Introduction
Visceral obesity and insulin resistance is
associated with a chronic low-grade inflammatory state, suggesting
that inflammation is a potential mechanism whereby obesity leads to
insulin resistance (1). Although
originally known as a passive depot for energy storage, white
adipose tissue (WAT) has now been defined as a complex and active
secretory organ that sends and receives signals that regulates
energy expenditure, insulin sensitivity and inflammation by
secreting a series of substances (2). These include tumor necrosis factor
(TNF) α, interleukin (IL) 6, leptin and adiponectin. These
adipokines are expressed in visceral adipose tissue (VAT) and are
associated with fasting glucose and insulin action (3). Consequently, inflammation within VAT
may play a key role in the development of a number of the
pathological features that results in type 2 diabetes.
Polysaccharides are members of a structurally
diverse class of macromolecules, in which polymers of
monosaccharide residues are joined to each other by glycosidic
linkages. Polysaccharides have great potential for structural
variability, as well as the necessary flexibility for the precise
regulatory mechanisms of specific cell-cell interactions and the
capacity for carrying biological information (4). Over recent decades, polysaccharides
have been shown to exert a number of biological activities,
including immunomodulatory (5),
antitumor (6), anti-inflammatory
(7) and anti-diabetic (8) activities. In addition, selenium was
shown to be important in the improvement of glucose homeostasis and
in the anti-inflammatory treatment (9,10).
Previously, we reported that oral administration of
Se-enriched exopolysaccharides (Se-ECZ-EPS) produced by
Enterobacter cloacae (E. cloacae) Z0206 (11) resulted in a reduction of blood
glucose and an improvement of serum insulin levels in diabetic mice
(12), with EPS showing
anti-inflammatory effects on broilers (13). To investigate the mechanism of the
anti-diabetic effects of EPS, the experiment was designed to
examine whether EPS exhibited any effects on adipose inflammation
and glucose uptake in high-fat-diet (HFD)-induced diabetic KKAy
mice.
Materials and methods
Materials
The Se-ECZ-EPS-producing bacterial strain E.
cloacae Z0206 was identified and collected by the China General
Microbiological Culture Collection Center (Beijing, China)
(14).
Preparation of Se-ECZ-EPS
Preparation and purification of Se-ECZ-EPS were
performed according to the previous study conducted in our
laboratory (14). Cultivation
medium containing 2.5% sucrose, 0.5% peptone, 0.5% yeast extract,
0.2% K2HPO4, 0.1%
KH2PO4 and 0.05%
MgSO4•7H2O was prepared. Exopolysaccharide
production was performed in a 10-dm3 bioreactor (Sangon
Biotech Ltd., Shanghai, China) in 7 dm3 growth volume
with stirring rate of 200 rpm at 30°C for 2 days. Concentration and
time of adding selenium into culture were optimized through
experiments. Aeration rate (1 vvm), growth temperature, foam level,
dissolved oxygen tension (DOT) and pH were measured and/or
controlled by the bioreactor control unit.
The fermentation liquid was centrifuged at 4,500 × g
to remove mycelia. The supernatant was concentrated and
precipitated with chilled 95% EtOH and maintained at 4°C overnight.
The precipitate was collected by centrifugation and freeze-dried to
yield a yellow powder. Subsequently, the collected yellow powder
was dissolved in 0.125 mol/l solution of NaOH and extracted at 60°C
for 8 h (1:1, v/v). The insoluble material was removed by
centrifugation and the supernatant was added to solid ammonium
sulfate until precipitation was observed (15). The suspension was allowed stand
overnight at 4°C and centrifuged at 7,600 × g for 20 min. The
precipitation was collected and dissolved in water and the
suspension was dialyzed against water and lyophilized to obtain
Se-ECZ-EPS (EPS).
Animals
Male KKAy and C57BL/6J mice (aged, 6 weeks) were
supplied by the Animal Experimental Center of Zhejiang University
(Hangzhou, China). The mice were housed under a controlled
environment at 22±2°C and relative air humidity of 60±10% under a
12-h light/dark cycle with free access to food and water. The
C57BL/6J mice were fed a normal chow diet, whereas the KKAy mice
were fed a high-fat diet. The experiments were approved by the
Committee of Experimental Animal Care, Zhejiang University.
Experimental design
The KKAy mice (aged, 8 weeks) were randomized into 2
groups according to fasting blood glucose values and initial body
weight. Following two weeks feeding with a high-fat diet, the KKAy
mice with significantly increased blood glucose level (≥20 mM) were
gavaged once daily with distilled water or EPS (0.2 mg/g body
weight). At the same time, the lean mice were also treated with
distilled water. Blood glucose levels were tested regularly for the
fed (tested at 8:30 a.m.) and fasted (tested at 14:30 p.m.
following 5 h fasting) mice using a One-Touch Basic Glucose Monitor
(Roche Diagnostics, Mannheim, Germany). Six weeks later, as the
blood glucose of the EPS-supplemented KKAy mice steadily dropped to
a level (≤10 mM) similar to that of the lean mice, the animals were
sacrificed following fasting for 12 h. Visceral fat was collected
and immediately frozen in liquid nitrogen and then stored at
−80°C.
Cell culture
3T3-L1 preadipocytes (obtained from the Chinese
Academy of Medical Sciences, Beijing, China) were cultured in high
glucose DMEM supplemented with 10% newborn bovine serum (growth
medium) at 37°C in a humidified atmosphere containing 5%
CO2. Confluent cells were induced by incubation in
growth medium supplemented with 1 μM insulin, 0.5 mM IBMX and 1 μM
dexamethasone for 3 days. The cells were then incubated in growth
medium containing 1 μM insulin for 3 days. The cells were
subsequently maintained in growth medium until >90% of the cells
differentiated into adipocytes.
RNA interference (RNAi)
Based on the complete sequences of mouse SirT1 and
AMPKα1 (NCBI accession nos. NM_001159589.1 and NM_001013367.3),
four potential small interference (siRNA) target sites were
determined using the Qiagen siRNA design program. These were
confirmed by BLAST for specificity. Oligonucleotides that would
produce plasmid-based siRNA were cloned into pSilencer™ 4.1-CMV neo
plasmids (Ambion, Austin, TX, USA) and all constructs were
confirmed by sequencing. The most effective target sequence
(GATGCTGTGAAGTTACTGC) of mouse SirT1 and (TATGTCTCTGGAGGAGAGC) of
mouse AMPKα1 for RNAi (SirT1-siRNA and AMPKα1-siRNA) were screened
and the RNAi conditions were optimized.
3T3-L1 adipocytes were seeded in 6-well plates. For
the SirT1 and AMPKα1 knockdown experiments, the cells were
transiently transfected with 20 μM SirT1 or AMPKα1 siRNA or
negative control siRNA using Lipofectamine™ 2000 transfection
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) as per
the manufacturer’s instructions. Following 24 h, the protein
expression of SirT1 and AMPKα1 were detected.
Cell treatment
3T3-L1 adipocytes were treated with insulin to
induce insulin resistance and inflammation according to a previous
study (16). EPS (0.1 mg/ml) was
added with 100 nM insulin to determine the effects of EPS on
insulin-induced inflammation. To confirm the effect of EPS on
inflammation, SirT1-siRNA or AMPKα1-siRNA was tranfected into the
3T3-L1 adipocytes prior to treatment with insulin and EPS.
Glucose uptake measurement
Glucose uptake assays were performed using the
glucose analog 2-[N-(7-nitrobenz-2-oxa-1, 3-diazol-4-yl)
amino]-2-deoxy-d-glucose (2-NBDG; Cayman, Ann Arbor, MI, USA), a
fluorescent indicator for direct glucose uptake, as previously
described (17). Differentiated
3T3-L1 cells were treated with vehicle or EPS (0.1 mg/ml) and 100
nM insulin in the presence or absence of 10 μM 2-NBDG. The
concentration of 2-NBDG and the time of incubation (1 h) were
selected according to previous studies (18,19).
The incubation medium was then removed and cells were washed twice
with PBS. The cells in each well were subsequently resuspended in
200 μl pre-cold growth medium and maintained at 4°C for further
analysis performed within 1 h. The fluorescence intensity of 2-NBDG
was recorded using a FACS flow cytometer (FACSCanto™ II Flow
Cytometry System; BD Biosciences, San Diego, CA, USA). To rule out
false-positives, the fluorescence intensity of cells in the absence
of 2-NBDG was measured and this value was considered as the
background level. The relative fluorescence intensities, minus the
background level, were used for data analysis.
Quantified polymerase chain reaction
(qPCR)
Total RNA was isolated using the TRIzol reagent
(Invitrogen Life Technologies) and the RNA concentration was
quantified by the NanoDrop ND-1000 spectrophotometer. A one-step
qPCR assay was employed using the SYBR Premix Ex Taq™ (Takara Bio
Inc., Otsu, Japan). TNFα, IL1β, IL6, IL10, ATGL and HSL transcripts
were quantified using qPCR technology on the LightCycle1.5
(MasterCycler EP gradient RealPlex4; Eppendorf, Hamburg, Germany).
The following primers were designed using Primer Premier 5.0 (from
5′ to 3′): TNFα forward, GCATGGTGGTGGTTGTTTCTGACGAT and reverse
GCTTCTGTTGGACACCTGGAGACA; IL1β forward, CCTAGGAAACAGCAATGGTCGGGAC
and reverse GTCAGAGGCAGGGAGGGAAACAC; IL6 forward,
GAGTCACAGAAGGAGTGGCTAAGGA and reverse CGCACTAGGTTTGCCGAGTAGATC;
IL10 forward, GGACCAGCTGGACAACATACTGCTA and reverse
CCGATAAGGCTTGGCAACCCAAGT; ATGL forward, GAGCCCCGGGGTGGAACAAGAT and
reverse AAAAGGTGGTGGGCAGGAGTAAGG and HSL forward,
GCCGGTGACGCTGAAAGTGGT and reverse CGCGCAGATGGGAGCAAGAGGT. The PCR
system consisted of 10 μl of SYBR Premix Ex Taq (2X) mix, 0.4 μl
ROX Reference Dye (50X), 1.0 μl cDNA, 7.8 μl doubled-distilled
water and 0.4 μl primer pairs (10 mM), all in a total volume of 20
μl. PCR conditions were 95°C for 30 sec, followed by 40 cycles of 5
sec at 95°C and 34 sec at 60°C. All results were normalized to the
levels of 18S rRNA and relative quantification was calculated using
the ΔΔCt formula. All samples were run in triplicate and the
average values were calculated.
Western blot analysis
Protein supernatants were run on 10% SDS acrylamide
gels and electro-blotted onto nitrocellulose membranes (Pall, Co.,
Port Washington, New York, USA). The membranes were incubated with
primary antibodies overnight at 4°C, followed by incubation with
anti-rabbit or anti-mouse IgG. Primary antibodies specific against
GAPDH, SirT1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
AMPKα1, pAMPKα1 (phospho S487) (Abcam, Cambridge, MA, USA) and
Glut4 (Signalway Antibody, College Park, MD, USA) were used.
Statistical analysis
Data are presented as the mean ± SEM. A one-way
analysis of variance (ANOVA) was used to determine whether a
significant difference was present among the treatment groups using
SPSS 13.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of a supplemented diet EPS on VAT
inflammation and lipolysis of HFD-induced diabetic KKAy mice
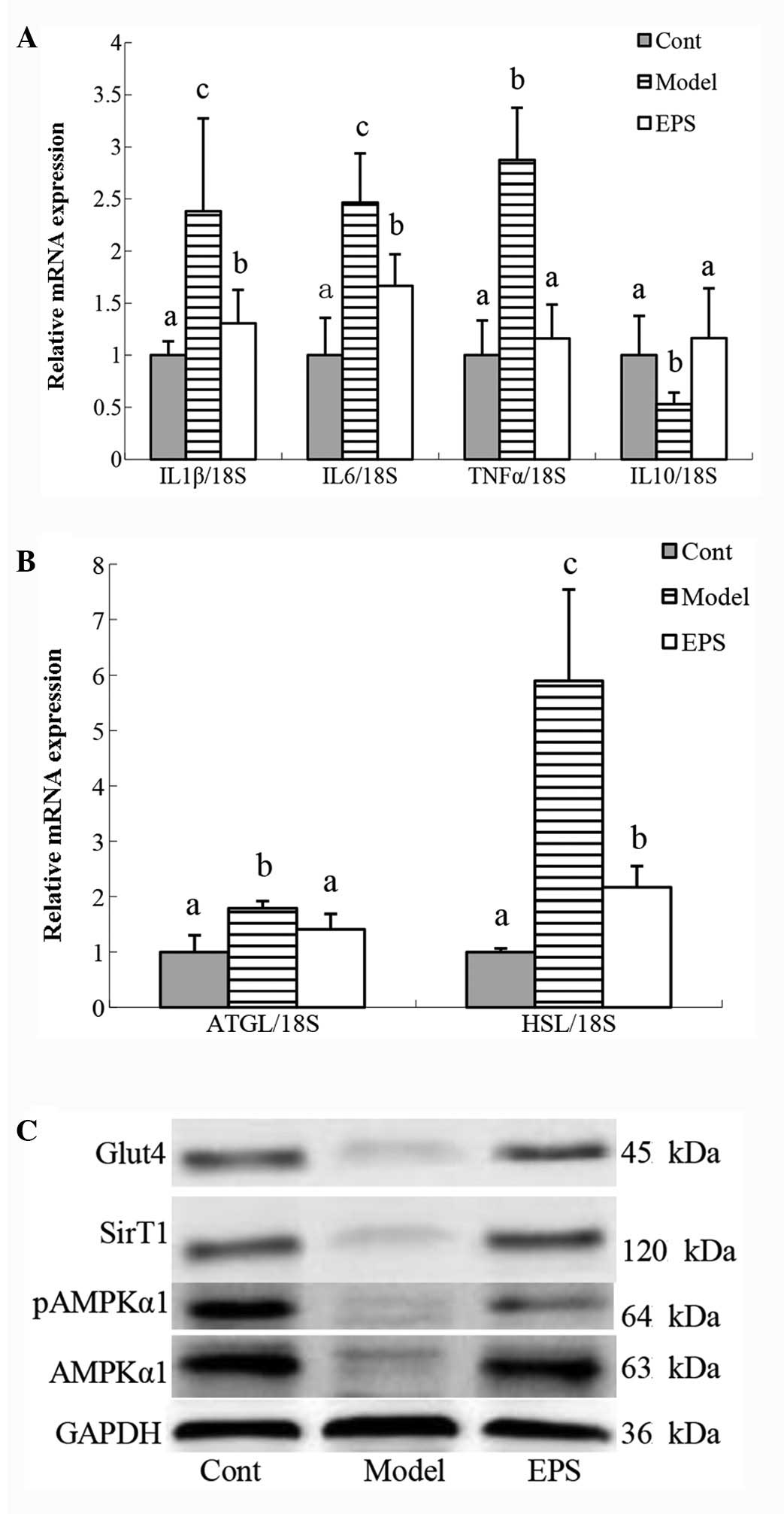
To investigate the effects of EPS on VAT
inflammation, EPS was supplemented to HFD-induced diabetic KKAy
mice. There was a significant increase in IL1β, IL6 and TNFα gene
expression in the VAT of HFD-induced diabetic mice compared with
C57BL/6J mice, while EPS supplementation significantly alleviated
this increase (Fig. 1A). Compared
with C57BL/6J mice, ATGL and HSL abundance in diabetic mice were
significantly increased, while ATGL and HSL abundance in mice
supplemented with EPS were significantly reduced compared with
diabetic mice (Fig. 1B). Diabetic
mice also showed a significantly reduced protein expression of
Glut4, SirT1, AMPKα1 and pAMPKα1, while EPS supplementation
alleviated these reductions (Fig.
1C).
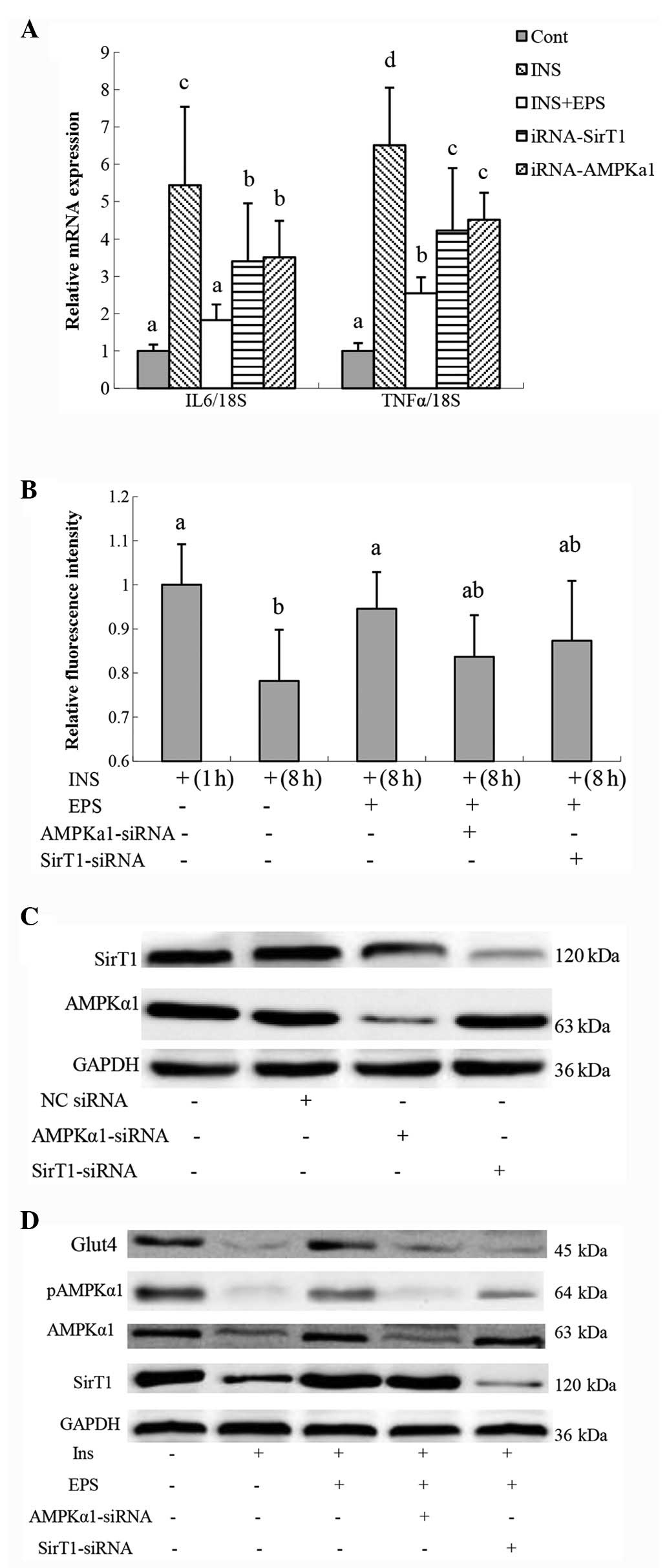
Effects of EPS on insulin-induced
inflammation and glucose uptake in 3T3-L1 cells
To induce insulin resistance, fully differentiated
3T3-L1 adipocytes were treated with insulin. IL6 mRNA expression
was significantly increased following treatment with insulin for 8
h (16). EPS was observed to
significantly reduce insulin-induced IL6 and TNFα mRNA expression
(Fig. 2A) and increased glucose
uptake (Fig. 2B). Moreover,
treatment with insulin decreased Glut4, SirT1, AMPKα1 and pAMPKα1
protein expression, while EPS was capable of counteracting the
effects of insulin (Fig. 2D).
To investigate the possible mechanisms underlying
alleviation of VAT inflammation in KKAy mice by EPS
supplementation, AMPKα1 or SirT1 were knocked down by siRNA. AMPKα1
protein expression was not affected by SirT1-siRNA and SirT1
protein expression was not affected by AMPKα1-siRNA (Fig. 2C). Following treatment with
AMPKα1-siRNA or SirT1-siRNA, IL6 and TNFα, gene expression remained
significantly reduced when compared with 3T3-L1 cells treated with
insulin. However, IL6 and TNFα gene expression was significantly
higher than 3T3-L1 cells treated with insulin and EPS.
Discussion
Local chronic inflammation within adipose tissue may
be the primary and crucial event that leads to systemic insulin
resistance and systemic inflammation (1). This inflammation is claimed to be the
connection between insulin resistance, obesity and diabetes
(20). Our previous studies have
demonstrated, that EPS has an immunomodulatory and antioxidant
effect (14,21) and notably, administration of EPS
resulted in a reduction of blood glucose levels and showed
significant anti-inflammatory and anti diabetic effects (12,13).
Thus, EPS was used as a therapeutic drug for the HFD-induced
diabetes of KKAy mice in the present experiment. Notably, the
results showed that VAT inflammation was significantly decreased
following EPS usage. EPS may alleviate inflammation primarily
through the AMPK/SirT1 pathway. The anti-inflammatory effects of
Se-ECZ-EPS are hypothesized to be the cause of the anti-diabetic
effects of EPS (22).
In the development of obesity, chronic over feeding
and glucose intake causes hypertrophy of adipocytes and
inflammatory changes at the cellular and molecular level (23–25).
TNFα and IL6 mRNA overexpression in adipocytes is associated with
an increased concentration of circulating cytokines, which may
interfere with insulin action by impairing insulin signal
transduction (20,26). These inflammatory signals may
primarily be promoted by VAT (27), since this fat depot expands in
response to chronic positive energy balance and is a more important
site for IL6 and TNFα secretion than subcutaneous fat (1,28).
Insulin resistance results in a high concentration
of free fatty acid (FFA), as the ability of insulin to inhibit
lipolysis is impaired (29). In
turn, increased concentrations of non-esterified fatty acids
released by expanded VAT negatively affects the insulin signaling
cascade (30,31). Insulin resistance also causes
reduced glucose transport into adipocytes, which may inhibit
glycerol synthesis and impair re-esterification of FFA into
triglycerides (32).
In the current study, TNFα and IL6 gene expression
was observed to significantly increase in VAT in HFD-induced
diabetes of KKAy mice, while following supplementing with EPS, the
pro-inflammatory cytokines were markedly reduced. The results
showed that EPS may alleviate the inflammatory condition in VAT.
Similarly, in the HFD-induced diabetes of KKAy mice, ATGL and HSL
gene expression was observed to be significantly increased. This
increase meant a higher lipolysis in VAT resulting from insulin
resistance. However, EPS supplementation decreased the gene
expression of the two lipolytic enzymes. This may result from
alleviation of inflammation in the VAT.
SirT1 and AMPK, as metabolic sensors, in conjunction
with PGC-1α, are crucial links in a regulatory network for nutrient
metabolic homeostasis (33). Since
chronic overnutrition leads to subclinical inflammation, which is a
characteristic of obesity and type 2 diabetes (25), there has been considerable interest
in the role of AMPK and SirT1 involved in obesity-associated
inflammation. Mounting evidence has suggested that AMPK and SirT1
have anti-inflammatory effects in adipocytes (34–36).
AMPKα1 antagonizes fatty acid-induced inflammation through SirT1
(37) and AMPK also mediates the
inhibition of nuclear factor (NF)-κB signaling through action on
its other downstream targets of PGC-1α, p53 and forkhead box O
factors (38). Activation of AMPK
may inhibit the synthesis of pro-inflammatory cytokines, including
IL6 and IL8 in adipocytes (39).
AICAR, an activator of AMPK, was also observed to reduce TNFα and
IL6 secretion in human subcutaneous adipose tissue cultured ex
vivo (40). Reduction of
adipose tissue SirT1 expression leads to ectopic inflammatory gene
expression and overexpression of SirT1 prevents HFD-induced
increases in adipose tissue inflammation (41).
In the present study, AMPKα1-siRNA or SirT1-siRNA in
3T3-L1 adipocytes in vitro were used to determine the
pathway by which EPS exerts its effects on inflammation. The
results showed that IL6 and TNFα mRNA expression were significantly
increased following induction by insulin, while IL6 and TNFα mRNA
abundance exhibited no change following treatment with insulin and
EPS. siRNA-mediated knockdown of SirT1 or AMPKα1 alone affected the
effects of EPS on inflammation, but adipocytes showed a significant
reduction in IL6 and TNFα gene expression compared with 3T3-L1
cells treated with insulin. These results demonstrated that EPS may
alleviate adipocyte inflammation predominantly through the
AMPK/SirT1 pathway. In addition, the glucose uptake and Glut4
expression were negatively associated with IL6 and TNFα abundance,
which further proved that Glut4 expression is decreased in
insulin-resistant states (32).
In conclusion, the current observations suggest that
EPS possibly exerts its anti-diabetic effect by alleviating
adipocyte inflammation via the AMPK/SirT1 pathway. EPS is composed
of glucose, mannose and galactose with α-configuration, pyranoside
and more branches (14). The
diversity of monosaccharide residues provide great flexibility in
the accurate modulatory mechanism of various cell-cell interactions
in higher organisms (42), which
may be associated with its anti-inflammatory effects. In addition,
selenium may partially exert anti-inflammatory effects, since the
organic selenium compounds, including selenoproteins, has been
hypothesized to play a preventive role in inflammatory diseases
(9). Consequently, the future
challenge may be to purify EPS and define the 3D structure of
polysaccharides and the structure-function correlation. The present
results suggest great potential for investigators to clarify the
biological activities of polysaccharides and develop clinical
application in the treatment of diabetes.
Acknowledgements
This study was financially supported by grants from
the National Basic Research Program of China (grant no.
2012CB124705) and the Modern Agro-industry Technology Research
System (no. CARS-36).
References
|
1
|
Wisse BE: The inflammatory syndrome: the
role of adipose tissue cytokines in metabolic disorders linked to
obesity. J Am Soc Nephrol. 15:2792–2800. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shoelson SE, Herrero L and Naaz A:
Obesity, inflammation, and insulin resistance. Gastroenterology.
132:2169–2180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samaras K, Botelho NK, Chisholm DJ and
Lord RV: Subcutaneous and visceral adipose tissue gene expression
of serum adipokines that predict type 2 diabetes. Obesity (Silver
Spring). 18:884–889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ooi VE and Liu F: Immunomodulation and
anti-cancer activity of polysaccharide-protein complexes. Curr Med
Chem. 7:715–729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Na HS, Lim YJ, Yun YS, Kweon MN and Lee
HC: Ginsan enhances humoral antibody response to orally delivered
antigen. Immune Netw. 10:5–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ni W, Zhang X, Wang B, Chen Y, Han H, Fan
Y, Zhou Y and Tai G: Antitumor activities and immunomodulatory
effects of ginseng neutral polysaccharides in combination with
5-fluorouracil. J Med Food. 13:270–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ananthi S, Raghavendran HR, Sunil AG,
Gayathri V, Ramakrishnan G and Vasanthi HR: In vitro antioxidant
and in vivo anti-inflammatory potential of crude polysaccharide
from Turbinaria ornata (Marine Brown Alga). Food Chem
Toxicol. 48:187–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu J, Fu J, Yuan J, Zhang N, Gao B, Fu G,
Tu Y and Zhang Y: Anti-diabetic activities of Acanthopanax
senticosus polysaccharide (ASP) in combination with metformin.
Int J Biol Macromol. 50:619–623. 2012.PubMed/NCBI
|
|
9
|
Kaur R and Sandhu HS: In vivo changes in
antioxidant system and protective role of selenium in
chlorpyrifos-induced subchronic toxicity in bubalus bubalis.
Environ Toxicol Pharmacol. 26:45–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Douillet C, Tabib A, Bost M, Accominotti
M, Borson-Chazot F and Ciavatti M: A selenium supplement associated
or not with vitamin E delays early renal lesions in experimental
diabetes in rats. Proc Soc Exp Biol Med. 211:323–331. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Yang H and Wang Y: Structure
characterization of a fucose-containing exopolysaccharide produced
by Enterobacter cloacae Z0206. Carbohydr Polym. 92:503–509.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin M, Lu Z, Huang M and Wang Y and Wang
Y: Effects of Se-enriched polysaccharides produced by
Enterobacter cloacae Z0206 on alloxan-induced diabetic mice.
Int J Biol Macromol. 50:348–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu Z, Jin M, Huang M and Wang Y and Wang
Y: Bioactivity of selenium-enriched exopolysaccharides produced by
Enterobacter cloacae Z0206 in broilers. Carbohydr Polym.
96:131–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu CL, Wang YZ, Jin ML and Yang XQ:
Preparation, characterization and immunomodulatory activity of
selenium-enriched exopolysaccharide produced by bacterium
Enterobacter cloacae Z0206. Bioresour Technol.
100:2095–2097. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rigas DA and Osgood EE: Purification and
properties of the phytohemagglutinin of Phaseolus vulgaris.
J Biol Chem. 212:607–615. 1955.PubMed/NCBI
|
|
16
|
Fasshauer M, Klein J, Lossner U and
Paschke R: Interleukin (IL)-6 mRNA expression is stimulated by
insulin, isoproterenol, tumour necrosis factor alpha, growth
hormone, and IL-6 in 3T3-L1 adipocytes. Horm Metab Res. 35:147–152.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshioka K, Takahashi H, Homma T, Saito M,
Oh KB, Nemoto Y and Matsuoka H: A novel fluorescent derivative of
glucose applicable to the assessment of glucose uptake activity of
Escherichia coli. Biochim Biophys Acta. 1289:5–9. 1996.
View Article : Google Scholar
|
|
18
|
Ball SW, Bailey JR, Stewart JM, Vogels CM
and Westcott SA: A fluorescent compound for glucose uptake
measurements in isolated rat cardiomyocytes. Can J Physiol
Pharmacol. 80:205–209. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou C, Wang Y and Shen Z: 2-NBDG as a
fluorescent indicator for direct glucose uptake measurement. J
Biochem Biophys Methods. 64:207–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dandona P, Aljada A and Bandyopadhyay A:
Inflammation: the link between insulin resistance, obesity and
diabetes. Trends Immunol. 25:4–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin M, Lu Z, Huang M and Wang Y and Wang
Y: Sulfated modification and antioxidant activity of
exopolysaccahrides produced by Enterobacter cloacae Z0206.
Int J Biol Macromol. 48:607–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bijland S, Mancini SJ and Salt IP: Role of
AMP-activated protein kinase in adipose tissue metabolism and
inflammation. Clin Sci (Lond). 124:491–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Esposito K, Nappo F, Marfella R, Giugliano
G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A and Giugliano D:
Inflammatory cytokine concentrations are acutely increased by
hyperglycemia in humans: role of oxidative stress. Circulation.
106:2067–2072. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mohanty P, Hamouda W, Garg R, Aljada A,
Ghanim H and Dandona P: Glucose challenge stimulates reactive
oxygen species (ROS) generation by leucocytes. J Clin Endocrinol
Metab. 85:2970–2973. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Glass CK and Olefsky JM: Inflammation and
lipid signaling in the etiology of insulin resistance. Cell Metab.
15:635–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang W, Zhang X, Wang H, Guo X, Li H,
Wang Y, Xu X, Tan L, Mashek MT, Zhang C, Chen Y, Mashek DG, Foretz
M, Zhu C, Zhou H, Liu X, Viollet B, Wu C and Huo Y: AMP-activated
protein kinase α1 protects against diet-induced insulin resistance
and obesity. Diabetes. 61:3114–3125. 2012.
|
|
27
|
Fontana L, Eagon JC, Trujillo ME, Scherer
PE and Klein S: Visceral fat adipokine secretion is associated with
systemic inflammation in obese humans. Diabetes. 56:1010–1013.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Winkler G, Kiss S, Keszthelyi L, Sápi Z,
Ory I, Salamon F, Kovács M, Vargha P, Szekeres O, Speer G, Karádi
I, Sikter M, Kaszás E, Dworak O, Gerö G and Cseh K: Expression of
tumor necrosis factor (TNF)-alpha protein in the subcutaneous and
visceral adipose tissue in correlation with adipocyte cell volume,
serum TNF-alpha, soluble serum TNF-receptor-2 concentrations and
C-peptide level. Eur J Endocrinol. 149:129–135. 2003.
|
|
29
|
Groop LC, Saloranta C, Shank M, Bonadonna
RC, Ferrannini E and DeFronzo RA: The role of free fatty acid
metabolism in the pathogenesis of insulin resistance in obesity and
noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab.
72:96–107. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ravussin E and Smith SR: Increased fat
intake, impaired fat oxidation, and failure of fat cell
proliferation result in ectopic fat storage, insulin resistance,
and type 2 diabetes mellitus. Ann N Y Acad Sci. 967:363–378. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rajala MW and Scherer PE: Minireview: the
adipocyte - at the crossroads of energy homeostasis, inflammation,
and atherosclerosis. Endocrinology. 144:3765–3773. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abel ED, Peroni O, Kim JK, Kim YB, Boss O,
Hadro E, Minnemann T, Shulman GI and Kahn BB: Adipose-selective
targeting of the GLUT4 gene impairs insulin action in muscle and
liver. Nature. 409:729–733. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cantó C and Auwerx J: PGC-1alpha, SIRT1
and AMPK, an energy sensing network that controls energy
expenditure. Curr Opin Lipidol. 20:98–105. 2009.PubMed/NCBI
|
|
34
|
Salt IP and Palmer TM: Exploiting the
anti-inflammatory effects of AMP-activated protein kinase
activation. Expert Opin Investig Drugs. 21:1155–1167. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yeung F, Hoberg JE, Ramsey CS, Keller MD,
Jones DR, Frye RA and Mayo MW: Modulation of NF-kappaB-dependent
transcription and cell survival by the SIRT1 deacetylase. EMBO J.
23:2369–2380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ghosh HS, Spencer JV, Ng B, McBurney MW
and Robbins PD: Sirt1 interacts with transducin-like enhancer of
split-1 to inhibit nuclear factor kappaB-mediated transcription.
Biochem J. 408:105–111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Z, Kahn BB, Shi H and Xue BZ:
Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK)
antagonizes fatty acid-induced inflammation through SIRT1. J Biol
Chem. 285:19051–19059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Salminen A, Hyttinen JM and Kaarniranta K:
AMP-activated protein kinase inhibits NF-κB signaling and
inflammation: impact on healthspan and lifespan. J Mol Med (Berl).
89:667–676. 2011.
|
|
39
|
Lihn AS, Pedersen SB, Lund S and Richelsen
B: The anti-diabetic AMPK activator AICAR reduces IL-6 and IL-8 in
human adipose tissue and skeletal muscle cells. Mol Cell
Endocrinol. 292:36–41. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lihn AS, Jessen N, Pedersen SB, Lund S and
Richelsen B: AICAR stimulates adiponectin and inhibits cytokines in
adipose tissue. Biochem Biophys Res Commun. 316:853–858. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gillum MP, Kotas ME, Erion DM, Kursawe R,
Chatterjee P, Nead KT, Muise ES, Hsiao JJ, Frederick DW, Yonemitsu
S, Banks AS, Qiang L, Bhanot S, Olefsky JM, Sears DD, Caprio S and
Shulman GI: SirT1 regulates adipose tissue inflammation. Diabetes.
60:3235–3245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang M, Cui SW, Cheung PCK and Wang Q:
Antitumor polysaccharides from mushrooms: a review on their
isolation process, structural characteristics and antitumor
activity. Trends Food Sci Tech. 12:4–19. 2008.
|