Introduction
Survivin, a member of the family of inhibitor of
apoptosis (IAP) proteins, contains 142 amino acid residues.
Survivin functions as a key regulator of mitosis and programmed
cell death by inhibiting apoptosis and promoting cell proliferation
(1). Studies have identified that
survivin is able to promote the migratory/invasive properties of
several types of human cancer, including melanoma, prostate cancer
and breast cancer (204). Survivin has been demonstrated to regulate
the invasion ability of colorectal cancer (CRC) (5,6).
However, little is known regarding the functional mechanism of
survivin in CRC invasion. A better understanding of the mechanisms
by which survivin regulates aggressive cellular behaviors may lead
to the production of novel therapeutic targets for colon
cancer.
The extracellular matrix (ECM) provides a structural
framework to support cells and maintains cell functions by
mediating cell-cell or cell-ECM interactions. Degradation of ECM
components is mainly controlled by matrix metalloproteinases
(MMPs), a large group of enzymes (7). The MMPs comprise a family of
zinc-dependent endopeptidases that consist of >21 human
proteases and are important in carcinogenesis. One of the smallest
known members of the MMP family, MMP-7, was first identified as an
enzyme of the involuting rat uterus and was subsequently determined
to be an important marker in human cancer progression (8). For example, MMP-7 has been reported
to be overexpressed in numerous types of human malignancy,
including ovarian, prostate, gastric and breast cancer (9–12),
and is important in cancer progression as it promotes tumor
invasion. Furthermore, the ability of CRC cells to migrate and
invade has been demonstrated to correlate with an increased
expression of MMP-7 (13–15). In addition, the overexpression of
MMP-7 has been reported to contribute to clinically aggressive
behaviors and is a significant predictor of patient survival in CRC
(16,17). Certain studies have indicated that
upregulation of survivin and MMP-7 expression levels correlates
with metastatic CRC and is significantly associated with liver
metastasis of colon cancer (18,19).
However, the functional correlation between survivin and MMP-7
remains largely unknown.
The present study identified an alternative role for
survivin in tumor progression. It demonstrates that the increased
invasion of colon carcinoma cells that overexpress survivin is
mediated by the activation of MMP-7. Furthermore, overexpression of
survivin enhances MMP-7 expression, whereas silencing endogenous
survivin in colon cancer cell lines results in a decreased MMP-7
expression. These findings suggest the molecular mechanism by which
survivin enhances cancer invasion. In addition, these data indicate
that MMP-7 is a potential mediator of this process.
Materials and methods
Cell culture
293FT cells were cultured and maintained in
Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, and
used as packaging cells for lentivirus production. RPMI-1640
supplemented with 10% fetal bovine serum was used to separately
culture SW620 and HCT-116 cells (American Type Culture Collection,
Manassas, VA, USA).
Expression vector construction and viral
production
Full-length survivin cDNA was generated by PCR and
subcloned into the pReceiver-Lv08 expression vector (Guangzhou
Fulengen Co., Ltd., Guangzhou, China). Survivin expression in colon
carcinoma cells was ablated using short hairpin RNA (shRNA). Sense
(GGACCACCGCAUCUCUACA) and antisense (UGUAGAGAUGCGGUGGUCC)
oligonucleotides for the double-stranded cassette were designed and
the scrambled sense (ACUACCGUUGUUAUAGGUG) and antisense
(CACCUAUAACAACGGUAGU) sequences were used. These oligonucleotides
were synthesized and generated by PCR and subcloned into the psi-U6
vector (Guangzhou Fulengen Co., Ltd.). The 293FT cells were
transfected with pReceiver-Lv105/survivin, psi-U6/survivin shRNA or
the control plasmid pReceiver-Lv105 and psi-U6 using the ViraPower™
Lentiviral Expression system, the BLOCK-iT™ Lentiviral RNAi
Expression system and Lipofectamine® 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The colon carcinoma cells (SW620 and
HCT-116) were infected by serial dilution with the virus-containing
medium and the stably-transfected cells were selected using
puromycin for one week.
siRNA transfection
A small interfering RNA (siRNA) pool against MMP-7
was purchased from Guangzhou Fulengen Co., Ltd. The siRNA was
dissolved in siRNA buffer (Guangzhou Fulengen Co., Ltd.) at a
concentration of 100 μM and transfected into cells using
Lipofectamine 2000. The gene-silencing effect was measured by
western blotting 48 h after transfection.
qPCR
To measure the mRNA levels of cell-cycle regulators,
total RNA was reverse transcribed using a PrimeScript™ RT Reagent
kit (Takara Bio, Inc., Shiga, Japan). qPCR was performed using
SYBR® Premix Ex Taq™ II (Takara Bio, Inc.) on a
StrataGene Mx3005P system. The primer sequences used were as
follows: Forward: TTCTCAAGGACCACCGCATC and reverse:
GCCAAGTCTGGCTCGTTCTC for survivin; and forward:
GAGTGAGCTACAGTGGGAACA and reverse: CTATGACGCGGGAGTTTAACAT for
MMP-7. GAPDH was used as an endogenous control. The time points for
colon carcinoma cells being transfected with MMP-7-specific siRNA
were 12, 24, 48, 72 and 96 h. All samples were normalized to
internal controls and fold-changes were calculated using relative
quantification (2−ΔΔCt).
Western blot analysis
Protein was extracted by solubilizing cells in RIPA
buffer containing a protease and phosphatase inhibitor cocktail
(Keygen Biotech. Co. Ltd., Nanjing, China). Protein concentration
was quantitated with Bradford protein assay reagent (Keygen
Biotech. Co. Ltd.), and 40 μg of total protein was loaded
onto 4–12% polyacrylamide gels, separated and then transferred onto
a polyvinylidene difluoride membrane. The membrane was probed with
mouse anti-Survivin, MMP-7 (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and proteins were detected with enhanced
chemiluminescence detection reagents (Keygen Biotech. Co. Ltd.).
Protein loading was normalized by probing the membrane with
anti-GAPDH antibodies.
Immunofluorescence analysis
Cell lines were plated on culture slides (Corning
Incorporated, Tewksbury, MA, USA) and, after 24 h, were rinsed with
phosphate-buffered saline (PBS) and fixed in methanol-acetone for
30 min at room temperature. The cells were permeabilized with 0.5%
Triton X-100 for 30 min and blocked for 45 min in 10% bovine serum
albumin (Sigma-Aldrich, St. Louis, MO, USA) in PBS, and then
incubated with primary monoclonal antibodies in PBS for 2 h at room
temperature. After three washes in PBS, the slides were incubated
for 1 h in the dark with secondary goat anti-mouse antibodies
(Invitrogen Life Technologies). After another three washes, the
slides were stained with 4′,6-diamidino-2-phenylindole
(Sigma-Aldrich) for 5 min to visualize the nuclei and were examined
using an Eclipse TE300 microscope (Nikon, Tokyo, Japan).
Boyden chamber assay
Biocoat™ Growth Factor-Reduced Matrigel™ invasion
chambers (8.0 μm PET membrane; 24-well cell culture inserts;
BD Biosciences, Franklin Lakes, NJ, USA) were preincubated in
RPMI-1640 supplemented with 1% fetal bovine serum for 1 h.
Harvested cells (2×103 cells) in 100 μl RPMI-1640
supplemented with 1% fetal bovine serum were replated onto the
upper chamber and placed in the lower chamber with 500 μl
RPMI-1640 supplemented with 10% fetal bovine serum. After 20 h, the
cells were fixed with methanol. Non-migrated cells on the upper
side of the filter were removed with a cotton swab and cells on the
underside of the filter were stained with hematoxylin. Images were
captured using an Eclipse TE300 microscope. Relative cell migration
was determined by the mean number of migrated cells in eight random
fields on the underside of the filter. For each experiment, three
independent filters were analyzed.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA)and the data
are expressed as the mean ± standard deviation. Comparisons were
conducted using Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
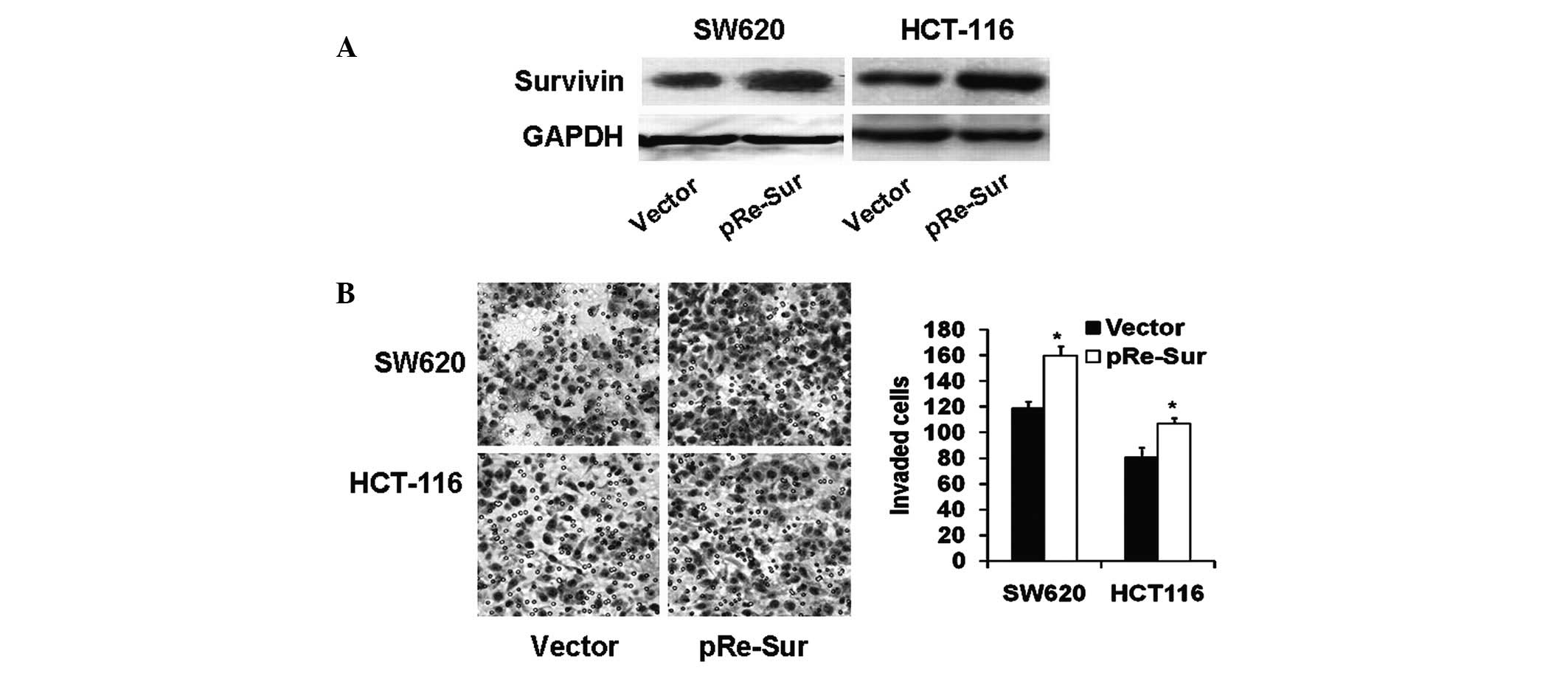
Overexpression of survivin enhances colon
carcinoma cell invasion
To investigate the potential role of survivin in
cell invasion, cell invasion was examined in human colon carcinoma
SW620 and HCT-116 cells overexpressing survivin. Western blot
analysis confirmed the upregulation of survivin (Fig. 1A) and the Boyden chamber assay
demonstrated that survivin overexpression significantly enhanced
cell invasion accordingly (Fig.
1B; P<0.01).
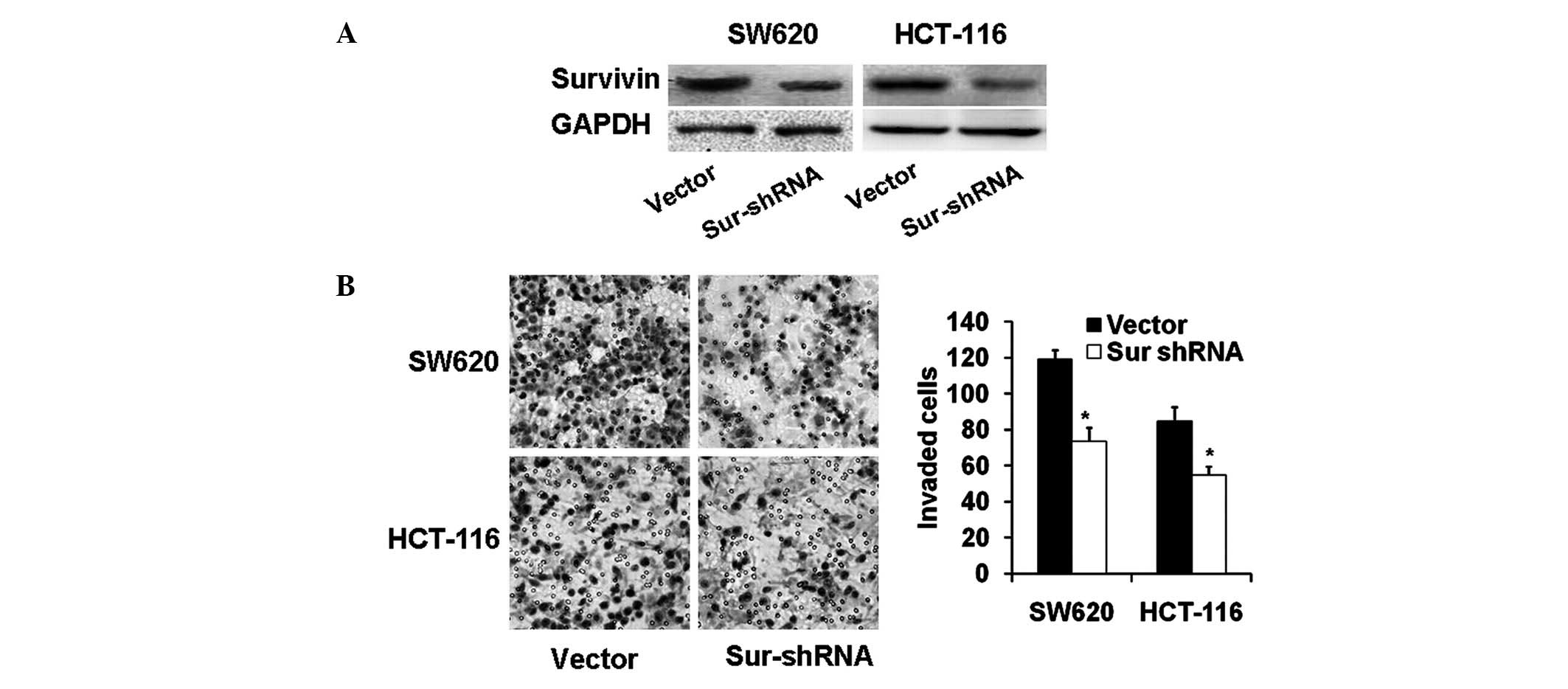
Knockdown of survivin impairs colon
carcinoma cell invasion
To confirm the role of survivin in the invasion of
colon carcinoma, RNAi-mediated knockdown of survivin was performed
to suppress survivin in SW620 and HCT-116 cells. As expected,
survivin was silenced markedly, as revealed by the western blot
analysis (Fig. 2A). As shown in
Fig. 2B, survivin-knockdown cells
showed significant reductions in cell invasion (P<0.01). These
data suggest that survivin may be involved in the invasion of colon
carcinoma cells.
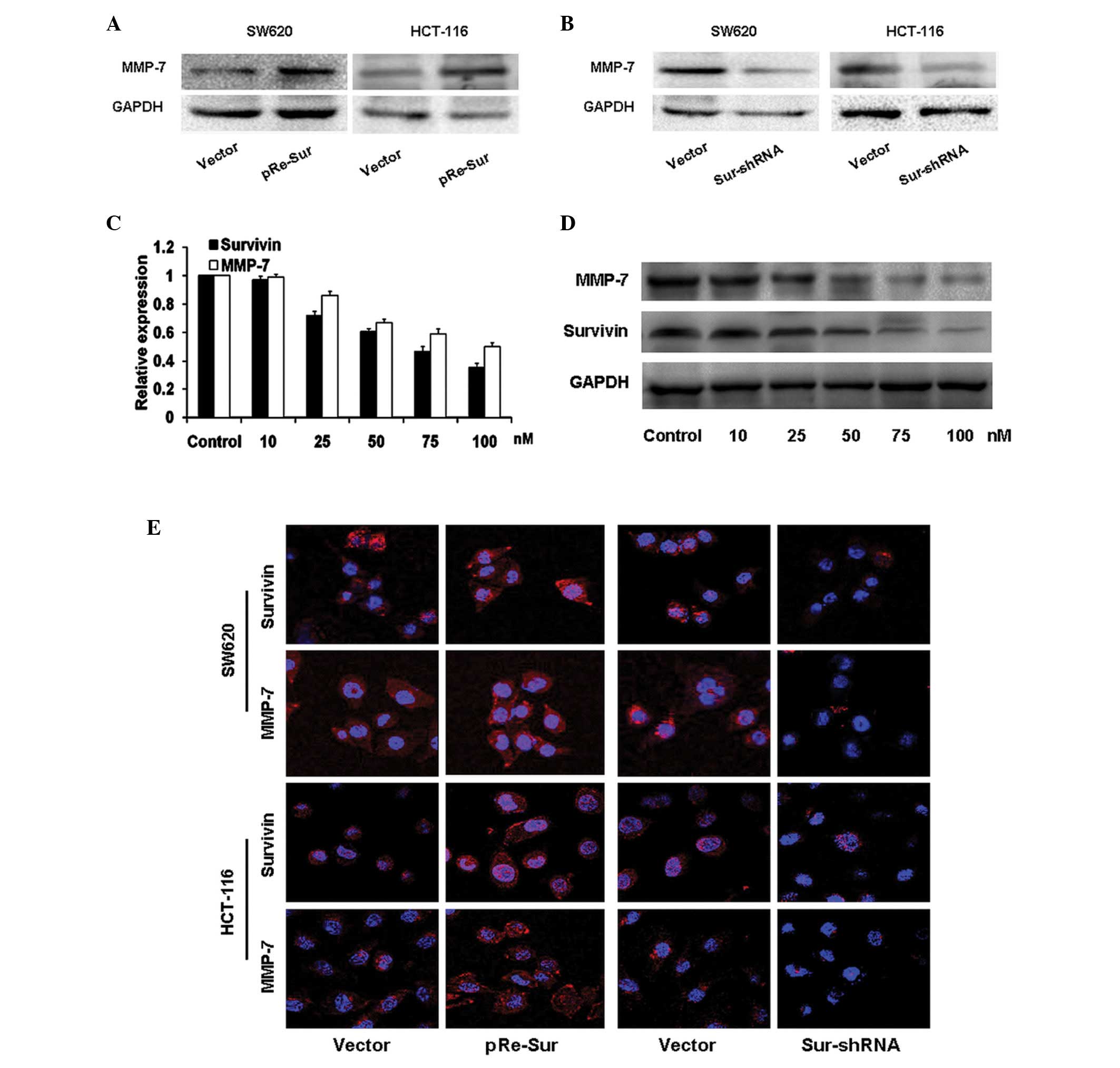
Survivin regulates MMP-7 expression in
colon carcinoma cells
To examine the effect of survivin on MMP-7
expression, ectopic expression and siRNA-based knockdown of
survivin were performed in colon carcinoma SW620 and HCT-116 cells.
Western blot analysis and immunofluorescence demonstrated that the
overexpression of survivin induced upregulation of MMP-7 (Fig. 3A and E). By contrast, MMP-7
expression levels were markedly suppressed in survivin-silenced
colon carcinoma cells (Fig 3B and
E). Furthermore, decreased expression levels of survivin led to
a dose-dependent reduction in MMP-7 expression levels (Fig. 3C and D; P<0.01).
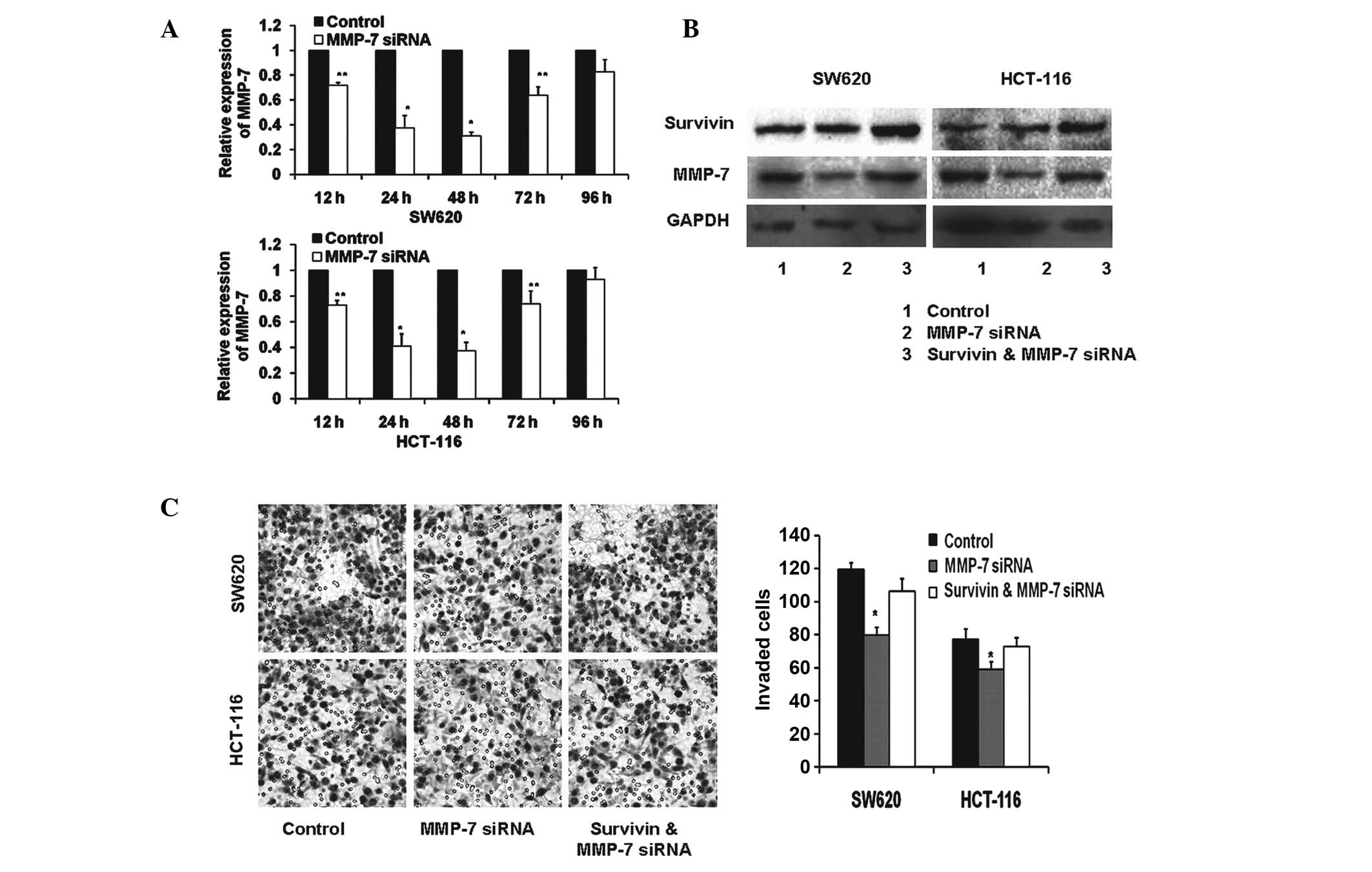
Role of MMP-7 in survivin-mediated
invasion
To confirm the importance of MMP-7 in
survivin-induced cell invasion, RNAi-mediated silencing of MMP-7
was performed in SW620 and HCT-116 cells and resulted in effective
reduction of the endogenous MMP-7, as determined by qPCR (Fig. 4A). Notably the optimum reduction
required 48 h of RNAi-mediated silencing. Subsequently, the protein
expression of survivin and MMP-7 was assessed following cell
culture for 48 h. As shown in Fig.
4B, MMP-7 expression levels were reduced successfully using
RNAi, as revealed by western blot analysis. Notably, the knockdown
of MMP-7 did not alter the expression levels of survivin in colon
carcinoma cells in comparison with the controls. Compared with the
controls, reduced MMP-7 expression levels in the colon carcinoma
cells significantly decreased the cell capacity for invasion, as
evaluated by Boyden chamber assay (P<0.01). However, no
significant difference was observed in the cells also
overexpressing survivin (P≥0.05) (Fig.
4C).
Discussion
CRC is the third most common type of cancer
worldwide. The majority of individuals who are diagnosed with this
disease are already at an advanced stage (20). Despite significant advances in
early diagnosis and treatment, the survival rate of patients has
not yet markedly improved, which is mainly due to the high rate of
localized recurrence and distant metastasis following surgical
resection (21). The exact
mechanisms underlying the aggressive progression of CRC remain to
be clarified. Therefore, elucidating the underlying mechanisms that
drive CRC cell invasion and metastasis may aid in the exploitation
of strategies for effective CRC treatment.
Survivin is the smallest member of the IAP family of
anti-apoptotic proteins. It is highly expressed in tumors and is
thought to promote tumor progression through apoptotic inhibition
and mitotic chromosomal alignment (1,22).
Studies have demonstrated that overexpression of survivin is
correlated with increased invasion and metastasis of human cancer,
such as melanoma, prostate cancer, breast cancer and endometrial
carcinoma (2–4,23).
Similar to these observations, the present study, to the best of
our knowledge, has demonstrated for the first time that the
overexpression of survivin is capable of enhancing cell invasion in
the SW620 and HCT-116 colon carcinoma cell lines. Studies performed
with the use of chemically synthesized siRNAs or plasmid/viral
vectors encoding shRNAs have suggested that RNAi-mediated survivin
knockdown is able to suppress cancer cell proliferation and promote
caspase-dependent apoptosis in a number of human tumor cell models
(24). Studies from our laboratory
and findings of other studies have demonstrated that inhibition of
survivin using shRNA and siRNA influences the biological features
of colorectal carcinoma cells and inhibits invasion and metastasis
of the human colon carcinoma cell line SW480 in vitro
(5,25). Similar to these observations, the
present study found that RNAi-mediated knockdown of survivin is
capable of significantly impairing cell invasion in SW620 and
HCT-116 colon carcinoma cells. Therefore, through two methods of
upregulating and inhibiting the expression of survivin in CRC
cells, the results of the present study strongly indicate that
survivin is required for tumor invasion in CRC. Notably, it has
been demonstrated that inhibition of survivin is an effective
therapeutic strategy in preclinical and clinical studies (26). The aforementioned studies suggest
that survivin may be used as a potential target for cancer
treatment in CRC patients.
Studies have revealed that survivin enhances cell
proliferation and suppresses apoptosis through a number of pathways
(27,28). The exact mechanisms by which
survivin exerts this effect on the invasion ability of CRC remain
largely unknown. In the present study, overexpression of survivin
enhanced cell invasion and resulted in increased MMP-7 activation
in SW620 and HCT-116 colon carcinoma cells. Conversely, knockdown
of survivin using RNAi decreased MMP-7 expression levels. Notably,
decreased expression levels of survivin led to a dose-dependent
decrease in MMP-7 expression levels. However, knockdown of MMP-7
expression did not affect the expression levels of survivin in
colon carcinoma cells compared with that in the controls.
Similarly, Chu et al demonstrated that survivin knockdown
downregulates the expression of MMPs in other types of colon
carcinoma cell (6). Futhermore,
the present study includes the primary investigation of the role of
MMP-7 in cell invasion induced by survivin. Silencing of MMP-7
expression in the colon carcinoma cells reduced the cell invasion
ability. By contrast, MMP-7 knockdown in transfected-survivin colon
carcinoma cells did not contribute to significant changes in cell
invasion. Overall, these results demonstrate that survivin may
promote the invasion of colon carcinoma cells through the
regulation of MMP-7 expression.
In conclusion, these data strongly suggest that
upregulation of MMP-7 expression levels by survivin contributes to
cell invasion, and that MMP-7 is a putative therapeutic target in
the invasive progression of colon carcinoma. Additonal
investigation is necessary to elucidate the regulatory mechanism
between survivin and MMP-7.
Acknowledgements
This study was supported by funding from the
National Natural Science Foundation of China (grant no.
81202125).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
MMP-7
|
metalloprotease-7
|
|
IAP
|
inhibitor of apoptosis
|
|
ECM
|
extracellular matrix
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McKenzie JA, Liu T, Goodson AG and
Grossman D: Survivin enhances motility of melanoma cells by
supporting Akt activation and α5 integrin upregulation. Cancer Res.
70:7927–7937. 2010.PubMed/NCBI
|
|
3
|
Zhang M, Coen JJ, Suzuki Y, et al:
Survivin is a potential mediator of prostate cancer metastasis. Int
J Radiat Oncol Biol Phys. 78:1095–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdraboh ME, Gaur RL, Hollenbach AD,
Sandquist D, Raj MH and Ouhtit A: Survivin is a novel target of
CD44-promoted breast tumor invasion. Am J Pathol. 179:555–563.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhonghong L, Lianjie L, Changqing Z, Ying
H, Yu J and Yan L: The influence of survivin shRNA on the cell
cycle and the invasion of SW480 cells of colorectal carcinoma. J
Exp Clin Cancer Res. 27:202008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chu XY, Chen LB, Wang JH, et al:
Overexpression of survivin is correlated with increased invasion
and metastasis of colorectal cancer. J Surg Oncol. 105:520–528.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen P and Parks WC: Role of matrix
metalloproteinases in epithelial migration. J Cell Biochem.
108:1233–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ii M, Yamamoto H, Adachi Y, Maruyama Y and
Shinomura Y: Role of matrix metalloproteinase-7 (matrilysin) in
human cancer invasion, apoptosis, growth, and angiogenesis. Exp
Biol Med (Maywood). 231:20–27. 2006.PubMed/NCBI
|
|
9
|
Wang FQ, So J, Reierstad S and Fishman DA:
Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by
activation of progelatinase. Int J Cancer. 114:19–31. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lynch CC, Hikosaka A, Acuff HB, et al:
MMP-7 promotes prostate cancer-induced osteolysis via the
solubilization of RANKL. Cancer Cell. 7:485–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi M, Liu D, Duan H, et al: Catecholamine
up-regulates MMP-7 expression by activating AP-1 and STAT3 in
gastric cancer. Mol Cancer. 9:2692010.PubMed/NCBI
|
|
12
|
Sizemore ST and Keri RA: The forkhead box
transcription factor FOXC1 promotes breast cancer invasion by
inducing matrix metalloprotease 7 (MMP7) expression. J Biol Chem.
287:24631–24640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyamoto S, Yano K, Sugimoto S, et al:
Matrix metalloproteinase-7 facilitates insulin-like growth factor
bioavailability through its proteinase activity on insulin-like
growth factor binding protein 3. Cancer Res. 64:665–671. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ho BY, Wu YM, Chang KJ and Pan TM:
Dimerumic acid inhibits SW620 cell invasion by attenuating
H2O2-mediated MMP-7 expression via JNK/C-Jun
and ERK/C-Fos activation in an AP-1-dependent manner. Int J Biol
Sci. 7:869–880. 2011.PubMed/NCBI
|
|
15
|
Lee SK, Han YM, Yun J, et al: Phosphatase
of regenerating liver-3 promotes migration and invasion by
upregulating matrix metalloproteinases-7 in human colorectal cancer
cells. Int J Cancer. 131:E190–E203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang WS, Chen PM, Wang HS, Liang WY and Su
Y: Matrix metalloproteinase-7 increases resistance to Fas-mediated
apoptosis and is a poor prognostic factor of patients with
colorectal carcinoma. Carcinogenesis. 27:1113–1120. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koskensalo S, Louhimo J, Nordling S,
Hagström J and Haglund C: MMP-7 as a prognostic marker in
colorectal cancer. Tumour Biol. 32:259–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang YJ, Lu ZH, Wang GQ, et al: Elevated
expressions of MMP7, TROP2, and survivin are associated with
survival, disease recurrence, and liver metastasis of colon cancer.
Int J Colorectal Dis. 24:875–884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang GQ, Lu ZH, Fang YJ, Chen G, Zhou ZW,
Pan ZZ and Wan DS: Expression and clinical significance of survivin
and matrix metalloproteinase-7 in colon cancer. Ai Zheng.
28:945–949. 2009.(In Chinese).
|
|
20
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
21
|
Kelley RK, Wang G and Venook AP: Biomarker
use in colorectal cancer therapy. J Natl Compr Canc Netw.
9:1293–1302. 2011.PubMed/NCBI
|
|
22
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Erkanli S, Bolat F, Kayaselcuk F, Demirhan
B and Kuscu E: COX-2 and survivin are overexpressed and positively
correlated in endometrial carcinoma. Gynecol Oncol. 104:320–325.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pennati M, Folini M and Zaffaroni N:
Targeting survivin in cancer therapy: fulfilled promises and open
questions. Carcinogenesis. 28:1133–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu JB, Nan QZ, Ma GF, et al: Short hairpin
RNA-mediated survivin gene silencing inhibits invasion and
metastasis of human colon carcinoma cell line SW480 in vitro. Nan
Fang Yi Ke Da Xue Xue Bao. 27:951–954. 2007.(In Chinese).
|
|
26
|
Church DN and Talbot DC: Survivin in solid
tumors: rationale for development of inhibitors. Curr Oncol Rep.
14:120–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Altieri DC: Survivin, versatile modulation
of cell division and apoptosis in cancer. Oncogene. 22:8581–8589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kelly RJ, Lopez-Chavez A, Citrin D, Janik
JE and Morris JC: Impacting tumor cell-fate by targeting the
inhibitor of apoptosis protein survivin. Mol Cancer. 10:352011.
View Article : Google Scholar : PubMed/NCBI
|