were incubated with the secondary horseradish
peroxidase-conjugated anti-rabbit antibody at room temperature for
1 h followed by washing again three times using TBST buffer.
Subsequently, the antibody-bound proteins were detected by an
Enhanced chemiluminescence substrate (Vector Labs, Burlingame, CA,
USA) and exposed to X-ray films. ImageJ 1.41o software (NIH) was
used to quantitatively analyze the expression levels of the
protein.
Statistical analysis
All the experiments were repeated three times. All
the data are presented as the mean ± standard error of the mean.
Statistical significance was determined using a one-way analysis of
variance followed by Duncan’s multiple range test, utilizing a SPSS
13.0 for Windows (SPSS Inc., Chicago, IL, USA). The differences
between groups were compared with the least significant difference
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cytotoxic activities of crocetin on
BGC-823 cells
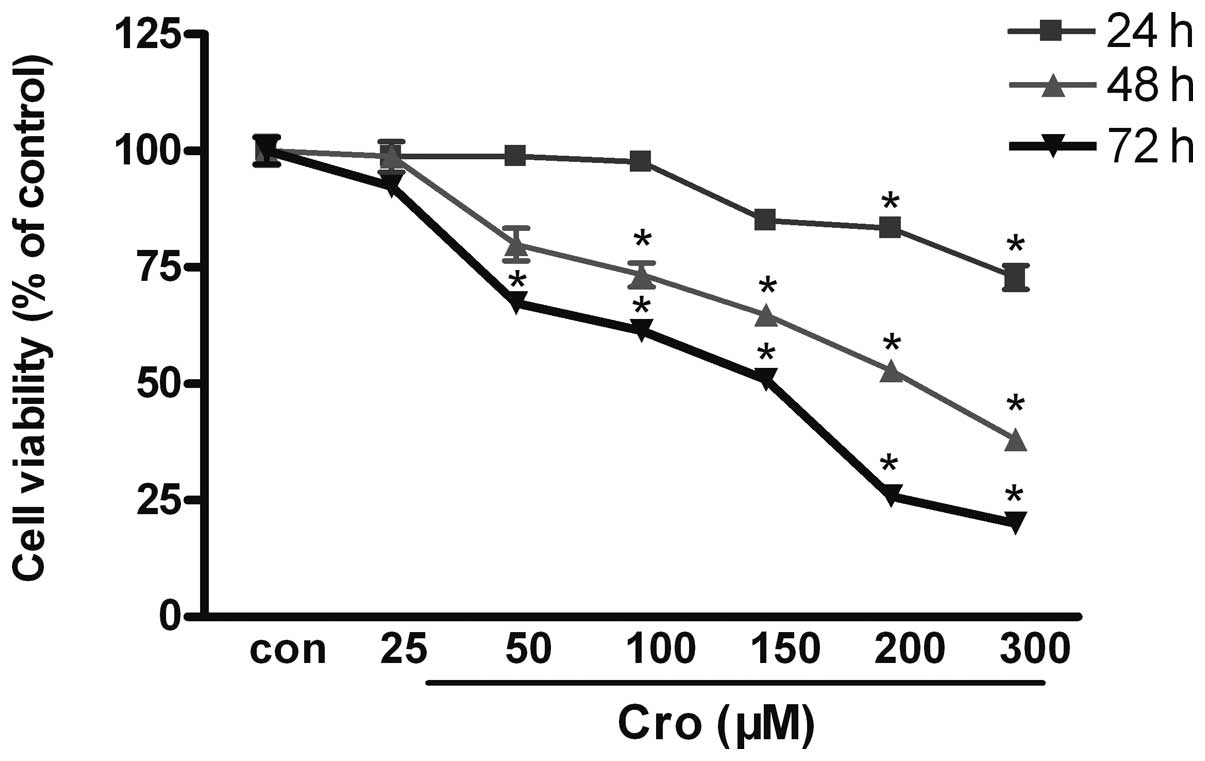
The MTT assay demonstrated that crocetin
significantly inhibited the viability of the BGC-823 cells
(Fig. 1). The cells were incubated
in the absence or presence of various concentrations of crocetin
(25–300 μM) for 24, 48 and 72 h. The MTT assay revealed that the
crocetin-treated cells exhibited a significant reduction in cell
proliferation in a concentration- and time-dependent manner, and
that the MTT absorbance value was significantly reduced,
demonstrating a significant difference compared with the control
group (P<0.05). The 50% maximal inhibitory concentration
(IC50) was 200 μM in the 48 h group (Fig. 1).
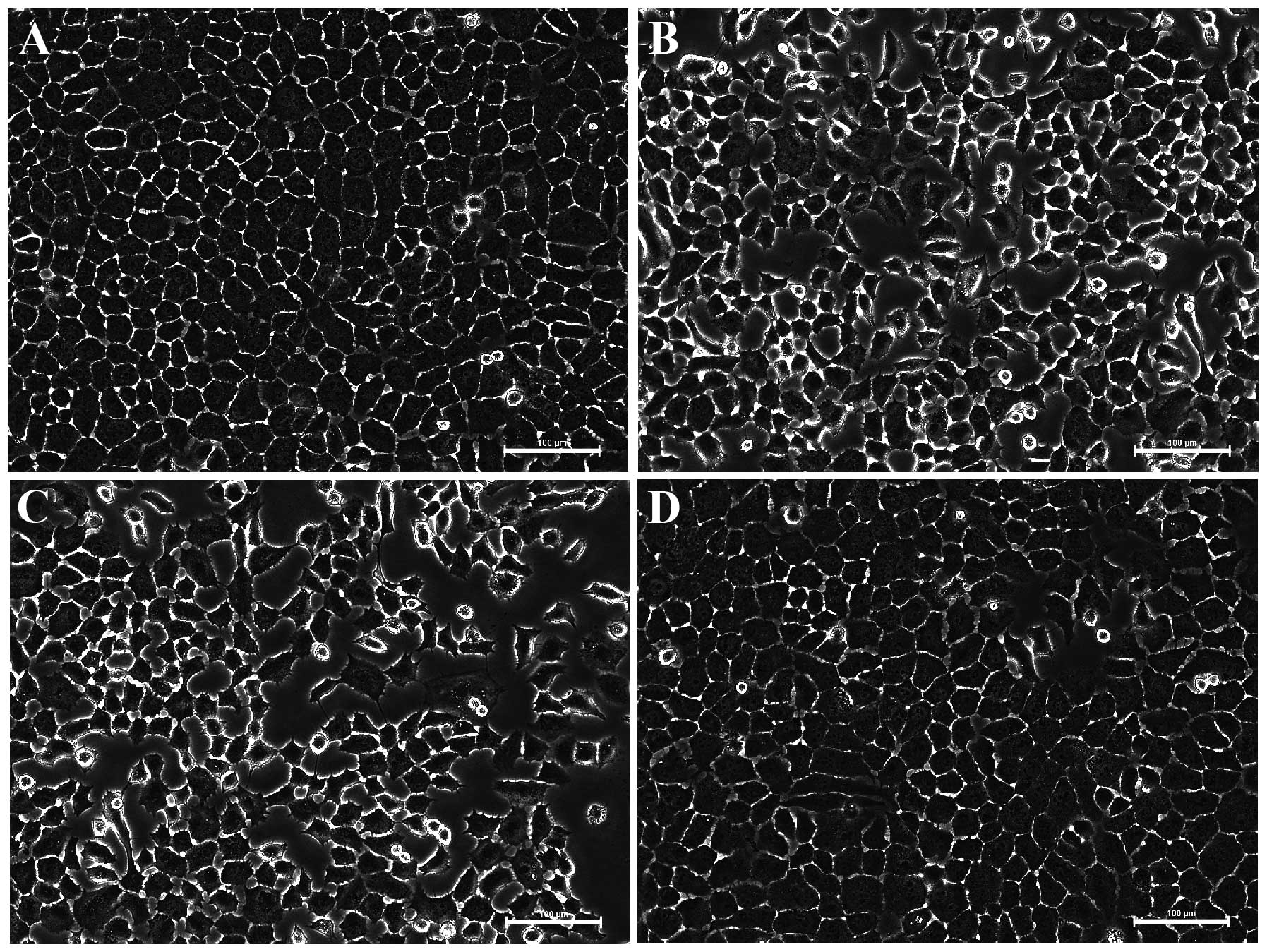
Morphology of the BGC-823 cells
The cells treated with vehicle (Fig. 2A) were healthy, as they had
networks of cell processes and vacuole-free cell bodies.
Connections between BGC-823 cells were clearly observed. Nucleus
fragmentation and shrinkage of the cell bodies were observed when
the BGC-823 cells were exposed to 200 μM crocetin (Fig. 2B) or docetaxel (Fig. 2C) for 48 h. DMSO itself did not
interfere with the BGC-823 cells (Fig.
2D).
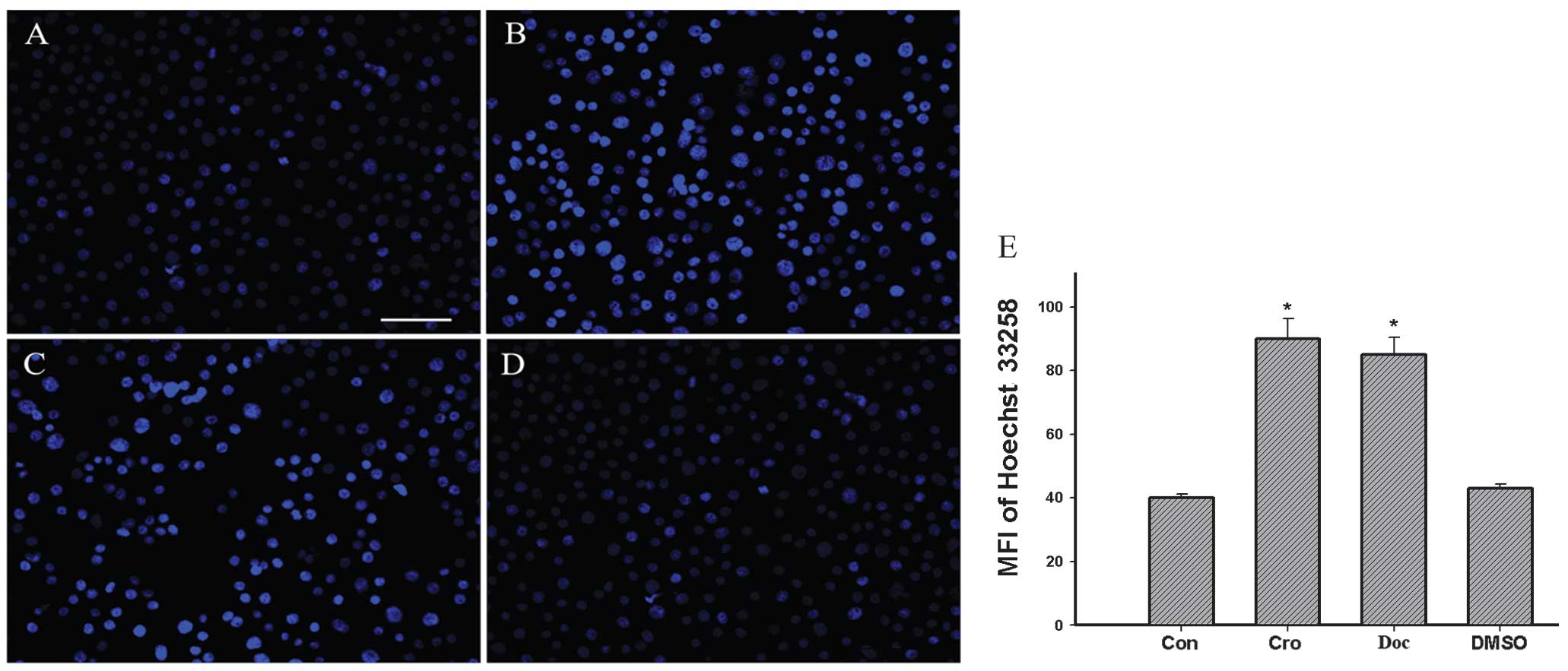
BGC-823 cell apoptosis is induced by
crocetin
The nuclear morphological changes associated with
apoptosis were observed by Hoechst 33258 staining. The control
group (Fig. 3A and E) revealed
intact and relatively large nuclei, whereas the crocetin- or
docetaxel-treated BGC-823 cells exhibited an increase in condensed
nuclei (Fig. 3B, C and E). DMSO
itself did not interfere with the BGC-823 cells (Fig. 3D and E).
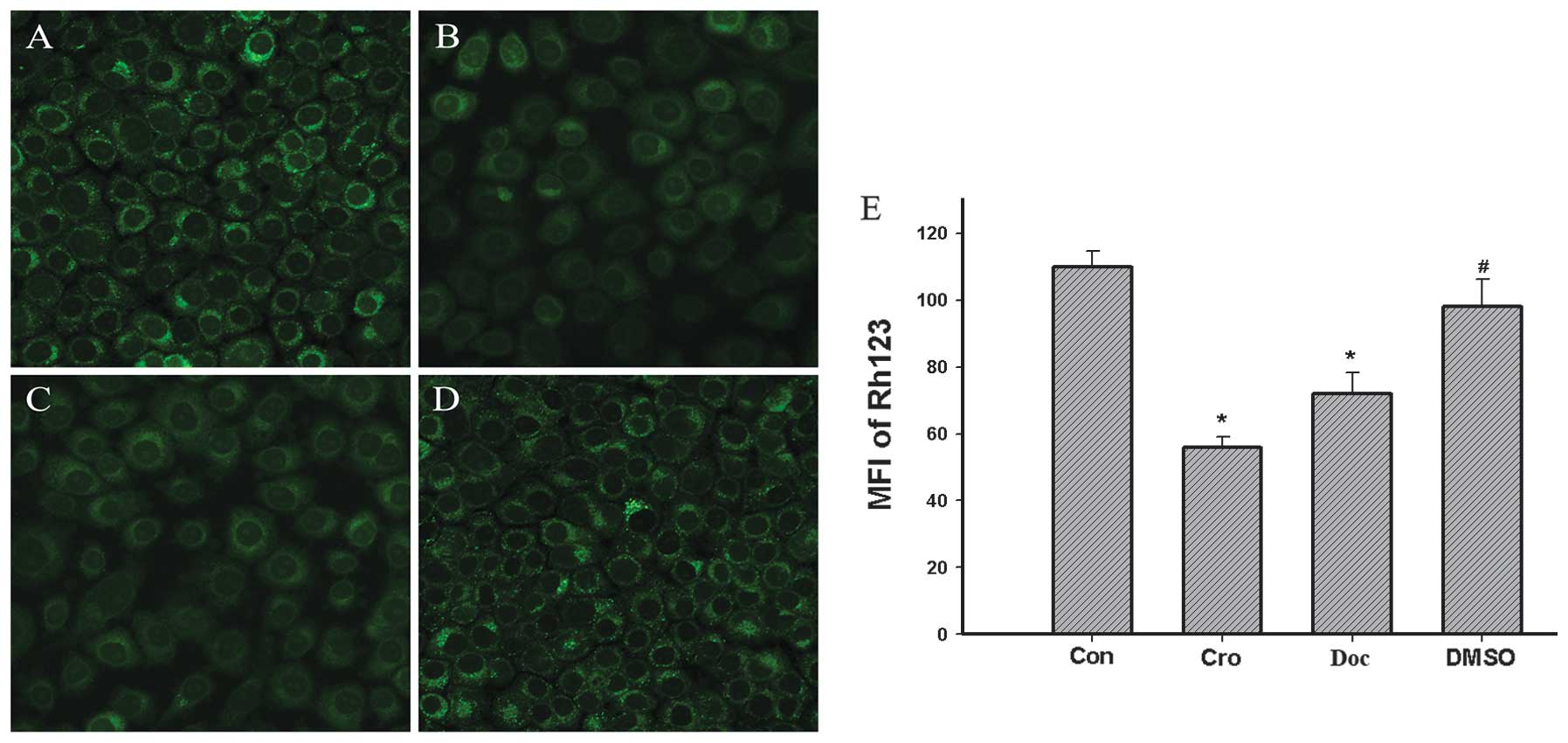
Crocetin-induction decreases the MMP
In this study, the effects on the 200 μM
crocetin-induced changes of the MMP in the BGC-823 cells were
investigated with the fluorescent dye, Rh123, a cell permeable
cationic dye, which preferentially enters into the mitochondria
based on the highly negative MMP. Following the exposure to 200 μM
crocetin for 48 h, the mitochondrial uptake of Rh123 was
significantly reduced (Fig. 4B)
and the intensity of the mean fluorescence was significantly
decreased (P<0.01) by 50% compared with the control (Fig. 4E).
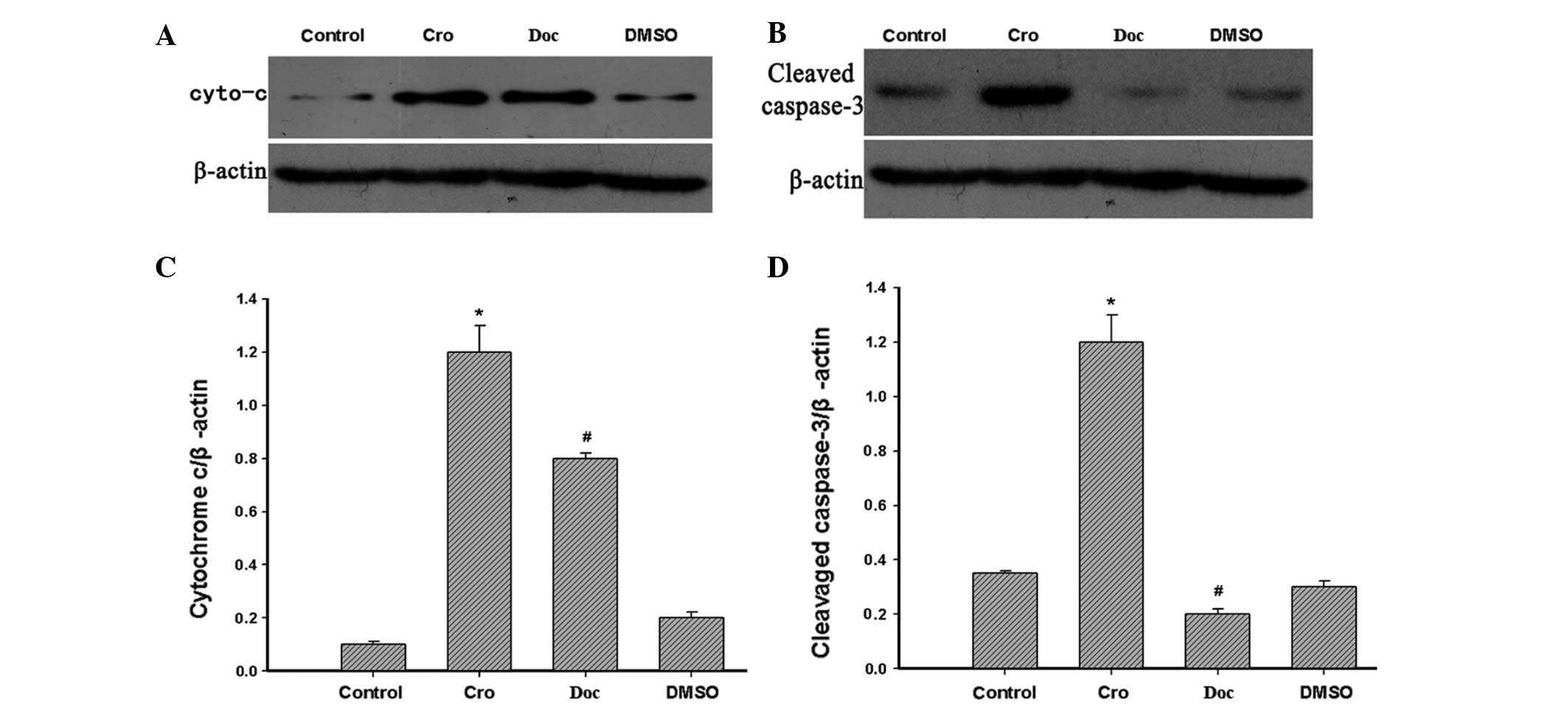
Apoptosis-related protein changes in
crocetin-treated BGC-823 cells
Cytochrome c is a component of the electron
transport chain in the mitochondria, and it is also involved in the
initiation of apoptosis. Therefore, the levels of cytochrome
c and cleaved caspase-3 in cortical neurons were examined in
the present study by western blotting. The levels of cytochrome
c and cleaved caspase-3 were shown to be markedly increased
in the cells that were incubated with 200 μM crocetin for 48 h
compared with the control group (Fig.
5A–D).
Discussion
GC is one of the most common types of malignant
tumors, with mortality rates in the forefront of cancer-related
mortality. Previous studies regarding the pathogenesis of GC
demonstrated that the incidence and progression of cancer have a
close correlation with cell apoptosis (20). Apoptosis is programmed cell death
and is distinguished from cell necrosis by morphological and
biochemical changes with its own specific characteristics (20). A number of the chemotherapeutic
agents, including crocetin and crocin, the principal active
ingredients of saffron, have potential antiproliferative effects
(5,21,22)
and have been revealed to suppress the growth of a variety of
cancer cells via arresting the cell cycle and inducing cell
apoptosis. To the best of our knowledge, the present study observed
for the first time that crocetin suppressed the proliferation of
BGC-823 cells in a dose- and time-dependent manner.
In the present experiments, a preliminary study
demonstrated the antitumor effect of crocetin in an in vitro
experimental BGC-823-cell model. Among the cytotoxicity detection
experiments, crocetin revealed significant antiproliferative
effects on the BGC-823 cells in a dose- and time-dependent manner.
It is noteworthy that accumulated data concerning the toxic effect
of crocetin has been reported, but there are different standpoints
with regard to its effect (14).
However, one previous in vitro study observed that crocetin
had no cytotoxic effect in the normal cells (23). This difference may be due to the
different cell lines and culture conditions used. Therefore, it was
hypothesized that there may be different mechanisms behind the
effects of crocetin on different cell lines, and for this purpose
there was a requirement to conduct the present study. Considering
the clinical value, the results imply that crocetin may be used as
a potential candidate for future drug development.
DNA damage and cell membrane death receptor
aggregation have long been considered to be a starting point for
the induction of MMP changes or the direct activation of apoptosis
(24). In the present study, the
apoptosis of BGC-823 cells induced by crocetin and stained by
Hoechst 33258 was observed, as well as the cell membrane staining
dense nuclei, nuclear pyknosis, fragmentation, chromatin
condensation and highlighted nuclear membrane staining under a
light microscope. The MMP of the BGC-823 cells was reduced by
crocetin, which indicated that crocetin induced apoptosis through a
mitochondrial damage pathway. In the latter part of the study,
crocetin was demonstrated to increase the activation of cleaved
caspase-3 and cytochrome c, as revealed by western blotting.
Increased cytoplasmic cytochrome c levels demonstrated that
cytochrome c released from the disruptive mitochondria
results in a decrease in the mitochondrial transmembrane potential.
The high level expression of cytochrome c resulted in
apoptosis of the BGC-823 cells through the caspase-9 and caspase-3
pathways (25).
Caspase-3, one of the key factors of apoptosis, uses
the mitochondrial-initiated intrinsic pathway to increase its
activity as cleaved caspase-3 and to lead to poly ADP ribose
polymerase cleavage, DNA damage and fragmentation, nuclear
condensation and ultimately induce apoptosis (26–29).
These results were consistent with other agents previously
reported, including docetaxel (30), γ-tocotrienol (31), magnolol (32), milk fermented by
Propionibacterium freudenreichii (33), triptolide and cisplatin (34).
Therefore, in the present study, it was shown that
mitochondrial damage, cytochrome c release and caspase-3
activation may be significant mechanisms for the crocetin-induced
apoptosis of BGC-823 cells. The underlying mechanism for crocetin
involved in signaling pathways modulating BGC-823 cell apoptotic
responses remains to be elucidated.
The data from the present study revealed that
crocetin suppressed the proliferation of the BGC-823 cells. This
inhibition was associated with reduced cell proliferation through
the downregulation or upregulation of certain apoptotic proteins,
including cytochrome c and cleaved caspase-3 expression. In
addition, a number of pharmacological studies have demonstrated
crocetin to have multiple activities, including anti-inflammatory
(35,36) and anti-oxidant (37,38)
effects. Therefore, it was hypothesized that the anti-oxidant and
anti-inflammatory effects of crocetin may also be considered to be
involved in several synergistic antitumor mechanisms (39,40).
Further in vivo studies are required to investigate in
greater detail the mechanisms and pharmacokinetics of crocetin and
to provide an experimental basis for the clinical application of
the drug. In conclusion, the extant results endorse our hypothesis
that crocetin has antitumor potential and may be considered as a
novel drug candidate for the treatment of GC and to reduce
chemotherapy side effects.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 30600524 and
81071990), the Science and Technology Planning Projects of
Guangdong (project no. 2010B080701088) and the Science and
Technology Projects of Guangzhou (project no. 2011J410010).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4
|
Sun X, Mu R, Zhou YS, et al: Analysis of
mortality rate of stomach cancer and its trend in twenty years in
China. Zhonghua Zhong Liu Za Zhi. 26:4–9. 2004.(In Chinese).
|
|
5
|
Wagner AD, Grothe W, Haerting J, et al:
Chemotherapy in advanced gastric cancer: a systematic review and
meta-analysis based on aggregate data. J Clin Oncol. 24:2903–2909.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji YB, Gao SY, Ji CF and Zou X: Induction
of apoptosis in HepG2 cells by solanine and Bcl-2 protein. J
Ethnopharmacol. 115:194–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Majewska A, Hoser G, Furmanowa M, Urbańska
N, Pietrosiuk A, Zobel A and Kuraś M: Antiproliferative and
antimitotic effect, S phase accumulation and induction of apoptosis
and necrosis after treatment of extract from Rhodiola rosea
rhizomes on HL-60 cells. J Ethnopharmacol. 103:43–52. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong L, Qu G, Li P, Han J and Guo D:
Induction of apoptosis and G2/M cell cycle arrest by Gleditsioside
E from Gleditsia sinensis in HL-60 cells. Planta Med.
69:561–563. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong L, Li P, Han J, Qu G and Guo D:
Structure-activity relationships of saponins from Gleditsia
sinensis in cytotoxicity and induction of apoptosis. Planta
Med. 70:797–802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdullaev FI and Espinosa-Aguirre JJ:
Biomedical properties of saffron and its potential use in cancer
therapy and chemoprevention trials. Cancer Detect Prev. 28:426–432.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Das I, Das S and Saha T: Saffron
suppresses oxidative stress in DMBA-induced skin carcinoma: A
histopathological study. Acta Histochem. 112:317–327. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Amin A, Hamza AA, Bajbouj K, Ashraf SS and
Daoud S: Saffron: a potential candidate for a novel anticancer drug
against hepatocellular cancer. Hepatology. 54:857–867. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdullaev Jafarova F, Caballero-Ortega H,
Riverón-Negrete L, et al: In vitro evaluation of the
chemopreventive potential of saffron. Rev Invest Clin. 54:430–436.
2002.(In Spanish).
|
|
14
|
Dhar A, Mehta S, Dhar G, et al: Crocetin
inhibits pancreatic cancer cell proliferation and tumor progression
in a xenograft mouse model. Mol Cancer Ther. 8:315–323. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chryssanthi D, Dedes PG, Karamanos NK,
Cordopatis P and Lamari FN: Crocetin inhibits invasiveness of
MDA-MB-231 breast cancer cells via downregulation of matrix
metalloproteinases. Planta Med. 77:146–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Y, Chen Y, Qu R, Lan T and Sang J: Type
II cGMP-dependent protein kinase inhibits EGF-triggered signal
transduction of the MAPK/ERK-mediated pathway in gastric cancer
cells. Oncol Rep. 27:553–558. 2012.PubMed/NCBI
|
|
17
|
Yang Y, Hu Y, Gu HY, et al: Oroxylin A
induces G2/M phase cell-cycle arrest via inhibiting Cdk7-mediated
expression of Cdc2/p34 in human gastric carcinoma BGC-823 cells. J
Pharm Pharmacol. 60:1459–1463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou Z, Xie L, Wei J, et al: Synergistic
anti-proliferative effects of gambogic acid with docetaxel in
gastrointestinal cancer cell lines. BMC Complement Altern Med.
12:582012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo T, Jiang W, Kong Y, Li S, He F, Xu J
and Wang HQ: The protective effects of jatrorrhizine on β-amyloid
(25–35)-induced neurotoxicity in rat cortical neurons. CNS Neurol
Disord Drug Targets. 11:1030–1037. 2012.
|
|
20
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amin AR, Kucuk O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Verrier F, Deniaud A, Lebras M, et al:
Dynamic evolution of the adenine nucleotide translocase interactome
during chemotherapy-induced apoptosis. Oncogene. 23:8049–8064.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nair SC, Panikkar KR and Parthod RK:
Protective effects of crocetin on the bladder toxicity induced by
cyclophosphamide. Cancer Biother. 8:339–343. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferri KF and Kroemer G: Organelle-specific
initiation of cell death pathways. Nat Cell Biol. 3:E255–E263.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Slee EA, Harte MT, Kluck RM, et al:
Ordering the cytochrome c-initiated caspase cascade:
hierarchical activation of caspase-2, -3, -6, -7, -8, and -10 in a
caspase-9-dependent manner. J Cell Biol. 144:281–292.
1999.PubMed/NCBI
|
|
26
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jänicke RU, Sprengart ML, Wati MR and
Porter AG: Caspase-3 is required for DNA fragmentation and
morphological changes associated with apoptosis. J Biol Chem.
273:9357–9360. 1998.
|
|
28
|
Candé C, Cecconi F, Dessen P and Kroemer
G: Apoptosis-inducing factor (AIF): key to the conserved
caspase-independent pathways of cell death? J Cell Sci.
115:4727–4734. 2002.PubMed/NCBI
|
|
29
|
Guoan X, Hanning W, Kaiyun C and Hao L:
Adenovirus-mediated siRNA targeting Mcl-1 gene increases
radiosensitivity of pancreatic carcinoma cells in vitro and in
vivo. Surgery. 147:553–561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Halder J, Landen CN Jr, Lutgendorf SK, et
al: Focal adhesion kinase silencing augments docetaxel-mediated
apoptosis in ovarian cancer cells. Clin Cancer Res. 11:8829–8836.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manu KA, Shanmugam MK, Ramachandran L, et
al: First evidence that γ-tocotrienol inhibits the growth of human
gastric cancer and chemosensitizes it to capecitabine in a
xenograft mouse model through the modulation of NF-κB pathway. Clin
Cancer Res. 18:2220–2229. 2012.
|
|
32
|
Rasul A, Yu B, Khan M, Zhang K, Iqbal F,
Ma T and Yang H: Magnolol, a natural compound, induces apoptosis of
SGC-7901 human gastric adenocarcinoma cells via the mitochondrial
and PI3K/Akt signaling pathways. Int J Oncol. 40:1153–1161.
2012.PubMed/NCBI
|
|
33
|
Cousin FJ, Jouan-Lanhouet S,
Dimanche-Boitrel MT, Corcos L and Jan G: Milk fermented by
Propionibacterium freudenreichii induces apoptosis of HGT-1
human gastric cancer cells. PLoS One. 7:e318922012.PubMed/NCBI
|
|
34
|
Li CJ, Chu CY, Huang LH, Wang MH, Sheu LF,
Yeh JI and Hsu HY: Synergistic anticancer activity of triptolide
combined with cisplatin enhances apoptosis in gastric cancer in
vitro and in vivo. Cancer Lett. 319:203–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nam KN, Park YM, Jung HJ, et al:
Anti-inflammatory effects of crocin and crocetin in rat brain
microglial cells. Eur J Pharmacol. 648:110–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hamid A, Qian ZY, Song L and Yang R:
Crocetin ameliorates TNBS induced chronic colitis in rats by
inhibiting expression of Cyclooxygenase-2. J Nat Rem. 12:12–19.
2012.
|
|
37
|
Ohno Y, Nakanishi T, Umigai N, Tsuruma K,
Shimazawa M and Hara H: Oral administration of crocetin prevents
inner retinal damage induced by N-methyl-D-aspartate in mice. Eur J
Pharmacol. 690:84–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Yan J, Xi L, Qian Z, Wang Z and
Yang L: Protective effect of crocetin on hemorrhagic shock-induced
acute renal failure in rats. Shock. 38:63–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation, and cancer: how are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tye H, Kennedy CL, Najdovska M, et al:
STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis
independent of tumor inflammation. Cancer Cell. 22:466–478. 2012.
View Article : Google Scholar : PubMed/NCBI
|