Introduction
The fetal programming hypothesis suggests that
diseases of the fetus originate through adaptation, which occurs
with fetal undernutrition (UN). These adaptations may be vascular,
metabolic or endocrinological and permanently change the function
and structure of the body in adult life.
In a previous study, Barker et al suggested
that infants with low birth weight (LBW) had an increased risk of
developing obesity, hypertension and diabetes (1). Population studies and animal models
have revealed critical periods when offspring are most vulnerable
to environmental effects, including maternal nutritional imbalance
(2,3). Thus, fetal programming is considered
to be a potential mechanism contributing to the development of
obesity. In rats, malnutrition during select periods of pregnancy
causes LBW in newborns (4).
When newborns were fed ad libitum,
growth-restricted offspring demonstrated accelerated growth,
currently termed ‘catch-up growth’ (5), such that their body weight exceeded
that of the control groups (6–8).
Therefore, evidence exists that early postnatal growth
acceleration, which is normally considered necessary, may
exacerbate metabolic dysfunction during later life (9). Moreover, excessive food intake and
subsequent obesity increase the risk of developing chronic
diseases. Thus, fetal programming may modify appetite-regulating
hormones and neurotransmitters in undernourished newborns (9). Findings of previous studies have
revealed that serotonin (5-HT) is a neurotransmitter that also acts
as an important hormone in an increasing number of physiological
processes outside of the brain. Thus, serotonergic and dopaminergic
receptors (D) may be targets for the treatment of cognitive
deficits and feeding disorders. This vulnerability may result from
abnormalities in the development and integration of serotonergic
and dopaminergic projections during the prenatal period (10,11).
Therefore, the aim of the present study was to
determine whether prenatal UN modified the expression of the
5-HT1A, D1, D2 and Ob-Rb receptors in the hypothalamus
of adult mice.
Materials and methods
Animals
The present study utilized a model of fetal
programming via maternal malnutrition, in which 50% of food was
restricted during pregnancy to produce LBW in the offspring
(12). The protocol was approved
by the Local Animal Research Committee and was conducted in
accordance with the American Association for Accreditation of
Laboratory Care and National Institutes of Health guidelines.
Animals were assigned to one of two nutritional groups: i) Control
(C) group, fed ad libitum during gestation; and ii) UN
group, fed with a 50% food-restricted diet during the final week of
gestation. The day of birth was designated as postnatal day (P)0.
Following birth, offspring were weighed and litter sizes were
normalized to eight offspring per litter for adequate and
standardized nutrition until weaning. For the offspring study,
animals were immediately classified into either the UN group, where
mothers received the restricted diet during the last gestation week
or to the C group, as aforementioned. Mothers from UN and C pups
were fed ad libitum during lactation. Each litter from the
two groups was weighed weekly. The first weight was recorded at P0
and subsequent weights were taken at P7 and P14, until P90. In the
period following weaning, food intake was monitored in the UN and C
offspring from the post-weaning period to P90. Offspring were
sacrificed at various postnatal ages by rapid decapitation.
RNA extraction
Hypothalamic studies were performed at P0, P14 and
P90. Pups were sacrificed by decapitation and brains were rapidly
removed and blotted free of excess blood. Sections of the
hypothalamus were removed from each group, rapidly frozen in dry
ice and stored at −70°C until use for RNA extraction.
Total cellular RNA was isolated from the
hypothalamic tissue using TRIzol reagent in 100 mg tissue according
to the manufacturer’s instructions. Briefly, RNA was precipitated
from the TRIzol solution following the addition of chloroform
followed by isopropyl alcohol and then washed in 75% ethanol in
diethyl pyrocarbonate-treated water (DEPC). The ethanol was then
removed and the RNA pellets were air-dried prior to the addition of
20 μl RNase-free water. To remove contaminating DNA, the samples
were treated with DNAase. Total RNA concentration was determined
spectrophotometrically at 260/280 nm, and the isolated RNA was
stored at −70°C.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis of the Ob-Rb receptor
Purified total RNA (2 μg) was used as a template to
generate first-strand cDNA (Fermentas First-Strand cDNA kit; Thermo
Fisher Scientific, Waltham, MA, USA) which was amplified with a
specific primer for Ob-Rb receptor and tubulin using a Fermentas
Pyrostart RT-PCR kit (Thermo Fisher Scientific). The PCR mixture
contained Taq DNA polymerase and buffers (PCR amplification
buffer with 30 mM MgCl), and 10 mM dNTP, and 15 μM each of the 5′
and 3′ primers against the Ob-Rb receptor were added to cDNA
samples generated from the hypothalamus samples. Primers used for
the Ob-Rb receptor were: Forward: 5′-CCAGGTGAGGAGCAAGAGAC-3′ and
reverse: 5′-CTGCACAGTGCTTCCCACTA-3′ (product size, 470 bp);
β-tubulin, forward: 5′-TCAGCGTGGTGCCCTCAC-3′ and reverse:
5′-GTGAGCTCAGGCACCGTC-3′ (product size, 370 bp). Initial
denaturation at 95°C for 5 min was followed by 35 cycles of
denaturation for 1 min at 95°C, annealing for 1 min at 55°C and
extension for 1 min at 72°C. PCR was terminated by a final
extension at 72°C for 5 min using a Mastercycler ep gradient S
thermocycler (Eppendorf, Hauppauge, NY, USA). Subsequent assay
results were analyzed relative to a housekeeping gene (tubulin)
within the same sample to normalize for possible variations in RNA
quality, quantity and efficiency. Tubulin levels were analyzed
independently and did not vary in any of the experimental
groups.
Electrophoresis
The samples were separated in 2% agarose gels in the
presence of ethidium bromide. The optical density (OD) of bands was
measured using a Kodak Transilluminator Gel Logic 200 (Kodak,
Rochester, NY, USA). Data are presented as a ratio of leptin
receptor expression to tubulin.
Autoradiography
Animals were sacrificed and whole brains were
rapidly removed, blotted free of excess blood, rapidly frozen in
pulverized dry ice and stored at −70°C for later use. For
5-HT1A, D1 and D2 receptor autoradiography, brains were
sliced into coronal sections on a cryostat (CM 1510; Leica Camera
AG, Solms, Germany) at −20°C, each with a section thickness of 20
μm. Sections were thaw-mounted on gelatin-coated slides and stored
at −70°C in plastic bags until the day of incubation. The tissue
was rehydrated at room temperature only during ligand incubation.
Standard conditions were used, for example the concentration of
tritium ligand (equivalent to its kDa) and the concentration of
ligand for non-specific binding, temperature, incubation time and
washing time.
For autoradiography studies, the incubation
experiments consisted of tissue sections pre-incubated in Coplin
jars at room temperature in 40 ml of a solution of Tris-HCl (pH
7.4) incubation buffers. Tissue sections were then incubated in the
same buffer containing the radioligand at an adequate final
concentration. Non-specific binding was generated by the addition
of butaclamol (1 μM) for D1 and D2 and WAY100635 (10 μM) for
5-HT1A. Following incubation, the sections were washed
in ice-cold (4°C) buffer solution twice for 5 min and immediately
dipped into cold distilled water to remove any salts. Tissue
sections were dried under a gentle stream of cool air. Slides were
arrayed in X-ray cassettes together with tritium standards
(Amersham Pharmacia Biotech, Piscataway, NJ, USA) and were exposed
to tritium-sensitive film (Kodak hyperfilm; Eastman Kodak) at room
temperature for 2 or 3 months (13–15).
Films were developed and fixed at room temperature (Table I). ODs of the selected areas
appeared on autoradiograms where these were determined using a
video-computer enhancement program (Jandel video analysis software;
Jandel Scientific, Corte Madera, CA, USA) and the OD values were
transformed into receptor density values expressed as fmol/mg
protein. Results were obtained from 10 animals per group. Brain
areas and nuclei were identified using the Paxinos and Watson
atlas.
 | Table IIncubation conditions for serotonergic
and dopaminergic receptors. |
Table I
Incubation conditions for serotonergic
and dopaminergic receptors.
| Receptor | Refs | Ligand binding | Non-specific
buffer | Incubation
conditions | Pre-incubation
buffer | Incubation | Washing | Exposure time,
days |
|---|
|
5-HT1A | 14 | 2 nM
[3H]8-OH-DPAT (specific activity 106 Ci/mmol) | 10 μM WAY100635 | 0.17 M Tris-HCl, 4 mM
CaCl2 and 0.01% ascorbic acid (pH 7.4) | 30 min at 22°C | 60 min at 22°C | 2×5 min at 4°C | 60 |
| D1 | 15 | 2 nM
[3H]SCH233902 (specific activity 85 Ci/mmol) | 1 μM butaclamol | 50 mM Tris HCl, 154
mM NaCl, 1 mM EDTA and 0.1% albumine (pH 7.4) | 10 min at 22°C | 90 min at 22°C and 30
nM ketanserine | 2×5 min at 4°C | 90 |
| D2 | 13 | 0.55 nM
[3H]raclopride (specific activity 60.1 Ci/mmol) | 1 μM butaclamol | 50 mM Tris HCl, 150
mM NaCl and 0.1% ascorbic acid (pH 7.4) | 20 min at 22°C | 45 min at 22°C | 2×5 min at 4°C | 90 |
Statistical analysis
Data are presented as mean ± SEM. Differences
between groups were considered statistically significant based on
one-way ANOVA followed by a parametric multiple comparison (Tukey’s
test). Statistical analyses were performed using Prism Software
(Graph Pad Prism 5 for Windows, San Diego, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Growth
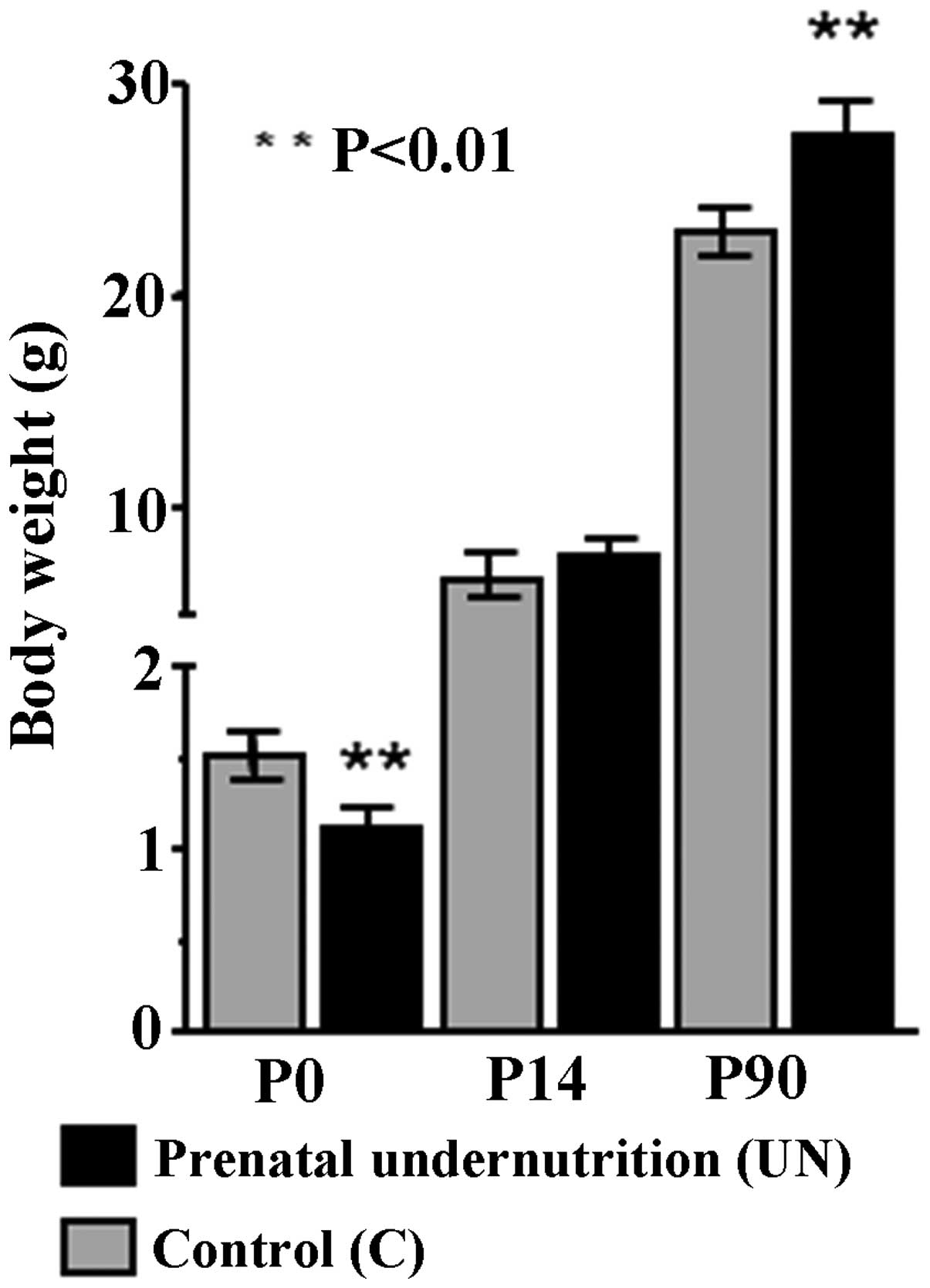
As a result of food restriction during gestation,
body weights of the UN group at birth were lower than those of the
C group (1.10±0.12 vs. 1.52±0.13 g; n=10; P<0.01) with a 17%
reduction in body weight. The subsequent growth pattern showed
‘catch-up growth’ in the UN group. Thus, although there was no
difference in body weight at P14 in the UN group compared with the
C group (7.5±0.4 vs. 6.8±0.3 g; n=10; P>0.1) (Fig. 1), offspring from the UN group
showed a significant increase in body weight compared with the C
group at P90 (UN, 28±1 g; C, 23±1.7 g; n=10; P<0.01) (Fig. 1). Weight was increased in the UN
group (>20%) compared with the C group at P90.
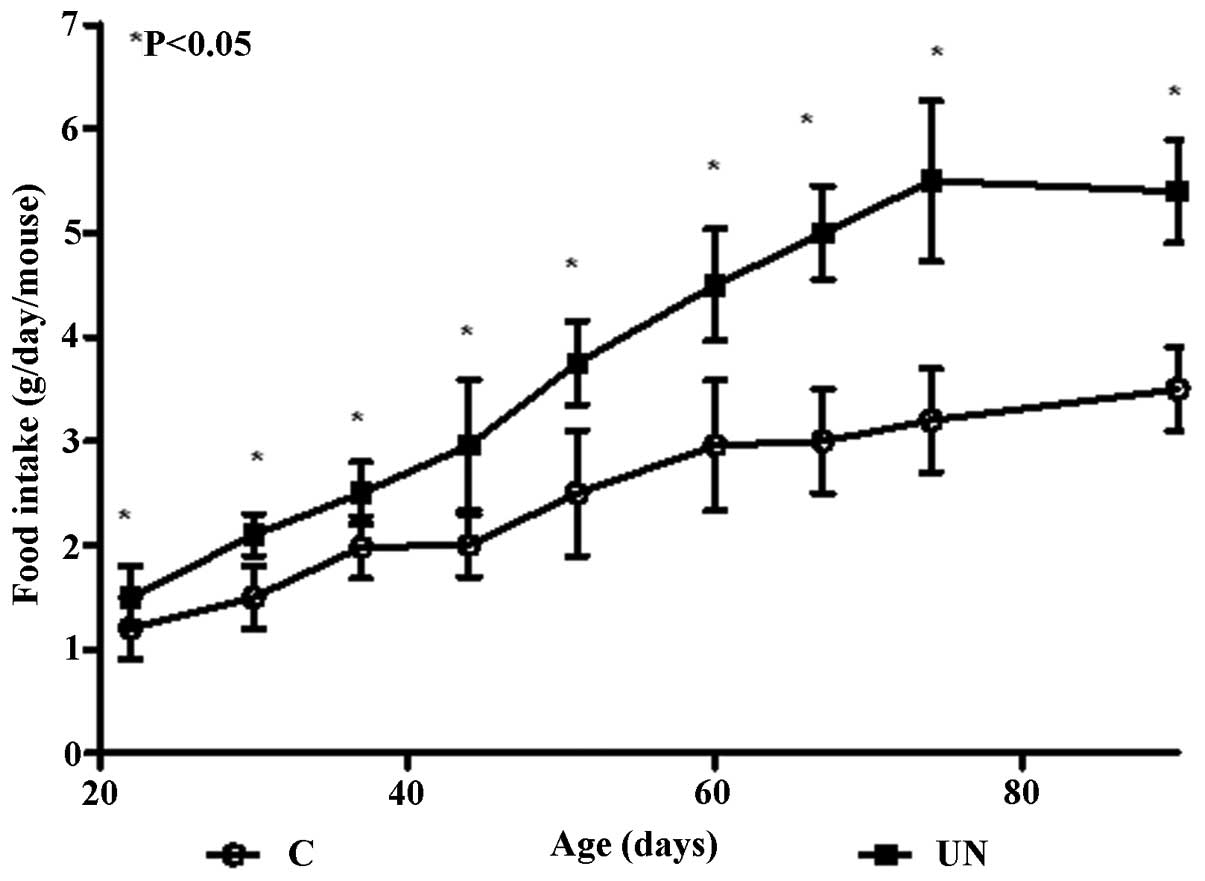
Food intake
Food intake was monitored following weaning in the
offspring from the UN group compared with the C group and increased
food intake was noted in the UN group. In early postnatal life, the
UN group continued with weight gain and this trend of hyperphagia
persisted throughout adult life. Food intake was significantly
increased in the UN group compared with the C group offspring in
adulthood at P90 (P<0.05; Fig.
2).
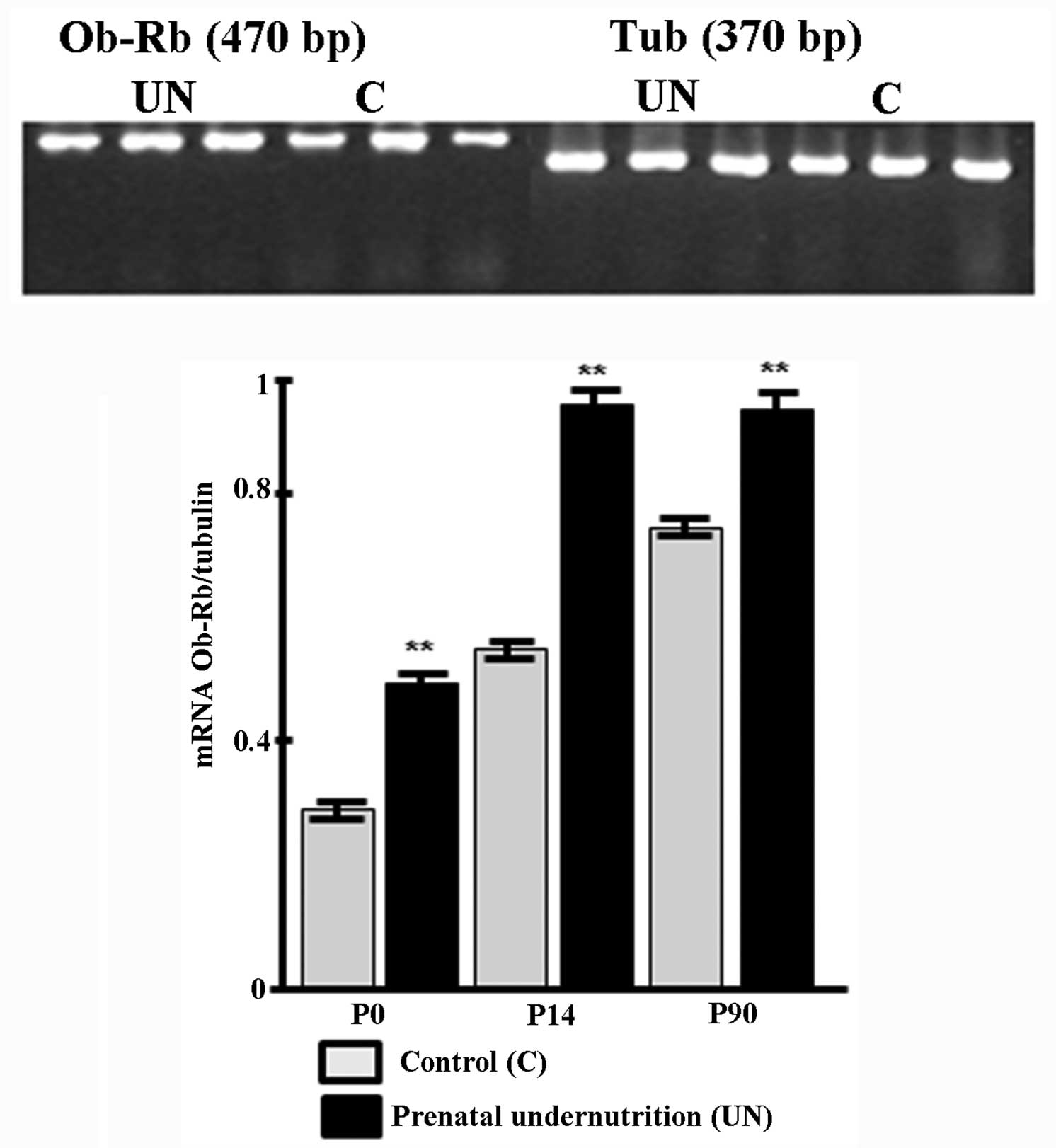
Ob-Rb leptin receptor expression
In the UN group, a significant increase in the Ob-Rb
receptor expression was observed in the hypothalamus at P14
(P<0.01) and P90 (P<0.01) (Fig.
3).
Effects of prenatal UN on
5-HT1A and D1 and D2 receptor expression in the
hypothalamus
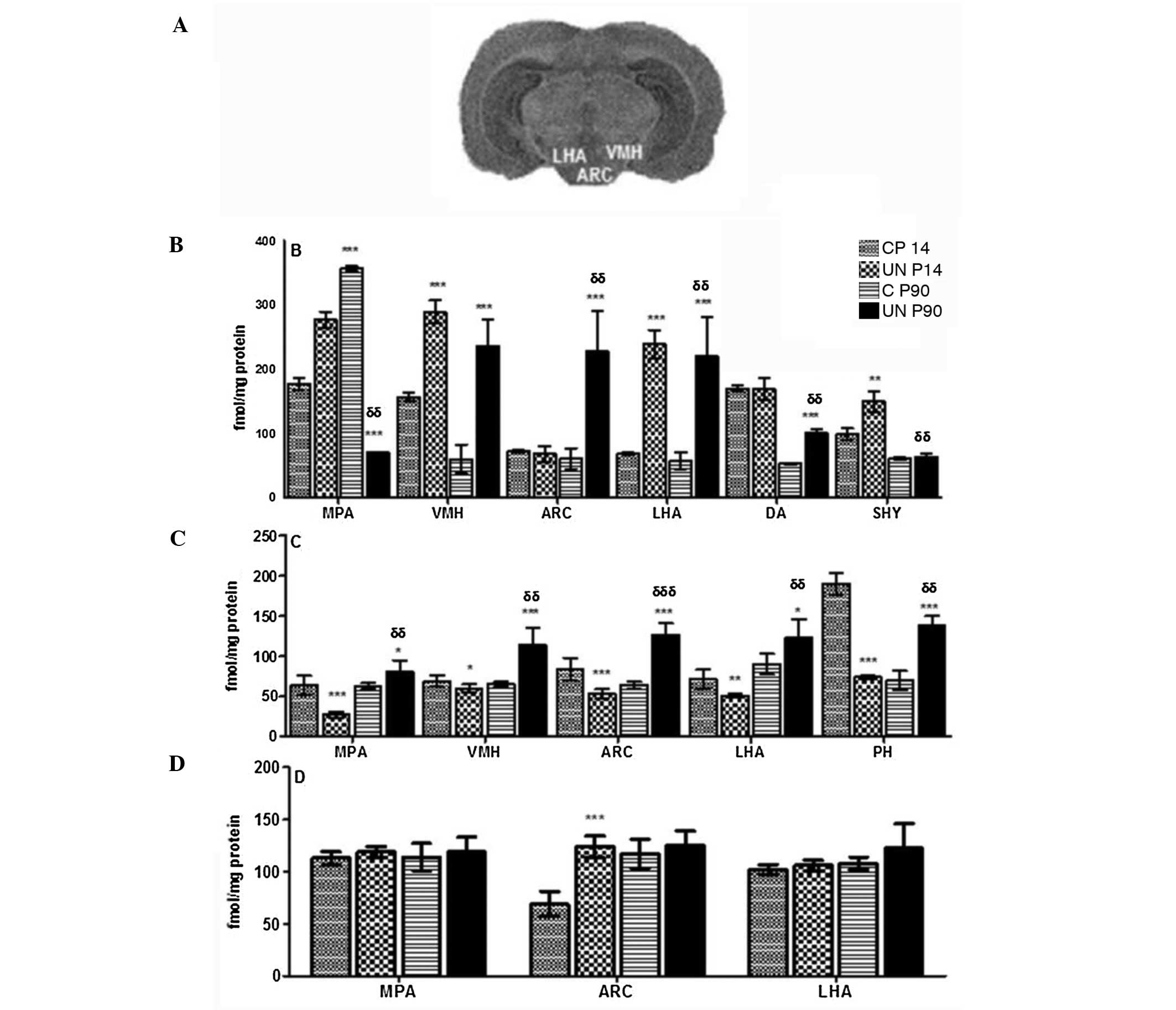
Comparison of the UN group with the C group at P14
revealed that the 5-HT1A receptor was increased in the
ventromedial nucleus of the hypothalamus (VMH; +84%; 289±18 vs.
157±7; P<0.001), in the medial preoptic area (MPA; +56%; 277±2
vs. 177±10; P<0.001) and the lateral area of the hypothalamus
(LHA; +251%; 239±22 vs. 68±2; P< 0.001). At P90, the UN group
had an increase in the dorsal hypothalamic area (+64%; 87±13 vs.
53±9; P<0.01), VMH (+293%; 236±42 vs. 60±22; P<0.001), LHA
(+279%; 220±26 vs. 58±13; P<0.001) and arcuate nucleus (ARC;
+273%; 228±62 vs. 61±16; P<0.001) (Fig. 4A and B).
In the UN group at P14, a decrease in D1 receptor
expression was observed in the MPA (−58%; 27±3 vs. 64±2;
P<0.001), VMH (−13%; 60±1 vs. 69±3; P<0.05), ARC (−37%; 53±6
vs. 84±4; P<0.001), LHA (−42%; 41±1 vs. 71±2; P<0.001) and
posterior hypothalamic area (−68%; 74±2 vs. 228±14; P<0.001) as
compared with group C, however, there was an increase at P90
(Fig. 4C). By contrast, in the UN
group at P14, there was an increase in D2 receptor expression in
the ARC (+80%; 124±1 vs. 69±2; P<0.001), although no differences
were observed at P90 (Fig.
4D).
Discussion
Results of the current study present three novel
observations. First, data demonstrate that the prenatal UN group
negatively impacted development of the offspring (UN), however,
following birth the rate and timing of postnatal catch-up growth
played critical and significant roles. Second, accelerated body
weight gain continued following weaning and was associated with
altered anorexigenic regulatory mechanisms (leptin receptor). The
data also show that developmental adaptation ensures fetal survival
of the 5HT1A, D1 and D2 receptors in the hypothalamus.
These observations emphasize the plasticity and potential of
critical appetite-regulating neurotransmitters in the pathogenesis
of fetal programming-induced obesity.
Fetal programming corresponded to an attempt of the
fetus to adapt to the adverse conditions encountered in
utero (9). These adaptations
are likely to be beneficial if the conditions prevail later in life
but become detrimental for normal or plentiful nutrition, favoring
the appearance of obesity. Furthermore, the environment encountered
during fetal life and infancy is significantly associated with the
risk of diseases in adult life (16). Thus, UN during pregnancy is
involved in the programming of offspring for the development of
obesity and diabetes (17). To
explain these causal relationships, it has been suggested that
adaptations during the critical phases of growth and development
may ensure the maintenance of homeostasis. Therefore, survival when
the environment is compromised. Studies exploring the ‘thrifty
phenotype’ hypothesis in animal models have indicated that
long-term obesity and disease risk markers may be programmed by
alterations in maternal nutrition, for example protein restriction
(18–20) or by reduced nutritional supply to
the fetus by uterine artery ligation in late pregnancy (18,19).
In addition, maternal high-fat diet consumption during gestation,
independent of obesity, increases the risk of offspring developing
behavioral disorders, including anxiety.
In pregnant mice, production of serotonin and the
expression of tryptophan hydroxylase 1 (Tph1), the rate-limiting
enzyme in the synthesis of the 5HT pathway, were highly elevated in
β-cells. Similar elevation in the expression of the Gαq protein
coupled 5HT receptor gene, Htr2b, was also noted. Moreover,
inhibition of Tph1 or Htr2b blocked normal increase of β-cell mass
during pregnancy and resulted in glucose intolerance in mice
(18,21).
Thus, current evidence has demonstrated that the
ghrelin orexigenic effect is mediated by the selective modulation
of hypothalamic fatty acid metabolism (18,22).
Moreover, ob/ob mice exhibited reductions in food intake and body
weight when treated with D1 and D2 agonists (18,23).
In the present study, exposure to maternal food
restriction during gestation resulted in LBW offspring. However,
with ad libitum feeding during early postnatal life, mice
recovered their body weight by P14. In the hypothalami, the VMH,
LHA and several other hypothalamic nuclei are well established as
centers for metabolism regulation and have potent modulator effects
on daily food intake mediated primarily via lower brainstem nuclei
(18,24–26).
In the group of UN mice in the present study, food
intake was increased and the group demonstrated hyperphagia. In
addition, the 5-HT1A receptor was elevated in VMH and
LHA at P14 and P90, but was reduced in the ARC, indicating a
decrease in 5-HT1A. This was directly associated with
negative regulation of food intake, indicating hyperphagia
associated with fetal programming. Previous studies have shown that
agents that mimic or enhance 5-HT activity produced hypophagia
(27) and weight loss, and
inhibited neuropeptide Y (NPY) neuronal activity. In addition,
drugs that block 5-HT release, stimulate feeding and NPY (28,29).
Results of this study suggest that 5-HT may
tonically inhibit NPY neurons and mediate the effects of 5-HT
serotonin on energy homeostasis in the ARC. The present study
indicates that the 5-HT1A receptor is likely to modify
the regulation between the NPY and 5-HT system in the ARC during
LBW, with an impact on adult life. Moreover, during neonatal
development, food intake must be maximized to support growth, yet
plasma leptin levels are relatively high. These high levels of
leptin during the postnatal period have been reported in rats and
mice. Similarly, Ob-Rb receptor expression was increased in the
hypothalamus. Little is known about the co-regulation of Ob-R and
Ob-Rb gene expression, receptor number or the impact of receptor
regulation on leptin sensitivity (12). In this study, Ob-Rb receptor
expression was increased with hyperphagia in mice with prenatal UN
and an increase of food intake in early postnatal life.
Previous studies (30) have shown the importance of leptin
and its association with dopamine in the modulation of food intake.
In the present study, the D1 receptor was reduced in the
hypothalamic nuclei in the UN group at P14. However, the D1
receptor at P90 was increased, which was important in ARC since
these have been involved in metabolic changes. Thus, if
dopaminergic receptor expression in the hypothalamus is controlled
by dopamine release, it is possible that upregulation of the D1
receptor mRNA in the VMH and a decrease in the LHA of obese rats
may be due to a low or high local dopamine concentration,
respectively (31,32). In addition, alterations in D2
receptor levels were compared in Zucker obese (fa/fa) and lean
(Fa/Fa) rats at 1 and 4 months of age, respectively, under two
varying feeding conditions (restricted and unrestricted food
access) using in vivo PET imaging and in vitro
autoradiography. D2 receptors were higher at 1 than at 4 months of
age and that food-restricted animals had higher D2 receptor levels
than unrestricted animals (31,32).
Thus, in the present study, increased D2 receptor expression in the
ARC at P14 indicated a correlation with catch-up growth following
prenatal UN. More studies are required to evaluate leptin effects
on brain structure, function and metabolism, and concomitantly, to
provide a solid foundation for studies aiming to assess possible
roles of leptin, 5-HT and dopamine in food intake and in UN.
In conclusion, prenatal UN during gestation has
defined time windows with long-term effects on weight gain and
metabolism. In addition, overfeeding immediately following fetal
growth retardation induces catch-up growth. Therefore, hyperphagia
resulting from early programming indicated changes in the
dopaminergic and serotonergic system that may program a state of
obesity during adulthood.
Acknowledgements
The present study was partially supported by a grant
from FIS (FIS/IMSS/PROT/G11/991). The authors thank Alberto Ramirez
for expert assistance.
References
|
1
|
Barker DJP, Hales CN, Fall CHD, Osmond C,
Phipps K and Clark PMS: Type 2 (non-insulin-dependent) diabetes
mellitus, hypertension and hyperlipidaemia (syndrome X): relation
to reduced fetal growth. Diabetologia. 36:62–67. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fontaine KR, Redden DT, Wang C, Westfall
AO and Allison DB: Years of life lost due to obesity. JAMA.
289:187–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poston L: Developmental programming and
diabetes. The human experience and insight from animal models. Best
Pract Res Clin Endocrinol Metab. 24:541–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Desai M, Gayle D, Babu J and Ross GM:
Programmed obesity in intrauterine growth-restricted newborns:
modulation by newborn nutrition. Am J Physiol Regul Integr Comp
Physiol. 288:R91–R96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hokken-Koelega AC, De Ridder MA, Lemmen
RJ, Den Hartong H, De Muinck Keizer-Schrama SM and Drop SL:
Children born small for gestation age: do they catch up? Pediatr
Res. 38:267–271. 1995. View Article : Google Scholar
|
|
6
|
Jones AP, Simson EL and Friedman MI:
Gestational undernutrition and the development of obesity in rats.
J Nutr. 114:1484–1492. 1984.PubMed/NCBI
|
|
7
|
Vickers MH, Breier BH, Cutfield WS, Hofman
PL and Gluckman PD: Fetal origin of hyperphagia, obesity, and
hypertension and postnatal amplification by hypercaloric nutrition.
Am J Physiol Endocrinol Metab. 279:E83–E87. 2000.PubMed/NCBI
|
|
8
|
Begum G, Stevens A, Smith EB, Connor K,
Challis JR, Bloomfield F and White A: Epigenetic changes in fetal
hypothalamic energy regulating pathways are associated with
maternal undernutrition and twinning. FASEB J. 26:1694–1703. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tamashiro K and Moran T: Perinatal
environment and its influences on metabolic programming of
offspring. Physiol Behav. 100:560–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berger MA, Barros VG, Sarchi MI, Tarazi FI
and Antonelli MC: Long-term effects of prenatal stress on dopamine
and glutamate receptors in adult rat brain. Neurochem Res.
27:1525–1533. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pôrto LC, Sardinha FL, Telles MM,
Guimarães RB, Albuquerque KT, Andrade IS, Oyama LM, Nascimento CM,
Santos OF and Ribeiro EB: Impairment of the serotonergic control of
feeding in adult female rats exposed to intrauterine malnutrition.
Br J Nutr. 101:1255–1261. 2009.PubMed/NCBI
|
|
12
|
Manuel-Apolinar L, Zarate A, Rocha L and
Hernández M: Fetal malnutrition affects hypothalamic leptin
receptor expression after birth in male mice. Arch Med Res.
41:240–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bauer A, Zilles K, Matusch A, Holzmann C,
Riess O and von Hörsten S: Regional and subtype selective changes
of neurotransmitter receptor density in a rat transgenic for the
Huntington’s disease mutation. J Neurochem. 94:639–650.
2005.PubMed/NCBI
|
|
14
|
Luna-Munguía H, Manuel-Apolinar L, Rocha L
and Meneses A: 5-HT1A receptors expression during memory formation.
Psychopharmacol. 181:309–318. 2005.PubMed/NCBI
|
|
15
|
Díaz-Romero M, Arias-Montaño JA, Eguibar
JR and Flores G: Enhanced binding of dopamine D1 receptors in
caudate putamen subregions in High-Yawning Sprague-Dawley rats.
Synapse. 56:69–73. 2005.PubMed/NCBI
|
|
16
|
Harder T, Rodekamp E, Schellong K,
Dudenhausen JW and Plagemann A: Birth weight and subsequent risk of
type 2 diabetes: a meta-analysis. Am J Epidemiol. 165:849–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stevens A, Begum G and White A: Epigenetic
changes in the hypothalamic pro-opiomelanocortin gene: a mechanism
linking maternal undernutrition to obesity in the offspring? Eur J
Pharmacol. 660:194–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hales CN and Barker DJP: The thrifty
phenotype hypothesis. Br Med Bull. 60:5–20. 2001. View Article : Google Scholar
|
|
19
|
Ozanne SE, Lewis R, Jennings BJ and Hales
CN: Early programming of weight gain in mice prevents the induction
of obesity by a highly palatable diet. Clin Sci (Lond).
106:141–145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jousse C, Parry L, Lambert-Langlais S,
Maurin AC, Averous J, Bruhat A, Carraro V, et al: Perinatal
undernutrition affects the methylation and expression of the leptin
gene in adults: implication for the understanding of metabolic
syndrome. FASEB J. 25:3271–3278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwak SH, Park BL, Kim H, German MS, Go MJ,
Jung HS, et al: Association of variations in TPH1 and HTR2B with
gestational weight gain and measures of obesity. Obesity (Silver
Spring). 20:233–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lage R, Vázquez MJ, Varela L, Saha AK,
Vidal-Puig A, Nogueiras R, Diéguez C and López M: Ghrelin effects
on neuropeptides in the rat hypothalamus depend on fatty acid
metabolism actions on BSX but not on gender. FASEB J. 24:2670–2679.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bina KG and Cincotta AH: Dopaminergic
agonists normalize elevated hypothalamic neuropeptide Y and
corticotropin-releasing hormone, body weight gain, and
hyperglycemia in ob/ob mice. Neuroendocrinol. 71:68–78. 2000.
View Article : Google Scholar
|
|
24
|
Schwartz MW, Woods SC, Porte D Jr, Seeley
RJ and Baskin DG: Central nervous system control of food intake.
Nature. 404:661–671. 2000.PubMed/NCBI
|
|
25
|
Davis JD and Smith GP: Learning to sham
feed: behavioral adjustments to loss of physiological
postingestional stimuli. Am J Physiol. 259:R1228–R1235.
1990.PubMed/NCBI
|
|
26
|
Smith GP: The controls of eating: a shift
from nutritional homeostasis to behavioral neuroscience. Nutrition.
16:814–820. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schellekens H, Clarke G, Jeffery IB, Dinan
TG and Cryan JF: Dynamic 5-HT2C receptor editing in a mouse model
of obesity. PLos One. 7:e322662012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dryden S, Burns SJ, Frankish HM and
Williams G: Increased hypothalamic neuropeptide Y concentration or
hyperphagia in streptozotocin-diabetic rats are not mediated by
glucocorticoids. Eur J Pharmacol. 340:221–225. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dryden S, Pickavance L, Frankish HM and
Williams G: Increased neuropeptide Y secretion in the hypothalamic
paraventricular nucleus of obese (fa/fa) Zucker rats. Brain Res.
690:185–188. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pfaffly J, Michaelides M, Wang GJ, Pessin
JE, Volkow ND and Thanos PK: Leptin increases striatal dopamine D2
receptor binding in leptin-deficient obese (ob/ob) mice. Synapse.
64:503–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sullivan EL, Smith MS and Grove KL:
Perinatal exposure to high-fat diet programs energy balance,
metabolism and behavior in adulthood. Neuroendocrinology. 93:1–8.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sullivan EL, Grayson B, Takahashi D,
Robertson N, Maier A, Bethea CL, Smit MS, Coleman K and Grove KL:
Chronic consumption of a high-fat diet during pregnancy causes
perturbations in in the serotonergic system and increased
anxiety-like behavior in nonhuman primate offspring. J Neurosci.
30:3826–3830. 2010. View Article : Google Scholar
|