Introduction
The activated form of vitamin D,
1,25-dihydroxyvitamin D3 [1,25(OH)2D3] has, in addition
to its central function in calcium and bone metabolism (1), important effects on the growth and
differentiation of a number of cell types, and immunoregulatory
properties. The biological effects of 1,25(OH)2D3 are
mediated by the vitamin D receptor (VDR), a member of the
superfamily of nuclear hormone receptors functioning as a
ligand-activated transcription factor that binds to specific DNA
sequence elements in vitamin D responsive genes and affects their
rate of RNA polymerase II-mediated transcription (2). A number of studies have demonstrated
an immunoregulatory effect for this steroid hormone (3–7).
Antigen presenting cells (APCs), in particular DCs, express the VDR
and are key targets of VDR agonists. Numerous studies have
demonstrated that 1,25(OH)2D3 inhibits the
differentiation and maturation of DCs (8–12).
These studies have shown that 1,25(OH)2D3-treated DCs
downregulated expression of the co-stimulatory molecules CD40,
CD80, CD86 and MHC class II, decreased IL-12 and enhanced IL-10
production, resulting in decreased T-cell activation in
vitro.
Besides the co-stimulatory molecules, inhibitory
molecules are crucial to the tolerogenic capacity acquired by DCs.
Members of the B7 family, programmed death ligand-1 (PD-L1) and
PD-L2 have been found to be important in DC-mediated immune
tolerance (13). In addition,
PD-L1 signaling regulates the generation of
CD4+Foxp3+ regulatory T cells (Treg)
(14). Chen et al (15) found that PD-L1 and PD-L2 contribute
to the poor stimulatory capacity of immature DCs (imDCs) via
engagement of the inhibitory PD-1. However, PD-1 ligand blockade
could not endow the same stimulatory capacity of imDCs as mature
DCs (mDCs), which implied that other negative molecules could be
involved in this process.
Herpesvirus entry mediator (HVEM), also known as
tumor necrosis factor receptor superfamily, member 14 (TNFRSF14),
is a member of the TNFR, is expressed by several types of cells,
including T cells, B cells and DCs (16–18),
can regulate the differentiation of T cells and DCs. HVEM can also
regulate DC-mediated T-cell immune responses. Certain studies have
demonstrated that HVEM−/− mice were more susceptible to
autoimmune diseases and APCs from HVEM−/− mice were more
active in stimulating T cells compared with those from wild type
(WT) mice (19). HVEM
overexpression on APCs inhibited ovalbumin (OVA) peptide-dependent
T-cell proliferation (20). HVEM
overexpression on DCs produced a regulatory cytokine, IL-10, which
had further effects on the induction of IL-10 producing
CD4+ T cells (21).
These findings supported the role of HVEM as an inhibitor molecule
involved in DC-mediated immunological tolerance. However, the
expression of HVEM on tolerogenic dendritic cells (tDCs) induced by
VDR agonists remain incompletely characterized. Therefore, the
expression of inhibitory molecules HVEM, PD-L1 and PD-L2 on
1,25(OH)2D3-treated mouse bone marrow-derived DCs was
analyzed.
The data of the present study verified that the
upregulated expression of the inhibitory molecule HVEM in
1,25(OH)2D3-treated DCs induced
CD4+CD25+Foxp3+ Treg and arrested
allogeneic CD4+ T-cell proliferation. All these results
further support the critical role of the inhibitory molecule HVEM
in DC-mediated immune tolerance.
Materials and methods
Experimental animals
Female C57BL/6J and Balb/c mice were used at ages
6–8 weeks (Chongqing Experimental Animal Co., Chongqing, China).
All the mice were bred under specific pathogen-free conditions. All
the experiments were approved by an Ethics Committee (The Ethics
Committee, Xinqiao Hospital, Third Military Medical University,
Jiangyin, Jiangsu, China).
Preparation of DCs from mouse bone
marrow
Murine bone marrow-derived DCs were prepared as
described previously with minor modifications. Briefly, bone marrow
mononuclear cells were prepared from C57BL/6 mouse tibia and femur
suspensions by depletion of red cells and cultured at a density of
2×106 per well in six-well plates in RPMI-1640 medium
supplemented with 10% fetal calf serum and 10 ng/ml recombinant
murine granulocyte/macrophage colony-stimulating factor and 1 ng/ml
recombinant murine IL-4 (22).
Non-adherent cells were gently washed out on the third day of
culture; the remaining loosely adherent clusters were cultured for
a further 4 days as non-treated (NT) DCs. To produce the different
subsets of DCs, imDCs were harvested on day 5 or 6 of culture.
1,25(OH)2D3-treated DCs (D3/imDCs) were generated by
adding 10−8 1,25(OH)2D3 on day 3 of NT-DCs
culture and harvested on day 8. OVA-D3/imDCs were generated by
adding 100 μg/ml OVA on day 7 of the D3/imDCs culture for 24 h and
harvested on day 8. Mature DCs (OVA/imDCs) was generated by adding
100 μg/ml OVA on day 7 of the NT-DCs culture for 24 h and harvested
on day 8. DCs prepared in this manner consisted of ~80%
CD11c+ cells, however, higher purities were required
thus the cells were sorted on CD11c+ by MACS beads
(Miltenyi Biotec, Germany) to yield ~90% purity.
Flow cytometric analysis
DCs were triple-stained with APC-anti-CD11c and
either PE-CD80, -MHC class II, -HVEM (CD270), -PDL1 (CD274) or
FITC-CD86, -CD40, -PDL2 (CD273) for phenotypic analysis. The
incidences of positive cells were determined by flow cytometry
using a FACS Calibur (BD Biosciences, San Jose, CA, USA).
Appropriately conjugated isotype-matched control antibodies were
used as negative controls.
Mixed lymphocyte reaction
DCs
Varying numbers of mitomycin C (25 mg/ml)-treated
DCs were seeded in triplicate in a flat-bottom 96-well plate
(Corning, Tewksbury, MA, USA) for use as stimulator cells. T cells
were prepared from spleens and isolated by CD4+ T cell
magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany),
and the purity of the CD4+ T cells was >95%,
according to the manufacturer’s instructions. In total,
1×105/well of T cells from BALB/c mice were added to the
DC cultures, with the final MLR occurring in 200 μl of RPMI-1640
medium (Life Technologies, Grand Island, NY, USA) supplemented with
10% FCS (Life Technologies), 100 U/ml penicillin and 100 μg/ml
streptomycin (both from Life Technologies). The cells were cultured
at 37ºC in a humidified atmosphere of 5% CO2 for 4 days,
and pulsed with 1 μCi [3H] thymidine (Amersham Pharmacia
Biotech, Piscataway, NJ, USA) for the last 18 h of the culture. The
cells were harvested onto glass fiber filters, and the
radioactivity incorporated was quantitated using a Beckman liquid
scintillation counter (Beckman Coulter, Miami, FL, USA). The
results were expressed as the mean cpm of triplicate cultures ±
standard error of the mean.
In vitro differentiation assessment of
CD4+CD25+Foxp3+ Treg
Naïve CD4+CD25-T cells were purified from
Balb/c spleen cells by magnetic-activated cell sorting (MACS)
according to the manufacturer’s instructions (Miltenyi Biotec). In
total, 1×106 allogeneic CD4+ CD25−
T cells were cocultured with mitomycin C (25 mg/ml)-treated
1×105 OVA/imDCs and OVA-D3/imDCs for 96 h. These
CD4+-T cells were then surface stained with
FITC-anti-CD4 and PE-anti-CD25 mAbs and resuspended in Fix/Perm
buffer (eBioscience, San Diego, CA, USA). Intracellular staining
with PEcy5-anti-Foxp3 mAb (eBioscience) and estimation of the
incidence of CD4+CD25+Foxp3+ Treg
was then determined by flow cytometry.
Enzyme-linked immunosorbent assay
Different subsets of DCs or DC coculture with naïve
CD4+CD25−-T cells supernatants were stored at
−80ºC. The levels of IL-2, IL-6 and IL-10 were measured using ELISA
kits (Cusabio Biotech Co., Ltd., Hubei, China), according to the
manufacturer’s instructions. The sensitivity limits for IL-2, IL-6
and IL-10 were 3.9 pg/ml, 0.39 and 0.8 pg/ml, respectively.
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical comparisons were performed using analysis of
variance and the χ2 test. P<0.05 was used to indicate
a statistically significant difference. Data analyses were
performed using SPSS version 13.0 (SPSS, Inc., Chicago, IL,
USA).
Results
1,25(OH)2D3-treated bone
marrow-derived dendritic cells exhibit features of tolerogenic
DCs
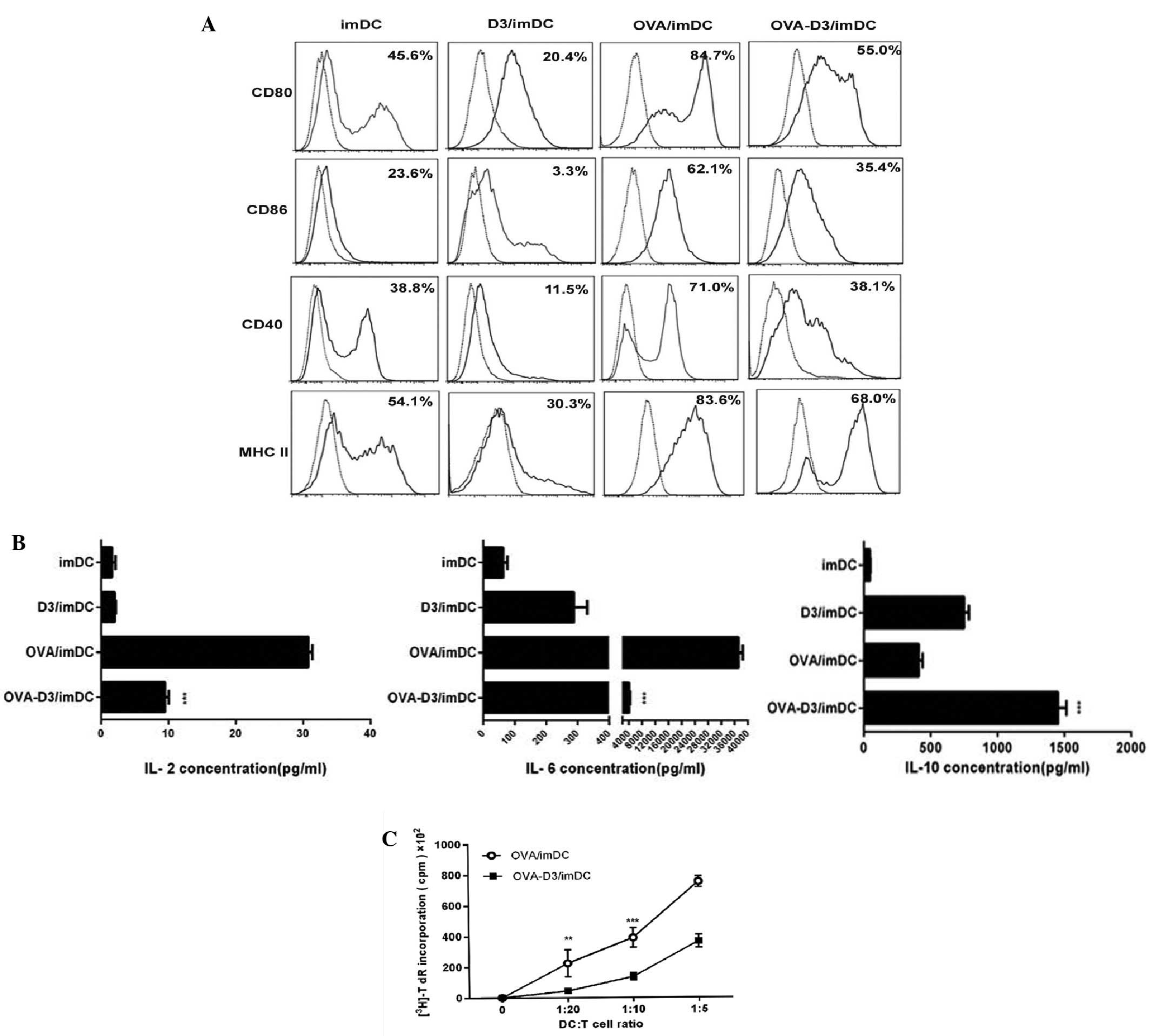
The phenotype and T-cell stimulatory capacity of
1,25(OH)2D3-treated BMDCs activated by OVA
(OVA-D3/imDCs) were analyzed and compared with those of mature DCs
(OVA/imDCs). 1,25(OH)2D3-treated DCs (D3/imDCs)
demonstrated immature phenotype, which downregulated the expression
of the co-stimulatory molecules (CD80,CD86 and CD40) and MHC class
II compared with those of immature DCs (all P<0.001). Upon
activation by OVA, D3/imDCs demonstrated a semi-mature phenotype,
with higher expression of co-stimulatory molecules CD86 and MHC
class II compared with those of imDCs (all P<0.05), but lower
than OVA/imDCs (all P<0.001) (Fig.
1A). This indicates that 1,25(OH)2D3 inhibits DC
maturation. Next, the cytokine production pattern of the
OVA-D3/imDCs was examined. D3/imDCs secreted low levels of IL-2,
IL-6 and intermediate levels of IL-10. Upon activation by OVA,
D3/imDCs produced more IL-10 and less IL-2 and IL-6 compared with
those produced by mature DCs (all P<0.001) (Fig. 1B). These results indicate that
1,25(OH)2D3 inhibits the secretion of pro-inflammatory
cytokines but promotes the secretion of anti-inflammatory
cytokines. OVA-D3/imDCs exhibited a semi-mature phenotype and
promoted secretion of anti-inflammatory cytokines. We speculated
that OVA-D3/imDCs would be less capable of allogeneic
CD4+ T-cell stimulation. Indeed, OVA-D3/imDCs
demonstrated poor capacity to stimulate CD4+ T-cell
proliferation compared with OVA/imDCs at different DC:T cell ratio
in a primary allogeneic MLR (P<0.01 or 0.001; Fig. 1C).
All these results support that
1,25(OH)2D3-treated dendritic cells possess tolerogenic
phenotypes.
1,25(OH)2D3-treated bone
marrow-derived dendritic cells promote expansion of
CD4+CD25+Foxp3+ Treg
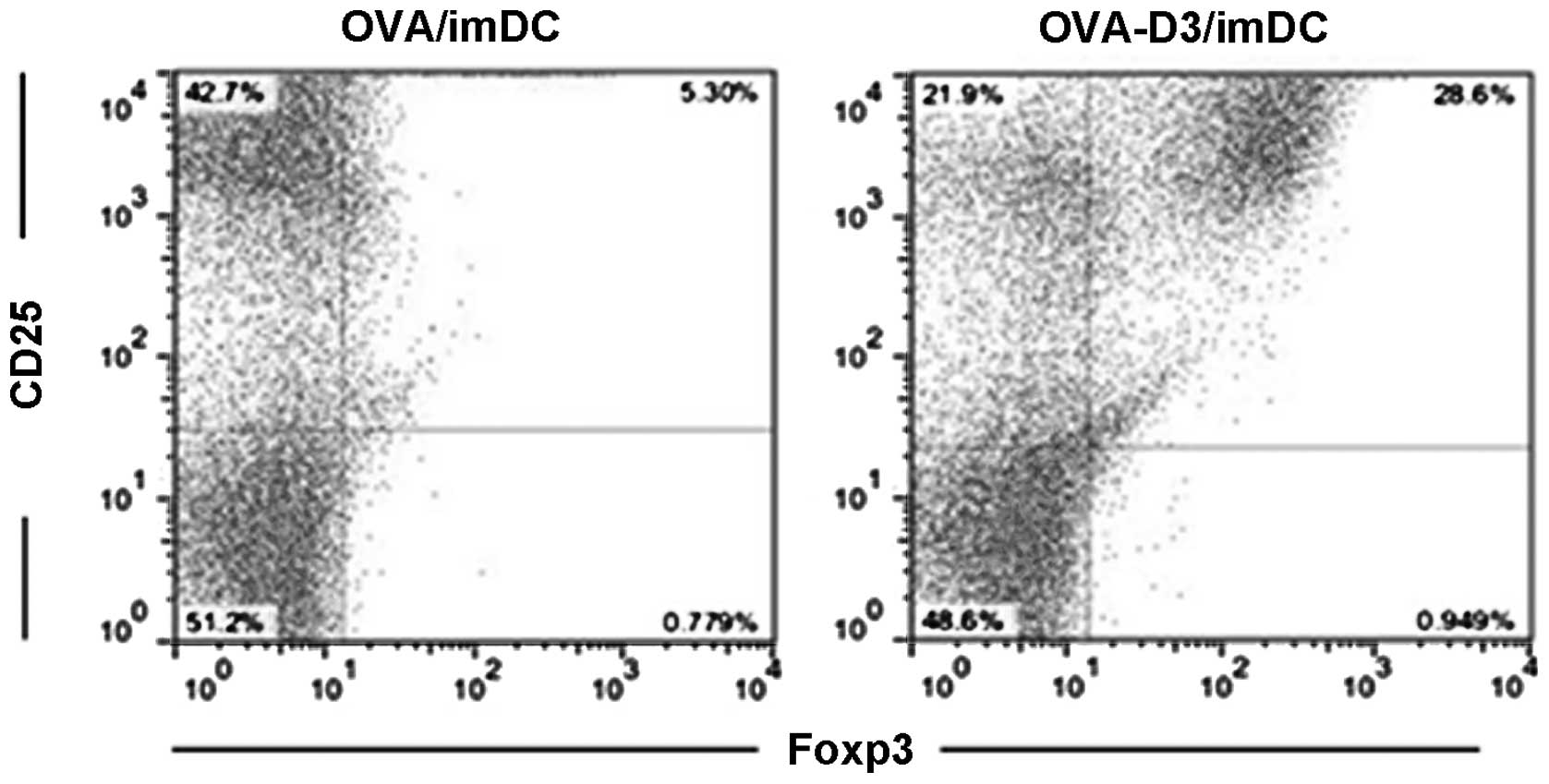
1,25(OH)2D3-treated BMDCs exhibit
features of tolerogenic DCs. Next, whether the tolerogenic DCs may
be correlated with specific interactions with
CD4+CD25+Foxp3+ Treg was
investigated. OVA-D3/imDCs or OVA/imDCs were cultured with
CD4+CD25−-T cells for 96 h, and then the
percentage of CD4+CD25+Foxp3+ Treg
was analyzed by flow cytometry. As expected, a significantly high
incidence of CD4+CD25+Foxp3+ Treg
and lower incidence of
CD4+CD25+Foxp3− effector-T cells
(Teff) were detected from OVA-D3/imDC cultures compared with those
of OVA/imDCs (Fig. 2). This
indicates that 1,25(OH)2D3-treated dendritic cells
promote expansion of
CD4+CD25+Foxp3+ Treg and inhibit
CD4+CD25+Foxp3+ Teff
proliferation.
1,25(OH)2D3 induces the
expression of the inhibitory molecules HVEM and PD-L1 on bone
marrow-derived dendritic cells
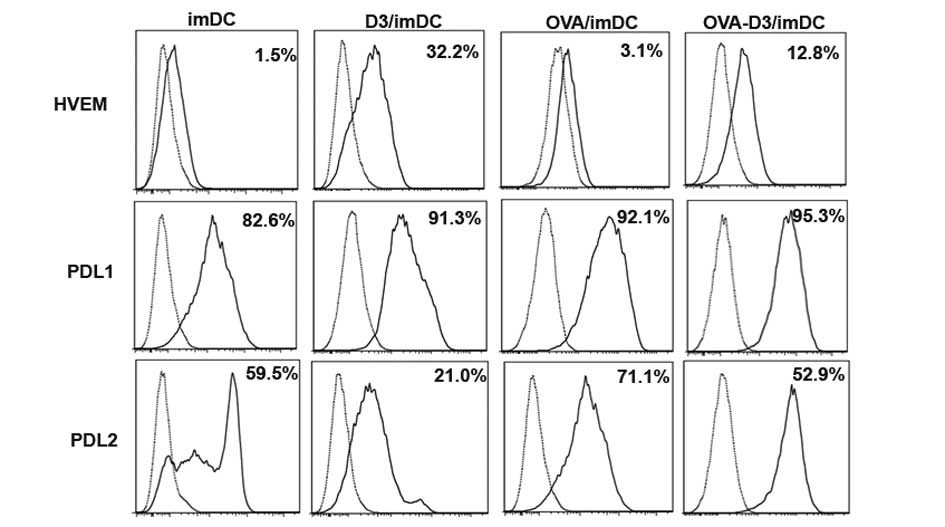
Inhibitory molecules HVEM, PD-L1, PD-L2 are known to
negatively regulate T-cell responses (19,23,24).
Therefore, the expression of HVEM, PD-L1 and PDL2 on
1,25(OH)2D3-treated dendritic cells (D3/imDCs) were
investigated by flow cytometric analysis. D3/imDCs induced up to
21.0-fold upregulation of HVEM expression and 1.1-fold upregulation
of PD-L1 expression. OVA-D3/imDCs led to a 4.1-fold enhancement of
HVEM expression and a slight 1.0-fold upregulation of PD-L1
expression (all P<0.001). Conversely, D3/imDCs led to 2.8-fold
downregulation of PD-L2 expression and OVA-D3/imDCs led to 1.5-fold
downregulation of PD-L2 expression, respectively (Fig. 3). These data implied that HVEM, but
not PD-L2, is involved in tDCs induced by VDR agonists and promotes
production of Treg.
Discussion
In the present study, 1,25(OH)2D3-treated
bone marrow-derived dendritic cells were verified to be tolerogenic
DCs, which induced Treg generation and inhibited allogeneic
CD4+ T-cell proliferation. In addition,
1,25(OH)2D3 was observed to markedly upregulate the
expression of the inhibitory molecule HVEM, which may be associated
with tolerogenic DCs.
DCs not only initiate T-cell responses but are also
involved in the silencing of T-cell immune responses. Mature DCs
induce differentiation of effector T cells (Th1, Th2) (25). Immature and semi-mature DCs appear
to induce the differentiation of Treg and thus promote tolerance
(26). The data of the present
study confirmed the findings of previous studies (8–9)
revealing that 1,25(OH)2D3-treated DCs significantly
downregulated the co-stimulatory molecules CD86, CD80, CD40 and MHC
II, which show an immature phenotype. Upon activation by OVA,
1,25(OH)2D3-treated DCs show a semi-mature phenotype,
with higher expression of co-stimulatory molecule CD86, and MHC
class II and decreased IL-2, IL-6 and enhanced IL-10 production.
Furthermore, 1,25(OH)2D3-treated DCs demonstrate a poor
capacity to stimulate CD4+ T-cell proliferation in a
primary MLR as previous studies have proved (10,27,28).
Indeed, 1,25(OH)2D3-treated DCs converted naïve
CD4+ T cells into Foxp3+ Treg and suppressed
allogeneic CD4+ T-cell proliferation. All these results
exhibited the tolerogenic properties of
1,25(OH)2D3-treated dendritic cells.
Besides the co-stimulatory molecules, it is observed
that inhibitory molecules, including PD-L1 and PD-L2 (members of
the B7 family) are involved in DC-mediated immune tolerance
(15,29). Unger et al (30) found PD-L1 was significantly
upregulated on 1,25(OH)2D3-treated DCs and regulated the
generation of Treg, as the blockade of PD-L1 eradicated the
suppressive capacity of Treg. This indicates that the inhibitory
molecule is critical for tDCs in the generation of Treg induced by
1,25(OH)2D3. However, the results of the present study
identified that PD-L1 was hardly affected by 1,25(OH)2D3
and PD-L2 was downregulated on 1,25(OH)2D3-treated DCs,
which implied that other negative molecules could be involved in
this process.
HVEM, also known as TNFRSF14, is a member of the TNF
family and their specific receptors (TNFR), which as an inhibitory
molecule is also involved in DC-mediated immune tolerance. Studies
have found that HVEM−/− mice were more susceptible to
autoimmune diseases and APCs in HVEM−/− mice were more
active in stimulating T cells compared with WT mice (15). HVEM overexpression on APCs inhibits
OVA peptide-dependent T-cell proliferation (20). HVEM overexpression on DC induced
IL-10 producing CD4+-T cells, and immune adoption of
these DCs in vivo can protect against experimental
autoimmune myocarditis (EAM) (21). However, very little is known
regarding the role of HVEM in the induction of Treg by
1,25(OH)2D3-treated BMDCs.
The present study’s observations that HVEM was
significantly upregulated in 1,25(OH)2D3-treated DCs
indicates that HVEM may be involved in the induction of Treg by
1,25(OH)2D3. However, it remains unclear what the exact
underlying mechanism is. Kuipers et al (31) found that by triggering PD-L1 on DCs
using soluble PD-1 immunoglobulin resulted in a decreased
expression of the positive co-stimulatory molecules CD80, CD86 and
CD40 and increased IL-10 production. An explanation may be reverse
signaling by HVEM into DCs, resulting in a suppressive
DC-phenotype. All these findings are based on the hypothesis that
vitamin D regulates immune responses by controlling the expression
of CYP27B1 and VDR (32–36).
In conclusion, increased HVEM on
1,25(OH)2D3-treated mouse-BMDCs induced
CD4+CD25+Foxp3+ Treg and impaired
allogeneic CD4+ T-lymphocyte proliferation, which
further supported the important role of inhibitory molecules in
DC-mediated immune tolerance.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (nos. 30872341 and 81270075).
References
|
1
|
Casteels K, Bouillon R, Waer M and Mathieu
C: Immunomodulatory effects of 1,25-dihydroxyvitamin D3. Curr Opin
Nephrol Hypertens. 4:313–318. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carlberg C: Current understanding of the
function of the nuclear vitamin D receptor in response to its
natural and synthetic ligands. Recent Results Cancer Res.
164:29–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Griffin MD, Xing N and Kumar R: Vitamin D
and its analogs as regulators of immune activation and antigen
presentation. Annu Rev Nutr. 23:117–145. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mathieu C and Adorini L: The coming of age
of 1,25-dihydroxyvitamin D3 analogs as immunomodulatory agents.
Trends Mol Med. 8:174–179. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adorini L: Immunomodulatory effects of
vitamin D receptor ligands in autoimmune diseases. Int
Immunopharmacol. 2:1017–1028. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adorini L: 1,25-Dihydroxyvitamin D3
analogs as potential therapies in transplantation. Curr Opin
Investig Drugs. 3:1458–1463. 2002.PubMed/NCBI
|
|
7
|
Deluca HF and Cantorna MT: Vitamin D: its
role and uses in immunology. FASEB J. 15:2579–2585. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Penna G and Adorini L: 1
Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation,
activation, and survival of dendritic cells leading to impaired
alloreactive T cell activation. J Immunol. 164:2405–2411. 2000.
View Article : Google Scholar
|
|
9
|
Piemonti L, Monti P, Sironi M, et al:
Vitamin D3 affects differentiation, maturation, and function of
human monocyte-derived dendritic cells. J Immunol. 164:4443–4451.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Griffin MD, Lutz WH, Phan VA, Bachman LA,
McKean DJ and Kumar R: Potent inhibition of dendritic cell
differentiation and maturation by vitamin D analogs. Biochem
Biophys Res Commun. 270:701–708. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Halteren AG, van Etten E, de Jong EC,
Bouillon R, Roep BO and Mathieu C: Redirection of human
autoreactive T-cells Upon interaction with dendritic cells
modulated by TX527, an analog of 1,25 dihydroxyvitamin D(3).
Diabetes. 51:2119–2125. 2002.PubMed/NCBI
|
|
12
|
Gauzzi MC, Purificato C, Donato K, et al:
Suppressive effect of 1alpha,25-dihydroxyvitamin D3 on type I
IFN-mediated monocyte differentiation into dendritic cells:
impairment of functional activities and chemotaxis. J Immunol.
174:270–276. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai H, Zhu H, Lei P, et al: Programmed
death-1 signaling is essential for the skin allograft protection by
alternatively activated dendritic cell infusion in mice.
Transplantation. 88:864–873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Pino-Lagos K, de Vries VC, Guleria
I, Sayegh MH and Noelle RJ: Programmed death 1 ligand signaling
regulates the generation of adaptive Foxp3+CD4+ regulatory T cells.
Proc Natl Acad Sci USA. 105:9331–9336. 2008.PubMed/NCBI
|
|
15
|
Chen C, Qu QX, Huang JA, et al: Expression
of programmed-death receptor ligands 1 and 2 may contribute to the
poor stimulatory potential of murine immature dendritic cells.
Immunobiology. 212:159–165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwon BS, Tan KB, Ni J, et al: A newly
identified member of the tumor necrosis factor receptor superfamily
with a wide tissue distribution and involvement in lymphocyte
activation. J Biol Chem. 272:14272–14276. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morel Y, Schiano de Colella JM, Harrop J,
et al: Reciprocal expression of the TNF family receptor herpes
virus entry mediator and its ligand LIGHT on activated T cells:
LIGHT down-regulates its own receptor. J Immunol. 165:4397–4404.
2000. View Article : Google Scholar
|
|
18
|
Jung HW, La SJ, Kim JY, et al: High levels
of soluble herpes virus entry mediator in sera of patients with
allergic and autoimmune diseases. Exp Mol Med. 35:501–508. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Subudhi SK, Anders RA, et al: The
role of herpesvirus entry mediator as a negative regulator of T
cell-mediated responses. J Clin Invest. 115:711–717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sedy JR, Gavrieli M, Potter KG, et al: B
and T lymphocyte attenuator regulates T cell activation through
interaction with herpesvirus entry mediator. Nat Immunol. 6:90–98.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai G, Wang H, Qin Q, et al: Amelioration
of myocarditis by HVEM-overexpressing dendritic cells through
induction of IL-10-producing cells. Cardiovasc Res. 84:425–433.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang M, Tang H, Guo Z, et al: Splenic
stroma drives mature dendritic cells to differentiate into
regulatory dendritic cells. Nat Immunol. 5:1124–1133. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Latchman Y, Wood CR, Chernova T, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Freeman GJ, Long AJ, Iwai Y, et al:
Engagement of the PD-1 immunoinhibitory receptor by a novel B7
family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaliński P, Hilkens CM, Wierenga EA and
Kapsenberg ML: T-cell priming by type-1 and type-2 polarized
dendritic cells: the concept of a third signal. Immunology Today.
20:561–567. 1999.PubMed/NCBI
|
|
26
|
Lutz MB and Schuler G: Immature,
semi-mature and fully mature dendritic cells: which signals induce
tolerance or immunity? Trends Immunol. 23:445–449. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anderson AE, Swan DJ, Sayers BL, et al:
LPS activation is required for migratory activity and antigen
presentation by tolerogenic dendritic cells. J Leukoc Biol.
85:243–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alroy I, Towers TL and Freedman LP:
Transcriptional repression of the interleukin-2 gene by vitamin D3:
direct inhibition of NFATp/AP-1 complex formation by a nuclear
hormone receptor. Mol Cell Biol. 15:5789–5799. 1995.PubMed/NCBI
|
|
29
|
Wölfle SJ, Strebovsky J, Bartz H, et al:
PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J
Immunol. 41:413–424. 2011.PubMed/NCBI
|
|
30
|
Unger WW, Laban S, Kleijwegt FS, van der
Slik AR and Roep BO: Induction of Treg by monocyte-derived DC
modulated by vitamin D3 or dexamethasone: differential role for
PD-L1. Eur J Immunol. 39:3147–3159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuipers H, Muskens F, Willart M, et al:
Contribution of the PD-1 ligands/PD-1 signaling pathway to
dendritic cell-mediated CD4+ T cell activation. Eur J
Immunol. 36:2472–2482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sadeghi K, Wessner B, Laggner U, et al:
Vitamin D3 down-regulates monocyte TLR expression and triggers
hyporesponsiveness to pathogen-associated molecular patterns. Eur J
Immunol. 36:361–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hewison M, Freeman L, Hughes SV, et al:
Differential regulation of vitamin D receptor and its ligand in
human monocyte-derived dendritic cells. J Immunol. 170:5382–5390.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong X, Craig T, Xing N, et al: Direct
transcriptional regulation of RelB by 1alpha,25-dihydroxyvitamin D3
and its analogs: physiologic and therapeutic implications for
dendritic cell function. J Biol Chem. 278:49378–49385. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Griffin MD, Lutz W, Phan VA, Bachman LA,
McKean DJ and Kumar R: Dendritic cell modulation by 1alpha,25
dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent
pathway that promotes a persistent state of immaturity in vitro and
in vivo. Proc Natl Acad Sci USA. 98:6800–6805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Szeles L, Keresztes G, Töröcsik D, et al:
1,25-dihydroxyvitamin D3 is an autonomous regulator of the
transcriptional changes leading to a tolerogenic dendritic cell
phenotype. J Immunol. 182:2074–2083. 2009. View Article : Google Scholar : PubMed/NCBI
|