Introduction
Although safe and effective vaccination for the
hepatitis B virus (HBV) is available for developing countries,
according to the World Health Organisation, >350 million people
are chronically infected with HBV (1). Long-term infection with HBV may lead
to cirrhosis and hepatocellular carcinoma, and there continues to
be no effective treatment for the millions of chronically infected
individuals (2). Therefore, there
is a pressing requirement for the development of safe and effective
anti-HBV agents. The genus Taraxacum is a member of the
family Asteraceae and widely distributed in the warmer temperate
zones of the Northern Hemisphere. The perennial weed has been known
for its curative properties since ancient times, and has been
utilized for the treatment of various ailments, such as hepatitis,
spleen and liver complaints, dyspepsia and anorexia (3). Taraxacum spp is used in a
number of traditional and modern herbal medical systems to treat
infections and bile and liver problems, and the use of
Taraxacum spp in Asia, Europe and North America has been
documented for the treatment of liver disorders (4).
Taraxacum mongolicum (T. mongolicum)
Hand.- Mazz., also known as Chinese dandelion, is a member of the
Taraxacum genus, which has been widely used in traditional
Chinese medicine for its curative effects, particularly with liver
disorders (5). Apart from being
used pharmaceutically, the inflorescences, leaves and roots of
T. mongolicum are processed into different food products.
Additionally, T. mongolicum extracts (TME) are often used as
flavor components in various food products, including alcoholic
beverages, soft drinks, frozen dairy desserts, candy, baked goods,
gelatins, puddings and cheese (6).
Detailed phytochemical studies regarding T. mongolicum have
been performed and have indicated that the phenolic and flavonoid
compounds are the major components of the plant (6–10).
Although T. mongolicum and herbal preparations containing it
have been used by herbalists and other traditional healers to treat
liver diseases for hundreds of years, modern pharmacological
studies regarding T. mongolicum have rarely been reported.
There have been limited numbers of reports regarding the biological
activity of T. mongolicum, including its antitumor and
antioxidative effects (9,11). Furthermore, the effect of a
traditional herbal preparation containing T. mongolicum was
observed in patients with chronic hepatitis B. The results showed
that liver enzymes were significantly more likely to return to
normal levels in patients who had been administered the
preparation. Of the 51 patients receiving the treatment, eight were
effectively cured, while in the control group, only one person was
cured (12). Though the curative
properties of T. mongolicum have scientific support, this
hypothesis is mainly based on the empirical findings over hundreds
of years. Therefore, further research is essential.
In order to validate the antiviral effect of T.
mongolicum against HBV, D-galactosamine (D-GalN), thioacetamide
(TAA) and tert-butyl hydroperoxide (t-BHP)-induced neonatal rat
primary hepatocyte damage models were used to assess the protective
activity of TME on hepatocytes. The primary duck fetal hepatocytes
infected with duck hepatitis B virus (DHBV) and HBV-transfected
HepG2.2.15 cells were employed in order to evaluate the antiviral
properties of TME against HBV. To quantify the active ingredients
of TME, the total phenolic and flavonoid contents of TME were
determined by colorimetry. In addition, the extract was analyzed by
high-performance liquid chromatography (HPLC) and its principle
components (caffeic acid and luteolin-7-O-β-D-glucopyranoside) were
isolated and authenticated.
Materials and methods
Chemicals and reagents
Fetal bovine serum, 1640 medium and Dulbecco’s
modified Eagle’s medium (DMEM) were purchased from Gibco-BRL (Grand
Island, NY, USA). G418 was obtained from Invitrogen Life
Technologies (Carlsbad, CA, USA). D-GalN, t-BHP, TAA,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
insulin, hydrocortisone and silybin were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Lamivudine was purchased from
GlaxoSmithKline (China) Investment Co., Ltd. (Beijing, China). HBV
DNA PCR-fluorescence quantitation kit and enzyme-immunoassay (EIA)
kits for the detection of hepatitis B surface antigen (HBsAg) and
hepatitis B envelope antigen (HBeAg) were provided by Shanghai
Kehua Bio-engineering Co., Ltd (Shanghai, China). A plasmid mini
preparation kit was purchased from Axygen Biosciences (Union City,
CA, USA). The viral DNA kit was obtained from Omega Bio-Tek, Inc.
(Norcross, GA, USA). SYBR® Premix Ex Taq™ II, Taq DNA
polymerase, PMD-18T vector kit, DNA marker and conventional PCR
reagents were obtained from Takara Biotechnology (Dalian) Co., Ltd.
(Dalian, China). All other reagents were of the highest commercial
grade available.
Preparation of TME
The whole herb of T. mongolicum Hand.-Mazz.
was purchased from Haozhou herbal medicine market (Anhui, China)
and verified by Professor Liurong Chen, College of Pharmaceutical
Sciences, Zhejiang University (Hangzhou, China). The voucher
specimen (TM-200405) of the plant was deposited at the herbarium of
the College of Pharmaceutical Sciences, Zhejiang University. The
dried whole herb of T. mongolicum (10 kg) was extracted
three times with 95% ethanol. The extract was combined and
concentrated under reduced pressure to generate a dark tar-like
mass, which was dissolved in hot water. This solution was treated
with 5% sodium carbonate, followed by three extractions with
chloroform to remove lipophilic constituents. The remaining aqueous
extract was further partitioned with ethyl acetate following
acidification to pH 2 using 1N hydrochloric acid. The ethyl acetate
fraction was washed with water to pH 7 and condensed under reduced
pressure (0.06 Mpa) to afford a dark-brown powder (160 g), which
was termed TME for further use.
Determination of total phenolics and
flavonoids
The total phenolic content of TME was determined
colorimetrically using ferric chloride-potassium ferricyanide
reagent by a modified colorimetric method (13). Absorbance was measured at 763 nm
with a UV-1600 Spectrophotometer (Beijing Rayleigh Analytical
Instrument Co., Beijing, China). The total phenolic content of TME
was calculated using the standard curve of caffeic acid. The total
flavonoid content in TME was determined using sodium
nitrite-aluminum nitrate-sodium hydroxide reagent by colorimetric
assay (14). Absorbance was
measured at 500 nm and the total flavonoid content of TME was
calculated using the standard curve of rutin. Chemical analyses
were repeated three times.
HPLC analysis of TME
TME was analyzed using a HPLC system (Waters 2695,
Waters, Milford, MA, USA) and a symmetry® C18 (5 μm, 4.6
× 150 mm) column was used (Waters). The mobile phase consisted of
two eluents: 0.1% acetic acid (B) and methanol (A). To achieve
separation, the flow rate was set at 0.8 ml/min and 5 μl sample was
injected. The following gradient elution method was adopted:
Initially, 90% B and 10% A maintained for 10 min followed by 55 min
linear change to 0% B and 100% A. The detection wavelength was set
at 280 nm with a column temperature of 30°C.
TME (100 g) was dissolved in 70% methanol. The
solute was applied to a polyamide column and washed with water
followed by 30, 50, 70 and 100% of methanol. The separated
fractions were repeatedly chromatographed on silica gel, reversed
phase silica gel (RP-18) and Sephadex LH-20. The 30 and 50%
methanol fractions afforded compound 1 (35 mg) and compound 2 (100
mg), respectively. Their structures were elucidated by spectroscopy
(Varian INOVA 400 MHz Nuclear Magnetic Resonance Spectrometer;
Varian, Palo Alto, CA, USA) and compared with literature data
(15,16). The standard solutions of compound 1
and compound 2 were prepared in the same mobile phase for HPLC
analysis.
Isolation and primary culture of neonatal
rat hepatocytes
The experimental protocol was approved by the Animal
Ethics Committee of Zhejiang Province (Zhejiang, China), in
accordance with the international standard on the care and use of
experimental animals. Rat hepatocytes were isolated from
three-day-old Sprague-Dawley (Zhejiang Experimental Animal Center,
Hangzhou, China) rats according to the method of Anil Kumar et
al (17). The isolated
hepatocytes were suspended in 1640 medium and subsequently
transferred to 96-well culture plates at a density of
~1.0×105 cells/ml. Following hepatocyte attachment to
the plate, cells were treated with hepatotoxic agents and analyzed
in the following assays.
Effect of TME on D-GalN-, TAA- or
t-BHP-injured rat hepatocytes
Cytotoxicity induced by TME treatment was detected
by MTT assay as follows: Hepatocytes were cultured in 1640 medium
in the presence of 1–100 μg/ml TME for 48 h and subsequently 10 μl
MTT (5 mg/ml) was added to the cells in each well. Following
culturing for 4 h, the medium was removed and the blue formazan
crystals that had formed were dissolved in dimethylsulfoxide. The
absorbency of formazan generated from MTT was measured at 570 nm
using an ELX 800 universal microplate reader (BioTek Instruments,
Winooski, VT, USA). Cell survival was defined as the quantity of
formazan produced relative to that of the untreated control
cells.
The hepatocytes were incubated for a further 48 h in
fresh culture medium containing 1–100 μg/ml TME after the cells had
been incubated for 8 h with 20 mM D-GalN, 2 h with 8 mM TAA and 1.5
h with 6.5 mM t-BHP, respectively. Silibin was used as a reference
drug. Hepatocyte injury was assessed according to cell viability.
Cell viability was expressed according to protection rate (%) and
proliferation index.
Isolation and primary culture of duck
fetal hepatocytes
Duck hepatocytes were isolated from 20-day-old
embryonated, unhatched, duck eggs laid by DHBV-infected ducks
according to a modified method (18). Cells were maintained at
1.0×105 cells/ml in 1640-medium containing 2 mM
glutamine, 1 μg/ml insulin, 7.5 μg/ml hydrocortisone, 10% (v/v)
fetal bovine serum, 100 U/ml penicillin and 100 U/ml streptomycin
at 37°C in a 5% CO2 atmosphere. Congenital DHBV
infection was confirmed by PCR assay on the viral DNA obtained from
the allantoic fluid of duck embryos using DHBV-specific primers.
The forward primer was 5′-AAC CAT TGA AGC AAT CAC TAG AC-3′ and the
reverse primer was 5′-ATC TAT GGT GGC TGC TCG AAC TA-3′.
Effect of TME on DHBV DNA replication in
duck fetal hepatocytes
Cytotoxicity induced by TME treatment was measured
using the MTT method as follows: Infected duck hepatocytes were
cultured in 1640-medium with a concentration of 1.0×105
cells/ml in the presence of 1–100 μg/ml TME for six days and
subsequently the MTT assay was performed as described previously.
Cytotoxicity was described according to the quantity of formazan
produced relative to that produced by the untreated control
cells.
In order to measure the DHBV DNA levels, the
infected duck hepatocytes were treated with varying concentrations
of TME and the medium with the test compound was replaced every
three days. On the third and sixth days, the DHBV DNA level in the
replaced culture supernatants was detected with a SYBR-green
dye-based quantitative PCR assay as follows: DHBV DNA was extracted
and amplified with a Bio-Rad iQ5 Real Time PCR system (Bio-Rad
Laboratories Inc., Irvine, CA, USA). The thermal program comprised
of an initial denaturation at 94°C for 10 min followed by 40
amplification cycles at 94°C for 30 sec, 55°C for 30 sec, and then
72°C for 45 sec. The forward primer was 5′-AGC TGG CCT AAT CGG ATT
AC-3′ and the reverse primer was 5′-TGT CCG TCA GAT ACA GCA AG-3′.
SYBR® Premix Ex Taq™ II was used to amplify and detect
the DNA level during the reaction. A plasmid containing the DHBV
genome was used to generate the standard curve for quantifying DHBV
levels. Lamivudine was used as the reference drug.
Effect of TME on HBV expression and
replication in HepG2.2.15 cells
HepG2.2.15 cells were provided by the State Key
Laboratory for the Diagnosis and Treatment of Infectious Diseases,
The First Affiliated Hospital, Zhejiang University (Hangzhou,
China). The cells were maintained in DMEM containing 2 mM
glutamine, 10% (v/v) heat-inactivated fetal bovine serum and 380
μg/ml of G418 at 37°C (95% humidity, 5% CO2). The
cytotoxicity of TME in HepG2.2.15 cells was analyzed by the MTT
assay. The cells were seeded in a 96-well plate at a concentration
of 1.0×105 cells/ml. Varying concentrations of TME were
applied to culture wells in triplicate. Following six days of
incubation, the MTT assay was performed as described previously.
Cytotoxicity was described according to the quantity of formazan
produced, compared with that produced by the untreated
cultures.
To determine HBV antigen and HBV DNA levels,
HepG2.2.15 cells were treated with various concentrations of TME
and the medium with test compound was replaced every three days. On
the third day, the replaced medium was assayed for HBsAg and HBeAg.
On the sixth day, the replaced medium was measured for HBsAg, HBeAg
and HBV DNA. Lamivudine served as a positive control. The HBsAg and
HBeAg levels in the replaced culture supernatants were determined
by using HBsAg and HBeAg EIA kits, respectively. The results were
measured at 450 nm by a MULTISKAN MK3 multi-well microplate reader
(Thermo Fisher Scientific Inc., Waltham, MA, USA).
HBV DNA levels in replaced culture supernatants were
detected with a HBV DNA PCR-fluorescence quantitation kit. HBV DNA
was extracted and amplified with Bio-Rad iQ5 Real Time PCR system
(Bio-Rad, Hercules, CA, USA). The thermal program comprised of an
initial denaturation at 94°C for 2 min followed by 40 amplification
cycles each of two steps: 95°C for 5 sec and 60°C for 30 sec. The
forward primer was 5′-CCG TCT GTG CCT TCT CAT CTG-3′, the reverse
primer was 5′-AGT CCA AGA GTA CTC TTA TAG AAG ACC TT-3′ and the
Taqman probe was FAM-CCG TGT GCA CTT CGC TTC ACC TCT GC. A plasmid
containing the full-length insert of the HBV genome was used to
prepare the standard curve.
Statistical analysis
Experimental results were expressed as the mean ±
standard deviation and subjected to a one-way analysis of variance
and Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Quantitation analysis of TME
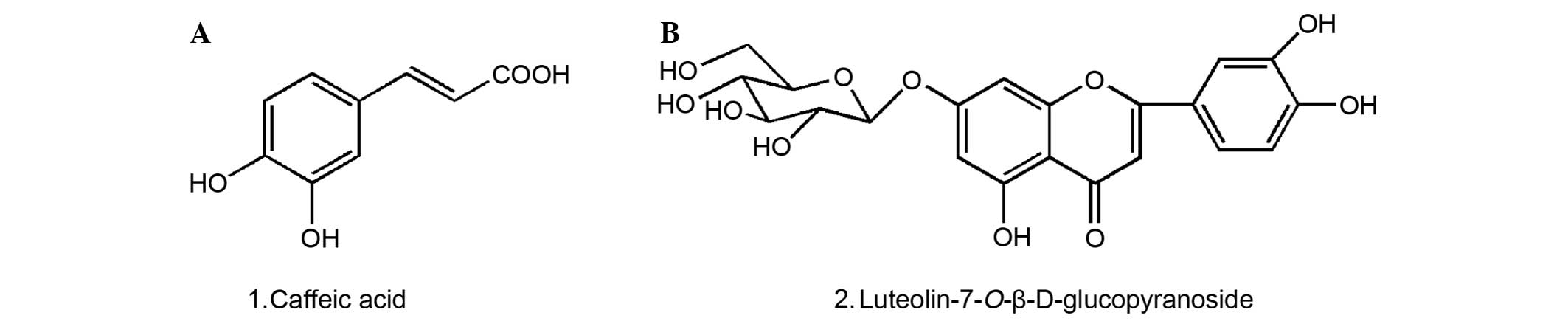
The structures of compounds 1 and 2 were identified
as caffeic acid and luteolin7-O-β-D-glucopyranoside, respectively
(Fig. 1). The results of
quantification indicated that the total phenolic acid content of
TME was 36.27%, using caffeic acid as the control standard, and the
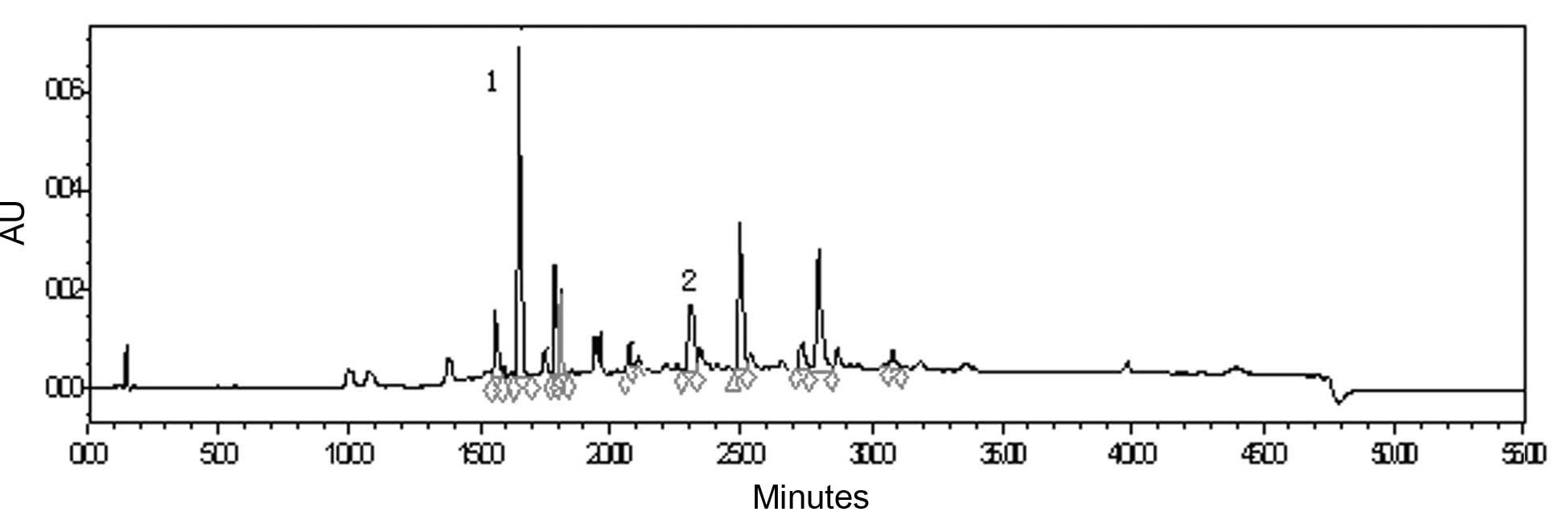
total flavonoid was 49.90% with rutin as the control. HPLC analysis
demonstrated that caffeic acid and luteolin-7-O-β-D-glucopyranoside
were the major phenolic and flavonoid components in the extract and
their absolute contents amounted to 0.6 and 1.0%, respectively
(Fig. 2).
Protective effect of TME on D-GalN-, TAA-
or t-BHP-injured rat hepatocytes
The cytotoxicity of TME toward neonatal rat primary
hepatocytes was tested. The result showed that TME, at
concentrations of 1–100 μg/ml was almost nontoxic to the cells.
Cytotoxicity was induced in neonatal rat primary hepatocytes by
exposure to 20 mM D-GalN, 8 mM TAA and 6.5 mM t-BHP, respectively.
Subsequently, the cells were treated with TME. As shown in Table I, TME concentrations between 1 and
100 μg/ml improved cell viability in a dose-dependent manner.
Silibin, used as the reference drug, induced a similar, but weaker,
effect, than that of TME at the same concentration.
 | Table IEffects of TME on D-GalN, TAA and
t-BHP-injured primary neonatal rat hepatocytes. |
Table I
Effects of TME on D-GalN, TAA and
t-BHP-injured primary neonatal rat hepatocytes.
| | D-GalN-induced
injury | TAA-induced
injury | t-BHP-induced
injury |
|---|
| |
|
|
|
|---|
| Group | Concentration
(μg/ml) | Protection rate
(%) | Proliferation
index | Protection rate
(%) | Proliferation
index | Protection rate
(%) | Proliferation
index |
|---|
| Vehicle | - | - | - | - | - | - | - |
| Chemical-control | - | - | - | - | - | - | - |
| Silybin | 100 | 22.8 | 3.20 | 20.6 | 2.98 | 24.1 | 3.60 |
| 50 | 7.9 | 1.76 | 9.7 | 1.94 | 14.6 | 2.58 |
| 10 | 1.0 | 1.10 | 1.8 | 1.18 | 7.8 | 1.84 |
| 1 | 0.1 | 1.01 | - | 1.00 | 1.2 | 1.13 |
| TME | 100 | 42.2 | 5.06 | 34.6 | 4.32 | 43.8 | 5.73 |
| 50 | 25.0 | 3.41 | 15.3 | 2.46 | 27.6 | 3.98 |
| 10 | 5.7 | 1.55 | 4.4 | 1.42 | 16.0 | 2.74 |
| 1 | 0.6 | 1.06 | 2.6 | 1.25 | 4.3 | 1.47 |
Antiviral effect of TME against DHBV in
primary duck fetal hepatocytes
The cytotoxicity test indicated that TME was
nontoxic to primary duck fetal hepatocytes at concentrations
between 1–100 μg/ml. The effect of TME on the DHBV DNA level is
shown in Table II. Following cell
treatment with TME for three days, TME at concentrations between
50–100 μg/ml significantly reduced DHBV DNA levels in culture
supernatants compared with vehicle. On the sixth day, TME markedly
inhibited the DHBV DNA replication at a concentration of 1–100
μg/ml. Lamivudine, used as the standard drug, also induced a
similar effect.
 | Table IIAntiviral effect of TME against DHBV
in infected primary duck fetal hepatocytes following cell treatment
with test compound for 3 and 6 days. |
Table II
Antiviral effect of TME against DHBV
in infected primary duck fetal hepatocytes following cell treatment
with test compound for 3 and 6 days.
| |
logDHBV-DNA (copy/μl) |
|---|
| |
|
|---|
| Compound | Concentration
(μg/ml) | 3 days | 6 days |
|---|
| Vehicle | - | 3.714±0.036 | 3.839±0.024 |
| Lamivudine | 100 | 3.607±0.001b | 3.562±0.042c |
| TME | 100 | 3.393±0.088b | 3.346±0.036c |
| 50 | 3.610±0.029a | 3.555±0.047c |
| 25 | 3.630±0.050 | 3.558±0.054b |
| 10 | 3.697±0.024 | 3.575±0.039c |
| 1 | 3.707±0.024 | 3.662±0.037b |
Antiviral effect of TME against HBV in
HepG2.2.15 cells
Cellular toxicity tests revealed that TME was
harmless to HepG2.2.15 cells at concentrations between 1–100 μg/ml.
Expressions of HBsAg and HBeAg in culture supernatants were assayed
after the cells had been incubated with TME for 3 days (Table III). The results indicated that
TME had significant inhibitory effects on HBsAg expression at
concentrations between 10–100 μg/ml and the HBeAg expression at
concentrations between 50–100 μg/ml. TME produced the maximum
inhibition rates, on HBsAg and HBeAg expression at a concentration
of 100 μg/ml, of 59.47 and 66.37%, respectively. The levels of
HBsAg, HBeAg and HBV DNA in culture supernatants were measured
after the cells had been treated with TME for 6 days (Table IV). At concentrations between
10–100 μg/ml, TME significantly inhibited HBsAg expression. At
concentrations between 25–100 μg/ml, TME significantly reduced
HBeAg levels. At a concentration of 100 μg/ml, TME produced the
maximum inhibition rates of 91.39 and 91.72% on HBsAg and HBeAg
expression, respectively. Furthermore, TME markedly inhibited HBV
DNA replication at 25–100 μg/ml.
 | Table IIIAntiviral effect of TME against HBV
in HepG2.2.15 cells, after treating the cells with test compound
for three days. |
Table III
Antiviral effect of TME against HBV
in HepG2.2.15 cells, after treating the cells with test compound
for three days.
| | HBsAg | HBeAg |
|---|
| |
|
|
|---|
| Compound | Concentration
(μg/ml) | Absorbency (570
nm) | Inhibition (%) | Absorbency (570
nm) | Inhibition (%) |
|---|
| Vehicle | - | 1.200±0.033 | - | 0.893±0.079 | - |
| Lamivudine | 100 | 0.794±0.147b | 33.81 | 0.729±0.045a | 18.36 |
| 50 | 0.945±0.064b | 21.20 | 0.793±0.027 | 11.20 |
| 25 | 1.096±0.013b | 8.69 | 0.849±0.107 | 4.96 |
| 10 | 1.153±0.123 | 3.89 | 0.964±0.059 | - |
| 1 | 1.229±0.079 | - | 0.916±0.104 | - |
| TME | 100 | 0.486±0.011c | 59.47 | 0.300±0.010c | 66.37 |
| 50 | 0.627±0.013c | 47.78 | 0.536±0.016b | 40.01 |
| 25 | 0.860±0.048c | 28.36 | 0.778±0.067 | 12.88 |
| 10 | 1.072±0.061a | 10.67 | 0.783±0.095 | 12.32 |
| 1 | 1.198±0.032 | 0.17 | 0.824±0.160 | 7.76 |
 | Table IVAntiviral effect of TME against HBV
in HepG2.2.15 cells, after the cells had been treated with the test
compound for six days. |
Table IV
Antiviral effect of TME against HBV
in HepG2.2.15 cells, after the cells had been treated with the test
compound for six days.
| | HBsAg | HBeAg | |
|---|
| |
|
| |
|---|
| Compound | Concentration
(μg/ml) | Absorbency | Inhibition (%) | Absorbency | Inhibition (%) |
logHBV-DNA (copy/μl) |
|---|
| Vehicle | - | 2.497±0.009 | - | 2.360±0.088 | - | 5.137±0.032 |
| Lamivudine | 100 | 1.728±0.173b | 30.60 | 1.921±0.145a | 18.24 | 4.923±0.127a |
| 50 | 1.905±0.095c | 23.48 | 2.002±0.175a | 14.79 | 5.078±0.048 |
| 25 | 2.150±0.091b | 13.67 | 2.084±0.126a | 11.32 | 5.081±0.036 |
| 10 | 2.175±0.142a | 12.66 | 2.244±0.102 | 4.52 | 5.228±0.024 |
| 1 | 2.475±0.142 | 0.61 | 2.387±0.169 | - | 5.281±0.149 |
| TME | 100 | 0.214±0.010c | 91.39 | 0.195±0.122c | 91.72 | 4.825±0.039c |
| 50 | 0.696±0.031c | 72.06 | 0.472±0.004c | 79.93 | 4.954±0.077a |
| 25 | 1.631±0.119c | 34.51 | 1.470±0.099c | 37.43 | 4.978±0.075a |
| 10 | 1.445±0.098a | 41.95 | 2.118±0.138 | 9.87 | 5.076±0.073 |
| 1 | 2.557±0.031 | - | 2.523±0.052 | - | 5.208±0.067 |
Discussion
The beneficial role of hepatoprotectors in viral
hepatitis is achieved through their inhibitory action on
inflammatory and cytotoxic cascades induced by viral infection
(19). Furthermore, these agents
are capable of improving the regeneration process and normalizing
liver enzymes through their effects on protein synthesis (19). The hepatocyte-protective effect of
TME was evaluated by using hepatotoxic agents, such as D-GalN, TAA
and t-BHP-induced neonatal rat hepatocyte injury models. Cell
viability was expressed by the protection rate (%) and
proliferation index. The results indicated that TME possesses
potent hepatocyte protective activity. Furthermore, TME afforded
stronger protection on D-GalN-, TAA- or
t-BHP-injured rat hepatocytes compared with the reference
drug Silibin at the same concentration (Table I), thereby demonstrating the
protective effect of TME on chemically injured hepatocytes. These
data provided partial evidence for the clinical use of TME as a
hepatoprotective drug.
D-GalN is a hepatotoxic agent that interferes with
the metabolism of uridine diphosphate glucose and is often used in
pharmacodynamics research to induce hepatic injury. It has been
suggested that oxygen-derived free radicals released from activated
hepatic macrophages are the primary cause of D-GalN-induced liver
damage (20). Increased reactive
oxygen species production has been reported in primary cultures of
rat hepatocytes with damage induced by D-GalN (21). TAA is a thiono-sulfur-containing
compound with liver-damaging and carcinogenic effects. Several
studies using rats and cultured cells have indicated the
involvement of oxidative stress in the etiology of TAA-induced
liver damage (22,23). t-BHP is often used in studies
investigating the mechanism of cell injury initiated by acute
oxidative stress. The compound is metabolized by cytochrome P-450
in hepatocytes or by hemoglobin in erythrocytes to free-radical
intermediates, which in turn initiate lipid peroxidation and
glutathione depletion. These changes compromise cellular integrity
and cause cell injury in cultured hepatocytes (24,25).
In cells exposed to D-GalN, TAA and t-BHP, subsequent treatment
with TME improved cell viability, suggesting that its ability to
ameliorate oxidative stress may have contributed to the improvement
of chemical-induced hepatocyte damage.
DHBV is similar to HBV in terms of the mechanism of
replication and genome organization (26,27).
The natural route of transmission is from the bloodstream of
persistently infected laying ducks to the egg, resulting in
congenital infection (28).
Congenitally infected ducks are at risk of developing hepatoma or
secondary amyloidosis due to chronic stimulation of the immune
system (29). The DHBV-infected
primary duck hepatocyte model is a valuable model for hepadnavirus
infection with high reproducibility and efficiency for evaluating
novel agents directed against HBV (30). In the present study, TME at
concentrations between 1–100 μg/ml significantly inhibited the
replication of DHBV DNA in duck fetal hepatocytes, thus indicating
that the antiviral effect of TME against DHBV is possibly
associated with blocking viral DNA replication.
HepG2.2.15 cells are derived from human
hepatoblastoma HepG2 cells that were transfected with a plasmid
containing HBV DNA. The cells are capable of stably secreting viral
particles in the culture medium (31). HBV is a small double-stranded DNA
virus composed of an outer envelope containing HBsAg and an inner
nucleocapsid consisting of HBeAg and hepatitis B core antigen. The
viral core also contains a double stranded DNA genome and DNA
polymerase. The presence of HBsAg is the most common marker for HBV
infection, whereas HBeAg is used as an ancillary marker primarily
to indicate active HBV replication and associated progressive liver
disease (32). In the present
study, TME at concentrations between 25–100 μg/ml significantly
reduced HBsAg, HBeAg and HBV DNA levels in HepG2.2.15 cells,
thereby suggesting that the antiviral effect of TME against HBV is
associated with blocking the steps of protein synthesis and DNA
replication.
Phytochemical and HPLC assaying of TME led to the
conclusion that this extract fraction contains a large quantity of
phenolic and flavonoid compounds, such as caffeic acid and
luteolin-7-O-β-D-glucopyranoside. Phenolic and flavonoid compounds
exhibit several pharmacological activities, such as antioxidative,
anti-inflammatory and antiviral effects (33,34).
TME exhibited a pronounced protective potential against oxidative
damage in accordance with the description of the pharmacological
effects of phenolic and flavonoid compounds in the aforementioned
references. These data supported the former hypothesis that TME
possesses efficacy against chemical-induced hepatocyte injury and
virus infection. Therefore, phenolic and flavonoid compounds may be
the major active components responsible for the biological activity
of TME.
In conclusion, this investigation verifies the
potent antiviral effect of TME against HBV in cell culture.
Furthermore, the protective effect on hepatocytes and antiviral
effects of TME were stronger than those of the reference drugs
silibin and lamivudine, respectively. The protective effect of TME
on hepatocytes was achieved by its ability to ameliorate oxidative
stress. The antiviral effect of TME may contribute to blocking
protein synthesis steps and DNA replication. This study provides
scientific evidence for the use of T. mongolicum in treating
hepatitis.
Acknowledgements
This study was supported by a grant from the Key
Project of the Chinese Ministry of Education (grant no. 212073),
the China Postdoctoral Science Foundation (grant no. 20110491806)
and the Project for Supporting Xinjiang through Science and
Technology (grant no. 201191260).
References
|
1
|
Kao JH and Chen DS: Global control of
hepatitis B virus infection. Lancet Infect Dis. 2:395–403. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liaw YF: Therapy of chronic hepatitis B:
current challenges and opportunities. J Viral Hepat. 9:393–399.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schütz K, Carle R and Schieber A:
Taraxacum - a review on its phytochemical and pharmacological
profile. J Ethnopharmacol. 107:313–323. 2006.PubMed/NCBI
|
|
4
|
Yarnell E and Abascal K: Dandelion
(Taraxacum officinale and T. mongolicum). Integr Med.
8:35–38. 2009.
|
|
5
|
Song L, Hong X and Ding X: Dictionary of
Modern Chinese Medicine. People’s Health Publishers; Beijing: pp.
22412001, (In Chinese).
|
|
6
|
Shi S, Zhou H, Zhang Y, Zhao Y, Huang K
and Liu S: A high-speed counter-current chromatography-HPLC-DAD
method for preparative isolation and purification of two
polymethoxylated flavones from Taraxacum mongolicum. J
Chromatogr Sci. 47:349–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ling Y, Bao Y, Guo X, Xu Y, Cai S and
Zheng J: Isolation and identification of two flavonoids from
Taraxacum mongolicum Hand.-Mazz. Zhongguo Zhong Yao Za Zhi.
24:225–226. 1999.(In Chinese).
|
|
8
|
Shi S, Zhang Y, Zhao Y and Huang K:
Preparative isolation and purification of three flavonoid
glycosides from Taraxacum mongolicum by high-speed
counter-current chromatography. J Sep Sci. 31:683–688. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi S, Zhao Y, Zhou H, Zhang Y, Jiang X
and Huang K: Identification of antioxidants from Taraxacum
mongolicum by high-performance liquid chromatography-diode
array detection-radical-scavenging detection-electrospray
ionization mass spectrometry and nuclear magnetic resonance
experiments. J Chromatogr A. 1209:145–152. 2008.
|
|
10
|
Kim YH, Choo SJ, Ryoo IJ, Ahn JS and Yoo
ID: Eudesmanolides from Taraxacum mongolicum and their
inhibitory effects on the production of nitric oxide. Arch Pharm
Res. 34:37–41. 2011.
|
|
11
|
Kim DH and Kim SH: Antitumor activity of
Taraxacum mongolicum. J Korean Med Sci. 16:386–413.
1995.
|
|
12
|
Chen Z: Clinical study of 96 cases with
chronic hepatitis B treated with jiedu yanggan gao by a
double-blind method. Zhong Xi Yi Jie He Za Zhi. 10:71–74. 1990.(In
Chinese).
|
|
13
|
Singh M, Singh SS and Sanwal GG: A new
colorimetric method for the determination of pheonlics. Indian J
Exp Biol. 16:712–714. 1978.
|
|
14
|
Dewanto V, Wu X, Adom KK and Liu RH:
Thermal processing enhances the nutritional value of tomatoes by
increasing total antioxidant activity. J Agric Food Chem.
50:3010–3014. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choudhary MI, Naheed N, Abbaskhan A,
Musharraf SG, Siddiqui H and Atta-Ur-Rahman: Phenolic and other
constituents of fresh water fern Salvinia molesta.
Phytochemistry. 69:1018–1023. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barberán FA, Hernández L, Ferreres F and
Tomás F: Highly methylated 6-hydroxyflavones and other flavonoids
from Thymus piperella. Planta Med. 51:452–454.
1985.PubMed/NCBI
|
|
17
|
Anil Kumar PR, Menon Bindu, Anil Kumar TV
and Kumari TV: Culture of neonatal rat liver cells: a preliminary
observation. Trends Biomater Artif Organs. 16:34–47. 2002.
|
|
18
|
Borel C, Schorr O, Durand I, Zoulim F, Kay
A, Trepo C and Hantz O: Initial amplification of duck hepatitis B
virus covalently closed circular DNA after in vitro infection of
embryonic duck hepatocytes is increased by cell cycle progression.
Hepatology. 34:168–179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Srivastava S, Srivastava AK, Srivastava S,
Patnaik GK and Dhawan BN: Effect of picroliv and silymarin on liver
regeneration in rats. Indian J Pharmacol. 26:19–22. 1994.
|
|
20
|
Hu HL and Chen RD: Changes in free
radicals, trace elements, and neurophysiological function in rats
with liver damage induced by D-galactosamine. Biol Trace Elem Res.
34:19–25. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quintero A, Pedraza CA, Siendones E, et
al: PGE1 protection against apoptosis induced by D-galactosamine is
not related to the modulation of intracellular free radical
production in primary culture of rat hepatocytes. Free Radic Res.
36:345–355. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akbay A, Cinar K, Uzunalimoğlu O, Eranil
S, Yurdaydin C, Bozkaya H and Bozdayi M: Serum cytotoxin and
oxidant stress markers in nacetylcysteine treated thioacetamide
hepatotoxicity of rats. Hum Exp Toxicol. 18:669–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cascales M, Martín-Sanz P, Craciunescu DG,
Mayo I, Aguilar A, Robles-Chillida EM and Cascales C: Alterations
in hepatic peroxidation mechanisms in thioacetamide-induced tumors
in rats. Effect of a rhodium (III) complex. Carcinogenesis.
12:233–240. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thornalley P, Trotta RJ and Stern A: Free
radical involvement in the oxidative phenomena induced by
tert-butyl hydroperoxide in erythrocytes. Biochem Biophys Acta.
759:16–22. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rush GF, Gorski JR, Ripple MG, Sowinski J,
Bugelski P and Hewitt WR: Organic hydroperoxide-induced lipid
peroxidation and cell death in isolated hepatocytes. Toxicol Appl
Pharmacol. 78:473–483. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mason WS, Seal G and Summers J: Virus of
Pekin ducks with structural and biological relatedness to human
hepatitis B virus. J Virol. 36:829–836. 1980.PubMed/NCBI
|
|
27
|
Summers J and Mason WS: Replication of the
genome of a hepatitis B-like virus by reverse transcription of an
RNA intermediate. Cell. 29:403–415. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Omata M, Uchiumi K, Ito Y, Yokosuka O,
Mori J, Terao K, Wei-Fa Y, O’Connell AP, London WT and Okuda K:
Duck hepatitis B virus and liver disease. Gastroenterology.
85:260–267. 1983.PubMed/NCBI
|
|
29
|
Duflot A, Mehrota R, Yu S, Barraud L,
Trepo C and Cova L: Spectrum of liver disease and duck hepatitis B
virus infection in a large series of Chinese ducks with
hepatocellular carcinoma. Hepatology. 21:1483–1491. 1995.PubMed/NCBI
|
|
30
|
Schultz U, Grgacic E and Nassal M: Duck
hepatitis B virus: an invaluable model system for HBV infection.
Adv Virus Res. 63:1–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sureau C, Romet-Lemonne JL, Mullins JI and
Essex M: Production of hepatitis B virus by a differentiated human
hepatoma cell line after transfection with cloned circular HBV DNA.
Cell. 47:37–47. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang RL, Chen CF, Feng HY, Lin LC and
Chou CJ: Anti-hepatitis B virus of seven compounds isolated from
Piper Kadsura (Choisy) Ohwi. Chin Med J. 12:179–190. 2001.
|
|
33
|
McDougall B, King PJ, Wu BW, Hostomsky Z,
Reinecke MG and Robinson WE Jr: Dicaffeoylquinic and
dicaffeoyltartaric acids are selective inhibitors of human
immunodeficiency virus type 1 integrase. Antimicrob Agents
Chemother. 42:140–146. 1998.PubMed/NCBI
|
|
34
|
Sandhar HK, Kumar B, Prasher S and Sharma
P: A review of phytochemistry and pharmacology of flavonoids. Int
Pharm Sci. 1:25–41. 2011.
|