Introduction
Fructus Evodiae, a traditional Chinese
medicine, has been used in the treatment of headache, abdominal
pain, postpartum hemorrhage, dysentery and amenorrhea (1). Evodiamine is the most important
quinoline alkaloid isolated from the fruit of Fructus
Evodiae. Evodiamine has been demonstrated to possess numerous
biological effects, including analgesic, anti-inflammatory,
antiobesity, vasodilatory, thermoregulatory and uterotonic effects
(1). In recent years, studies
investigating the anticancer mechanisms of evodiamine have become a
major topic of focus. Certain studies have suggested that
evodiamine has anticancer activity by inducing apoptosis, arresting
the cell cycle and inhibiting tumor invasion and metastasis.
Evodiamine has been demonstrated to inhibit the proliferation of
multiple tumor cells, including human leukemia (2), malignant melanoma (3–5),
thyroid carcinoma (6), prostate
cancer (7–9), breast cancer (10), hepatoma (11,12),
cervical cancer (5,6,13),
colon carcinoma (14,15) and pancreatic cancer (16) cells.
Cancer is not only a disease exhibiting abnormal
cellular proliferation and differentiation, but is also a disease
with abnormal apoptosis. Previous studies on apoptosis and its
mechanisms have provided a novel method for overcoming cancer, that
is, the induction of apoptosis in tumor cells rather than the
inhibition of tumor cell proliferation. Various methods may be used
to induce apoptosis in tumor cells or to establish the optimal
conditions for this induction.
Gastric carcinoma is one of the most common types of
malignancy and is the leading cause of cancer-related mortality in
China (17). Chemotherapy is one
of the main therapeutic approaches for treating gastric cancer.
However, the effect of evodiamine on gastric carcinoma remains
poorly defined and the exact mechanisms are unclear. Examining the
molecular mechanisms underlying the effects of evodiamine on
gastric carcinoma cells may provide novel methods for the treatment
of gastric cancer and add to the growing evidence that evodiamine
may be used as a systemic anticancer drug.
The SGC-7901 human gastric cancer cell line is an
ideal cellular model to study the proliferation and differentiation
of gastric cancer in vitro, which has the advantages of a
simple in vitro amplification and rapid entry to the
exponential phase. Consequently, the present study selected the
SGC-7901 cell line for investigation.
Our previous study (18) demonstrated that evodiamine inhibits
the proliferation and induces apoptosis of SGC-7901 gastric cancer
cells, and the half effective inhibitory concentration
(IC50) of evodiamine is 1.5 μmol/l. In the present
study, SGC-7901 cells were treated with 1.5 μmol/l evodiamine for
different time periods and the effects on proliferation and
apoptosis were observed. The present study investigates the
possible molecular mechanisms by which evodiamine affects SGC-7901
cells.
Materials and methods
Materials
Evodiamine was obtained from the National Institutes
for Food and Drug Control (Beijing, China). RPMI-1640 medium,
penicillin-streptomycin and 0.25% trypsin solution were purchased
from Hyclone (Logan, UT, USA). L-Glutamine, Hanks’ balanced salt
solution and dimethylsulfoxide (DMSO) were obtained from Solabio
(Beijing, China). Fetal bovine serum (FBS) was purchased from
Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.
(Hangzhou, China). The Hoechst staining kit, Bradford protein assay
kit, caspase-3 activity assay kit, caspase-8 activity assay kit,
caspase-9 activity assay kit, enhanced bicinchoninic acid (BCA)
protein assay kit and RIPA lysis buffer were purchased from the
Beyotime Institute of Biotechnology (Shanghai, China). The Annexin
V-fluorescein isothiocyanate apoptosis detection kit and cell cycle
detection kit were purchased from KeyGen Biotech Co., Ltd.
(Nanjing, China). Caspase-3, Bax, Bcl-2, β-actin, horseradish
peroxidase (HRP)-conjugated goat anti-mouse IgG and HRP-conjugated
goat anti-rabbit IgG antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture and morphological
analysis
The SGC-7901 human gastric cancer cell line was
obtained from the Shanghai Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). The cells were cultured in
RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and
100 mg/l streptomycin, at 37°C in a humidified atmosphere of 95%
air and 5% CO2. Following 48 h, the medium was removed
and replaced by medium containing 1.5 μmol/l evodiamine or a
drug-free medium (control condition) for 12, 24 and 36 h. The
morphology of SGC-7901 cells was monitored under an inverted
contrast phase microscope (Nikon TE 2000-U; Nikon Tokyo, Japan) at
12, 24 and 36 h.
Hoechst 33258 staining
The SGC-7901 cells seeded on the coverslips were
treated with 1.5 μmol/l evodiamine for the indicated time points
and the coverslips were then collected. Hoechst 33258 staining was
performed on the coverslips to observe the morphological cell
changes. Attached cells were washed twice with phosphate-buffered
saline (PBS) and fixed with fixation fluid (paraformaldehyde) for
10 min. The fixation fluid was then removed and the cells were
washed twice with PBS prior to staining with 500 μl Hoechst 33258.
Following staining for 5 min, the cells were washed twice again and
observed under a fluorescence microscope (Nikon TE 2000-U) with
ultraviolet light.
Flow cytometric cell cycle analysis
SGC-7901 cells were incubated in six-well plates.
Following treatment with 1.5 μmol/l of evodiamine for 12, 24 and 36
h, the cells were harvested and washed with PBS. The pelleted cells
were fixed in ice-cold 70% ethanol at 4°C overnight. The fixed
cells were washed twice with PBS and the cells were treated with
100 μl RNase stock solution and incubated at 37°C for 30 min. The
cells were stained with 400 μl propidium iodide staining solution
at 4°C for 30 min in the dark. The stained cells were analyzed by
flow cytometry based on red fluorescence.
Caspase activity assay
SGC-7901 cells treated with evodiamine were
harvested and washed with ice-chilled PBS. The cell pellets were
resuspended with an appropriate quantity of lysis buffer (Tris-HCl)
for 15 min in an ice bath. Following centrifugation (16,000 × g at
4°C) for 15 min, the supernatant was transferred into ice-chilled
centrifuge tubes. The protein concentration was measured by the
Bradford assay. Then the enzyme activity of caspase-3, -8 and -9
was detected according to the manufacturer’s instructions of the
caspase activity assay kit (Beyotime Institute of
Biotechnology).
Western blot analysis
SGC-7901 cells were cultured in RPMI-1640 medium
until mid-log phase and then incubated with 1.5 μmol/l evodiamine
for 12, 24 and 36 h. The cells were harvested and the proteins were
isolated using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Total protein was measured using the enhanced BCA
protein assay kit (Beyotime Institute of Biotechnology). Equal
quantities of protein samples were separated by SDS-PAGE and
transferred onto the polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). Western blotting was performed using
antibodies against caspase-3, Bax and Bcl-2 (Santa Cruz
Biotechnology, Inc.). The secondary antibodies used were goat
anti-mouse and goat anti-rabbit (Santa Cruz Biotechnology, Inc.)
HRP-labeled antibodies. The signals were visualized by enhanced
chemiluminescence detection (Millipore).
Statistical analysis
All experiments were repeated three times and all
values are expressed as the mean ± standard deviation. The
independent-samples t-test was calculated to compare the mean of
each group with that of the control group. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of evodiamine on cellular
morphology
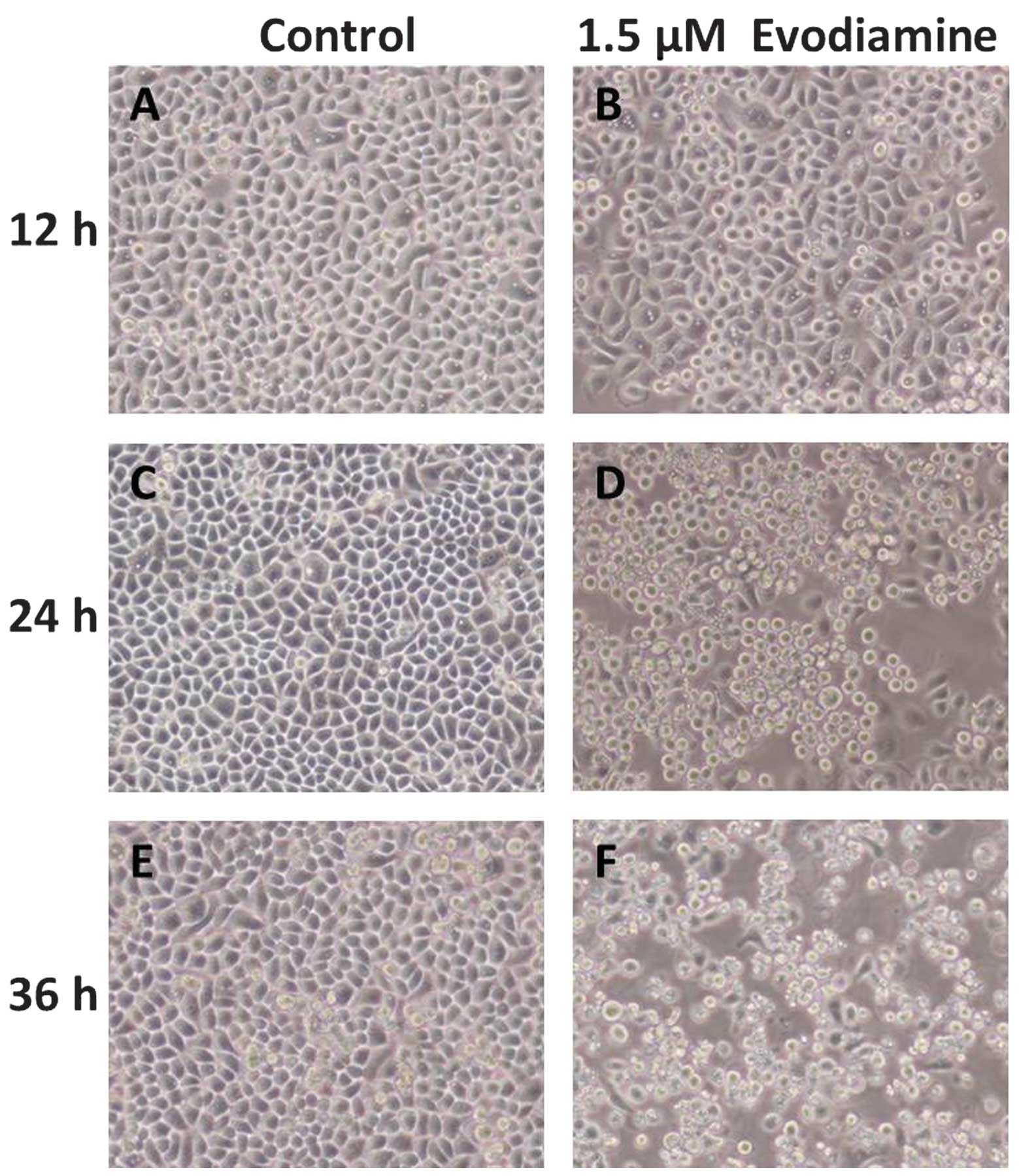
Following treatment with evodiamine, the
morphological changes in the cells were observed by inverted
microscopy. In the evodiamine groups, SGC-7901 cells became
irregular and exhibited shrinkage. Detachment of the cells from the
cell culture substratum was observed (Fig. 1). These changes were characteristic
of apoptotic cell death. In the control groups, cell morphology did
not change significantly.
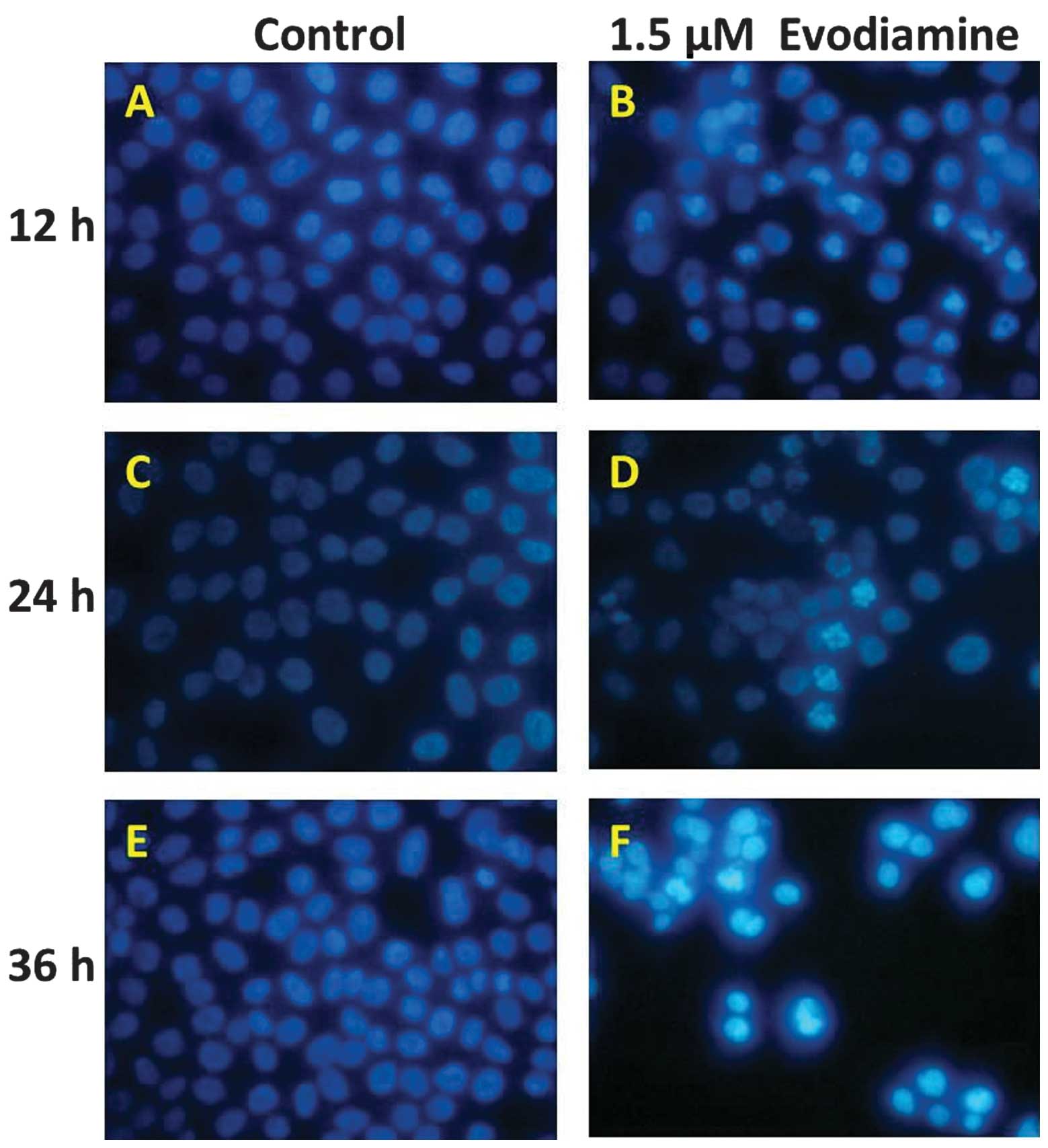
Hoechst 33258 staining was used to detect chromatin
condensation, one of the typical morphological features of
apoptosis. The Hoechst 33258 dye stained morphologically normal
nuclei dimly blue, whereas evodiamine-treated cells demonstrated
smaller nuclei with brilliant blue staining (Fig. 2). Compared with the control cells,
the cells exposed to evodiamine presented typical apoptotic
morphology. These results demonstrate that evodiamine induces the
morphological changes of apoptotic cell death in SGC-7901
cells.
Effects of evodiamine on cell cycle
progression
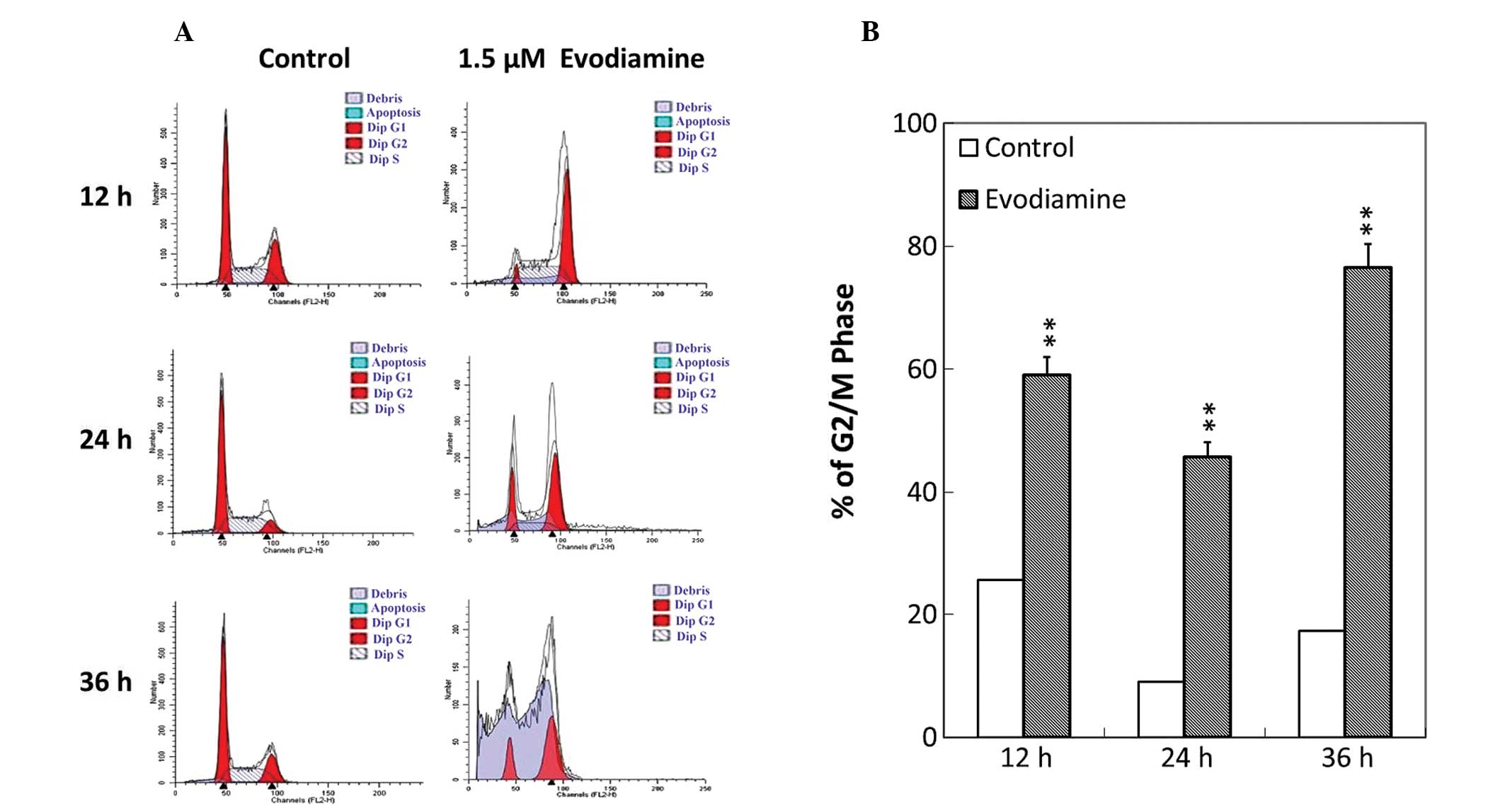
To investigate whether the antiproliferative effect
of evodiamine was associated with cell cycle arrest, analysis of
cell cycle phase distribution was performed following treatment
with evodiamine using flow cytometry. Evodiamine treatment resulted
in the accumulation of SGC-7901 cells at the G2/M phase (Fig. 3A). Following treatment with
evodiamine, the number of SGC-7901 cells that arrested at the G2/M
phase was elevated by 34.4, 44.4 and 59.2% at 12, 24 and 36 h,
respectively (Fig. 3B). These
results indicated that evodiamine was able to affect the
distribution of the cell cycle and induce G2/M arrest in SGC-7901
cells.
Effects of evodiamine on caspase
activity
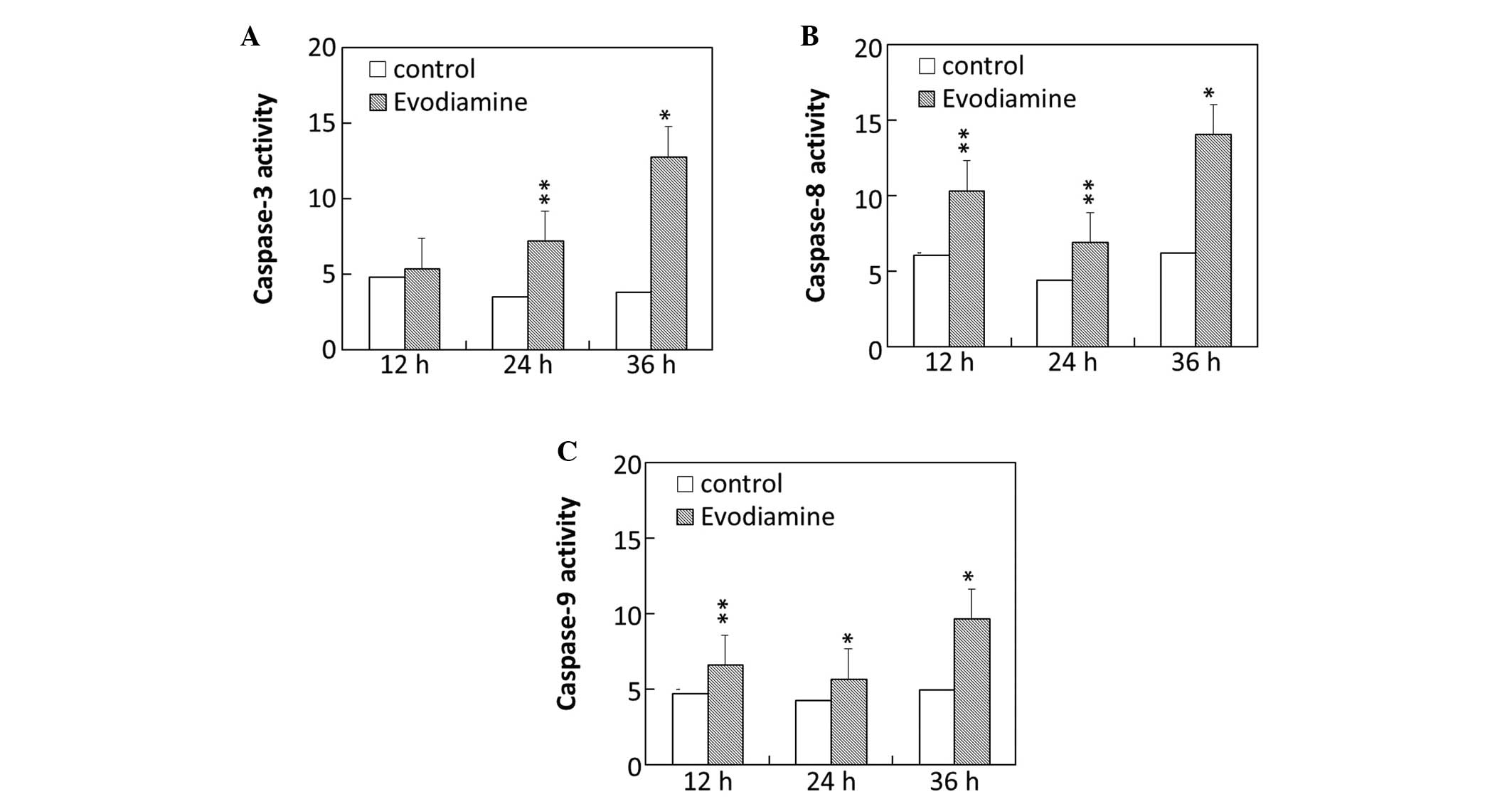
To investigate the molecular mechanism of
evodiamine-induced apoptosis, the activity of caspase-3, -8 and -9
in SGC-7901 cells was examined. The results demonstrated that the
caspase-3 activity of SGC-7901 cells was markedly increased
following treatment with evodiamine for 24 and 36 h (Fig. 4A). Evodiamine increased the
activity of caspase-8 in SGC-7901 cells following exposure for 12,
24 and 36 h (Fig. 4B). The
activity of caspase-9 was elevated following treatment with
evodiamine for 12, 24 and 36 h (Fig.
4C).
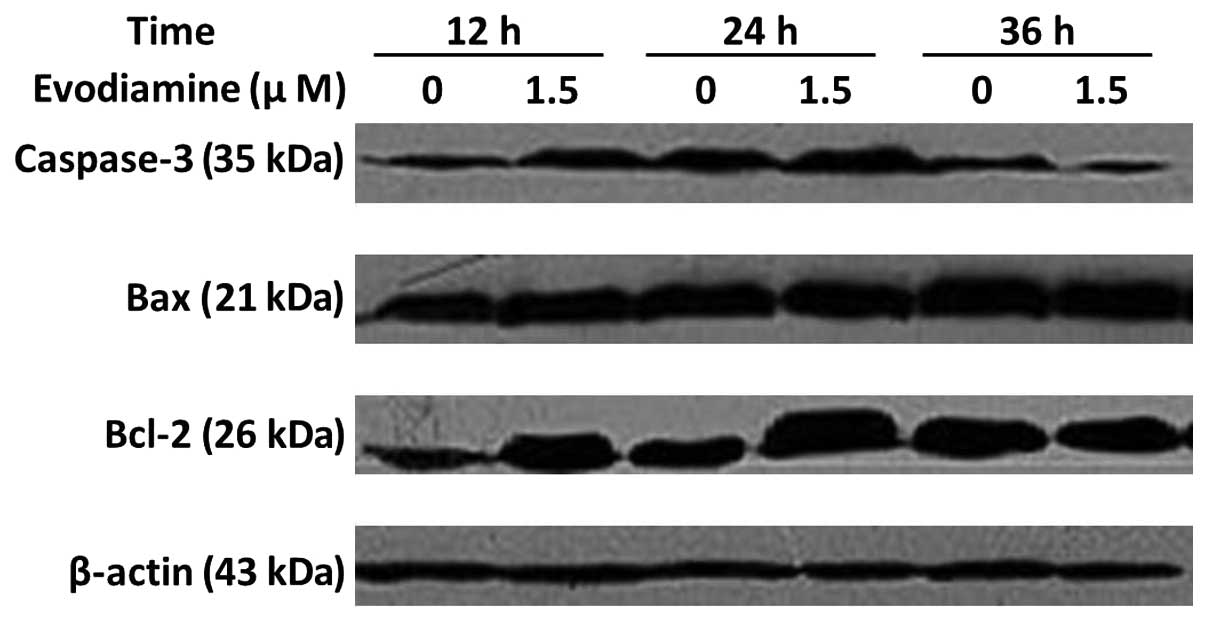
Western blot analysis of caspase-3, Bax
and Bcl-2 protein
The treatment of SGC-7901 cells with 1.5 μmol/l
evodiamine for 24 h resulted in an increase in caspase-3 expression
(Fig. 5). Bax expression was
elevated following incubation with evodiamine for 24 and 36 h. The
expression of Bcl-2 was increased following evodiamine treatment
for 12 and 24 h and decreased following 36 h. The expression of
β-actin served as an internal control. These data demonstrated that
evodiamine induced the apoptosis of SGC-7901 cells through a
caspase-dependent pathway.
Discussion
Apoptosis is an autonomic ordered, programmed,
physiological mode of cell death that is important in tissue
homeostasis and is controlled by serial genes. Cancer is not only a
disease with abnormal cellular proliferation and differentiation
but also a disease with abnormal apoptosis. Numerous types of
tumors are the result of an imbalance between cell proliferation
and cell death. Thus, investigating the basic mechanisms of
apoptosis may provide insight into potential novel therapeutic
targets and have vital significance.
Evodiamine is one of the major bioactive compounds
isolated and purified from the fruit Fructus Evodiae.
Several studies (2,6–8,10)
have indicated that evodiamine exhibits activity against human
tumor cells. Our previous study (18) demonstrated that evodiamine inhibits
the proliferation of SGC-7901 gastric cancer cells. In the present
study, the molecular mechanisms by which evodiamine affects
SGC-7901 cells were investigated and novel evidence was identified
for the future medical use of evodiamine. The previous study
determined whether evodiamine was toxic for normal peripheral blood
mononuclear cells (PBMCs). The results demonstrated that evodiamine
exhibited less toxicity in PBMCs that were treated with various
concentrations of evodiamine for 24 h. From the early tests it was
concluded that evodiamine-induced cell death was mediated by time-
and dose-dependent pathways, and the optimal concentration and
exposure time was 1.5 μm/l for 24 h. The present study also
demonstrated that DMSO, as a co-solvent, exhibited no cytotoxic
effect on SGC-7901 cells. Therefore, the IC50 of
evodiamine was selected to act upon SGC-7901 cells.
Morphological alteration is an important
characteristic of apoptosis. Alterations, including cell shrinkage,
cell detachment, chromatin condensation, nuclear fragmentation and
formation of apoptotic bodies are characteristic of cell apoptotic
death. Cellular morphological analysis demonstrated that SGC-7901
cells underwent typical apoptotic changes following treatment with
evodiamine.
Evodiamine has been demonstrated to induce cell
cycle arrest in prostate (7,8),
breast (10) and colorectal cancer
(14,15). Flow cytometry indicated that
evodiamine induced cell cycle arrest at the G2/M phase in SGC-7901
cells. The cells in G2/M phase mainly synthesize RNA and protein,
and prepare for cell division. The present study inferred that
evodiamine may affect the synthesis of proteins that are essential
for cells, thus inhibiting the proliferation of SGC-7901 cells.
In order to gain insight into the molecular
mechanisms underlying the effects of evodiamine in apoptosis, the
enzyme activity of caspase-3, -8 and -9 was determined. Numerous
internal and external signal stimuli are able to induce apoptosis.
Although there are various signals and pathways that follow, it is
generally considered that the common pathway of late apoptosis is
the activation of the caspase family. Caspases are the central
executioners in the apoptotic process. Caspases are divided into
two functional subfamilies, including promoter caspases (caspase-8
and -9) and effector caspases (caspase-3 and -7). The activated
promoter caspase is able to activate effector caspases, thus
completing apoptosis. Data from the present study demonstrated that
caspase-3, -8 and -9 were activated by evodiamine following
incubation for 24 and 36 h, indicating that evodiamine resulted in
apoptosis of SGC-7901 cells mediated by the activation of
caspase-3, -8 and -9. Furthermore, western blotting demonstrated
that the expression of caspase-3 also increased following
evodiamine treatment for 24 and 36 h.
The Bcl-2 protein family and its members form a
complicated interactive network, which is able to regulate
apoptosis. Among these proteins, the expression of Bax and Bcl-2 is
directly associated with apoptosis regulation. The increased
expression of Bax and decreased expression of Bcl-2 is able to
promote apoptosis. In the present study, data suggested that the
expression of Bax was increased following evodiamine treatment for
24 and 36 h. Evodiamine increased Bcl-2 expression at 12 and 24 h
and decreased Bcl-2 expression at 36 h. Bcl-2 is important in
tumorigenesis by inhibiting the signals that induce apoptosis.
Bcl-2 is also phosphorylated in vivo and this modification
has been demonstrated to effect its anti-apoptotic activity
(19). In addition, Bcl-2 is able
to inhibit or delay apoptosis induced by various stimuli, including
chemotherapeutic drugs. As a result, the present study inferred
that, in the early stages of evodiamine action, increased Bcl-2
expression may be a delayed action of cells on external stimulated
factors, so as to prevent the cytotoxic effect of evodiamine and
protect the cells. However, it was not able to prevent apoptosis
and the rate of apoptosis at this stage was elevated. When drug
treatment was prolonged to 36 h, the decreased Bcl-2 expression may
have been associated with the stimulatory effect of evodiamine
exceeding the cellular regulatory activity, which may have been
responsible for the induction of apoptosis in SGC-7901 cells. The
results indicate that the apoptosis caused by evodiamine in
SGC-7901 cells may be associated with the alteration of the Bcl-2
protein family.
In conclusion, the results of the present study
suggest that evodiamine inhibits the proliferation of SGC-7901
cells by arrest at the G2/M phase. In addition, evodiamine is able
to activate caspase-3, -8 and -9, upregulate the expression of
caspase-3 and Bax, and downregulate the expression of Bcl-2,
potentially the mechanism by which evodiamine induces the apoptosis
of SGC-7901 cells.
Acknowledgements
This study was supported by a research grant from
the National Natural Science Foundation of China (81260658).
References
|
1
|
Jiang J and Hu C: Evodiamine: a novel
anti-cancer alkaloid from Evodia rutaecarpa. Molecules.
14:1852–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee TJ, Kim EJ, Kim S, Jung EM, Park JW,
Jeong SH, Park SE, Yoo YH and Kwon TK: Caspase-dependent and
caspase-independent apoptosis induced by evodiamine in human
leukemic U937 cells. Mol Cancer Ther. 5:2398–2407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Intracellular regulation of evodiamine-induced A375-S2
cell death. Biol Pharm Bull. 26:1543–1547. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C, Wang MW, Tashiro S, Onodera S and
Ikejima T: Roles of SIRT1 and phosphoinositide 3-OH kinase/protein
kinase C pathways in evodiamine-induced human melanoma A375-S2 cell
death. J Pharmacol Sci. 97:494–500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Evodiamine induces tumor cell death through different
pathways: apoptosis and necrosis. Acta Pharmacol Sin. 25:83–89.
2004.PubMed/NCBI
|
|
6
|
Chen MC, Yu CH, Wang SW, Pu HF, Kan SF,
Lin LC, Chi CW, Ho LL, Lee CH and Wang PS: Anti-proliferative
effects of evodiamine on human thyroid cancer cell line ARO. J Cell
Biochem. 110:1495–1503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kan SF, Huang WJ, Lin LC and Wang PS:
Inhibitory effects of evodiamine on the growth of human prostate
cancer cell line LNCaP. Int J Cancer. 110:641–651. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kan SF, Yu CH, Pu HF, Hsu JM, Chen MJ and
Wang PS: Anti-proliferative effects of evodiamine on human prostate
cancer cell lines DU145 and PC3. J Cell Biochem. 101:44–56. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang DM, Guh JH, Huang YT, Chueh SC,
Chiang PC and Teng CM: Induction of mitotic arrest and apoptosis in
human prostate cancer pc-3 cells by evodiamine. J Urol.
173:256–261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao CH, Pan SL, Guh JH, Chang YL, Pai HC,
Lin CH and Teng CM: Antitumor mechanism of evodiamine, a
constituent from Chinese herb Evodiae fructus, in human
multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro
and in vivo. Carcinogenesis. 26:968–975. 2005.PubMed/NCBI
|
|
11
|
Wang XN, Han X, Xu LN, Yin LH, Xu YW, Qi Y
and Peng JY: Enhancement of apoptosis of human hepatocellular
carcinoma SMMC-7721 cells through synergy of berberine and
evodiamine. Phytomedicine. 15:1062–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu LH, Liu XD, Tan YH, Li JF, Du BY and
Wu YY: Proliferation-inhibited and apoptosis-inducted effects of
evodiamine on human hepatoma cell line HepG2. Chinese
Pharmacological Bulletin. 1:68–71. 2009.
|
|
13
|
Fei XF, Wang BX, Li TJ, Tashiro S, Minami
M, Xing DJ and Ikejima T: Evodiamine, a constituent of Evodiae
Fructus, induces anti-proliferating effects in tumor cells.
Cancer Sci. 94:92–98. 2003.
|
|
14
|
Ogasawara M, Matsubara T and Suzuki H:
Inhibitory effects of evodiamine on in vitro invasion and
experimental lung metastasis of murine colon cancer cells. Biol
Pharm Bull. 24:917–920. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ogasawara M and Suzuki H: Inhibition by
evodiamine of hepatocyte growth factor-induced invasion and
migration of tumor cells. Biol Pharm Bull. 27:578–582. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei WT, Chen H, Wang ZH, Ni ZL, Liu HB,
Tong HF, Guo HC, Liu DL and Lin SZ: Enhanced antitumor efficacy of
gemcitabine by evodiamine on pancreatic cancer via regulating
PI3K/Akt pathway. Int J Biol Sci. 8:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen W, Zheng R, Zhang S, et al: The
incidences and mortalities of major cancers in China, 2009. Chin J
Cancer. 32:106–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang H, Zhang Y, Liu X, Li Z, Xu W, He S,
Huang Y and Zhang H: Acid sphingomyelinase contributes to
evodiamine-induced apoptosis in human gastric cancer SGC-7901
cells. DNA Cell Biol. 30:407–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|