Introduction
Paraquat is one of the most widely used herbicides
worldwide, and has been approved for use by authorities in >120
countries. It is used on numerous crop types and is important for
controlling weeds on plantation estates (1–3). For
these reasons, it is particularly popular in the Chinese
countryside and is widely used by Chinese farmers (4). Paraquat is highly toxic for humans,
and many cases of acute poisoning, particularly cases of
intentional self-poisoning, have been reported over the past few
decades in China. In addition, nearly all treatments for paraquat
poisoning are unsuccessful (5).
Agricultural chemical intoxication is the major cause of poisoning,
and it remains a major cause of death of among Chinese farm
workers. Ingestion of large quantities of paraquat results in rapid
mortality, of which acute lung injury is the one of the major
causes. However, smaller doses often result in delayed lung
fibrosis that is also usually fatal. Little is known with regard to
the pathogenesis of acute lung injury and the fibrosis caused by
paraquat. Therefore, it is imperative to understand the underlying
mechanisms. Paraquat-induced pulmonary fibrosis involves two
factors, direct injury by oxygen free radicals and indirect injury
by inflammatory cells and fibroblasts (6,7).
Certain patients may develop pulmonary fibrosis, which may
progressively improve over time (8,9).
Paraquat is known to induce toxicity in cells by stimulating oxygen
utilization via redox cycling and the generation of reactive oxygen
intermediates (10–12). However, the exact role of paraquat
in the progression of pathogenesis has not been clearly established
(13–15).
Transforming growth factor-β1 (TGF-β1) contributes
to the fibrosis of injured organs (16). Abnormal expression of TGF-β1 is
hypothesized to be important in the pathogenesis of pulmonary
fibrosis (17). In order to
understand the mechanism of paraquat-induced pulmonary toxicity, an
animal model of paraquat-induced lung injury was developed by
intragastrically administering paraquat solution to Wistar rats.
The pathological progression of lung pathology in the rat model was
similar to that of patients presenting with paraquat poisoning. The
aim of this study was to establish the role of TGF-β1 in acute lung
injury caused by paraquat.
Materials and methods
Preparation of animal model
In total 32, 240–260 g, healthy adult male Wistar
rats (SPF, Code SCXK20100004, provided by the Experimental Animal
centre of Shandong University, Jinan, China) were randomly assigned
to the normal control group (n=8), 30 mg/kg paraquat (20% wt/vol,
imported from Syngenta AG, Basel, Switzerland) poisoning group
(n=8), 60 mg/kg paraquat poisoning group (n=8) and 120 mg/kg
paraquat poisoning group (n=8). Paraquat-treated rats were
administered the corresponding dose of 1 ml paraquat by lavaging
while the control group rats were administered 1 ml distilled
water. The rats were sacrificed with anaesthetic 48 h after
paraquat poisoning, and the serum and partial right lung tissues
were frozen at −70°C. Other lung tissues were maintained in
formaldehyde and glutaraldehyde for histopathological inspection.
The stuy was approved by Experimental Animal Ethics Committee of
Qilu Hospital of Shandong University (Jinan, China).
Measurement of serum TGF-β1
The rat serum TGF-β1 levels were determined by
enzyme linked immunosorbent assay (ELISA) according to the
manufacturer’s instructions [Shanghai SenXiong Biotech Industry
Co., Ltd (Shanghai, China), imported from R&D Systems
(Minneapolis, MN, USA)]. The assay method used was as follows: All
reagents were prepared and 100 μl of standard or activated samples
were added to the appropriate wells. The plate was covered and
incubated at 37°C for 2 h. Each well was aspirated and washed, and
the process was repeated three times for a total of four washes.
For washing, each well was filled with wash buffer (400 μl) using a
squirt bottle, multi-channel pipette, manifold dispenser or
autowasher. The complete removal of liquid at each step was
essential for successful analysis. After the final wash, any
remaining wash buffer by was removed by aspirating or decanting.
The plate was inverted and blotted with clean paper towels.
Biotin-conjugated anti-rat TGF-β1 (50 μl) from the kit was added to
each well, followed by incubation for 1 h at 37°C and a further
aspiration/wash. Working streptavidin-horseradish peroxidase
conjugate (100 μl) was added to each well, followed by incubation
for 1 h at 37°C and a further aspiration/wash. Next, 100 μl working
substrate solution was added to each well, followed by incubation
at 37°C for 5–10 minutes in the dark. Stop solution (50 μl) was
then added to each well and the absorbance was measured with a
Bio-Rad Model 680 microplate reader (Bio-Rad, Hercules, CA, USA) at
492 nm within 1 h.
Analysis of rat lung tissue TGF-β1 mRNA
expression
Fluorescence quantitative PCR (qPCR) was used to
measure lung tissue TGF-β1 mRNA expression. Takara RNAiso reagent
(Takara Bio, Inc., Shiga, Japan; code no. D312) was used initially
to extract sample total RNA and DNase I (Takara Bio Inc.; code no.
D2215) was used in the procedure. qPCR was performed using the
Takara SYBR® ExScript™ RT-PCR kit (Takara Bio Inc.; code
no. DRR053) (Table I).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name | Sequence | Length (mer) | GC (%) | Size (bp) |
|---|
| Rat-TGF-β1-F |
5′-TGCGCCTGCAGAGATTCAAG-3′ | 20 | 55.00 | 82 |
| Rat-TGF-β1-R |
5′-AGGTAACGCCAGGAATTGTTGCTA-3′ | 24 | 45.83 | |
| Rat-Actb-F |
5′-GGAGATTACTGCCCTGGCTCCTA-3′ | 23 | 56.52 | 150 |
| Rat-Actb-R |
5′-GACTCATCGTACTCCTGCTTGCTG-3′ | 24 | 54.17 | |
The RT reaction included 2 μl of 5X ExScript Buffer,
0.25 μl ExScript RTase, 0.25 μl RNase inhibitor (40 U/μl), 2 μl
dNTP mixture (10 mM), 0.5 μl Oligo dT Primer (50 μM), 0.5 μl random
6 mers (100 μM), 1 μl RNA (50 ng) and 3.5 μl RNase Free
dH2O. The total reaction volume was 10 μl and the
conditions were as follows: 37°C for 15 min and 85°C for 5 sec.
The PCR reaction included 12.5 μl 2X SYBR Premix
ExTaq, 0.5 μl Primer F/R (each 10 μM), 2 μl RT product and 10 μl
dH2O. The total reaction volume was 25 μl and the
conditions were as follows: 95°C for 10 sec, then 95°C for 5 sec
and 65°C for 30 sec, for 45 cycles. The main relative instruments
used are Takara PCR Thermal Cycler dice (Takara Bio Inc.; code no.
TP600) and Takara PCR Thermal Cycler Dice Real-Time system (Takara
Bio Inc.; code no. TP800).
Histopathological inspection of rat
model
Lung tissues underwent histopathological inspection
according to routine methods. Tissues from the right lung were
obtained and maintained in formaldehyde or glutaraldehyde. Sections
were created for histopathological inspection. We observed the
sections under a light microscope (XSP-44X9; Shanghai Optical
Instrument Factory, Shanghai, China), while the ultramicrostructure
were observed under an electron microscope (JEM-100SX, JEOL, Tokyo,
Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation
where indicated. Statistical differences were analyzed according to
the analysis of the t-test. P<0.05 was considered to indicate a
statistically significant difference. SPSS software, version 16.0
(SPSS, Inc., Chicago, IL, USA) was used to analysis the data.
Results
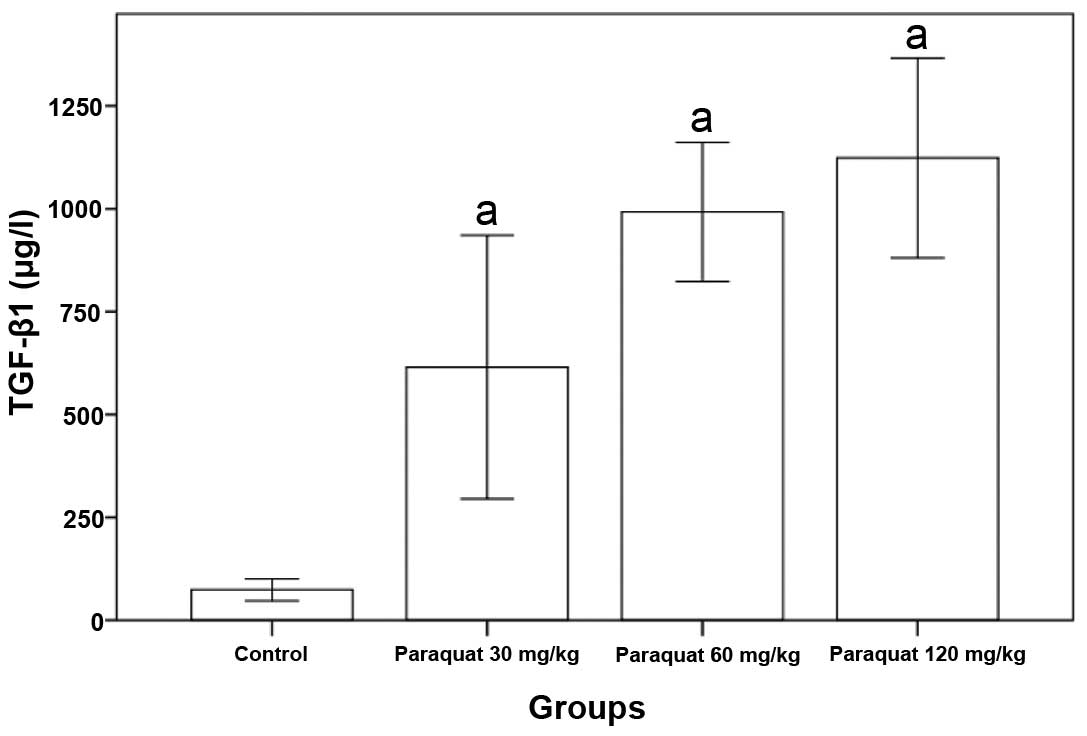
Changes in rats serum TGF-β1 levels
caused by paraquat poisoning
Rat serum TGF-β1 levels of the paraquat groups were
significantly higher than that of the control group (P<0.05,
Table II and Fig. 1).
 | Table IIChanges in rats serum TGF-β1 levels
following paraquat poisoning. |
Table II
Changes in rats serum TGF-β1 levels
following paraquat poisoning.
| Group | n | TGF-β1 (μg/l) |
|---|
| Control | 8 | 73.07±27.28 |
| Paraquat (30
mg/kg) | 8 | 707.25±195.91a |
| Paraquat (60
mg/kg) | 8 | 975.12±165.65a |
| Paraquat (120
mg/kg) | 8 |
1113.12±241.75a |
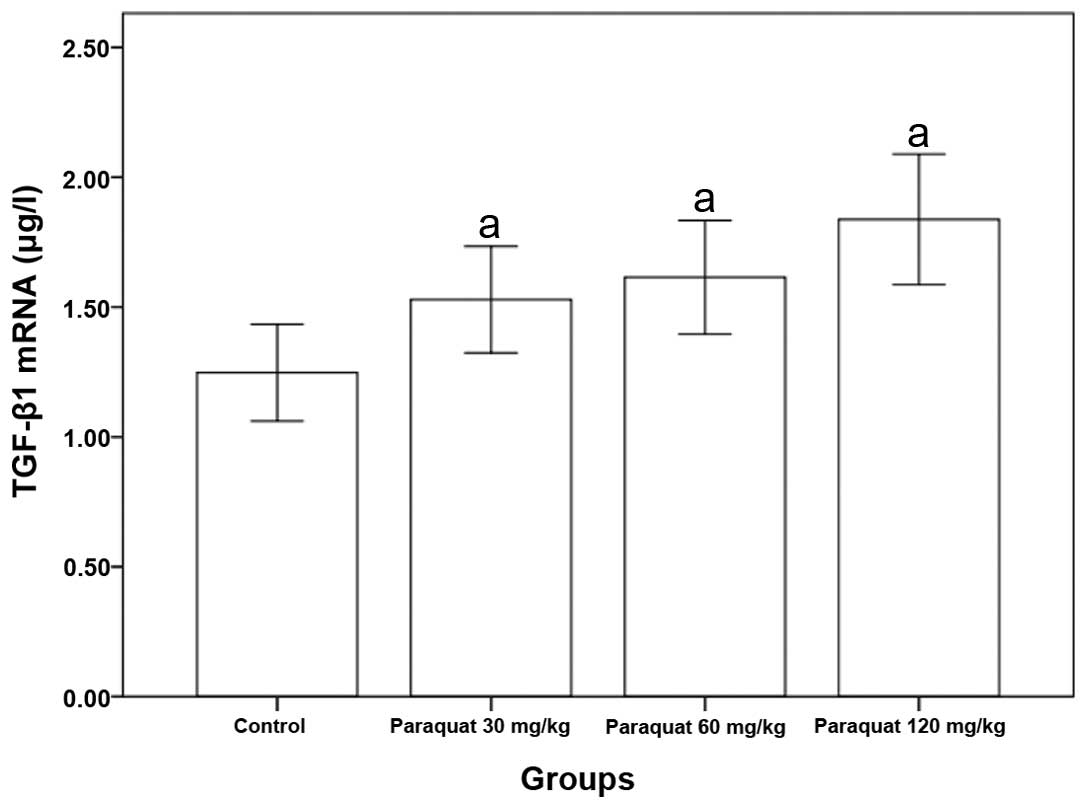
Rat pulmonary TGF-β1 mRNA expression
caused by paraquat poisoning
The expression of pulmonary TGF-β1 mRNA was markedly
higher than that of the control group, and a significant difference
was observed (P<0.05, Table
III and Fig. 2).
 | Table IIIExpression of rat TGF-β1 mRNA levels
caused by paraquat poisoning (μg/l, mean ± standard deviation). |
Table III
Expression of rat TGF-β1 mRNA levels
caused by paraquat poisoning (μg/l, mean ± standard deviation).
| Group | n | TGF-β1 (μg/l) |
|---|
| Control | 8 | 1.2455±0.1849 |
| Paraquat (30
mg/kg) | 8 | 1.5616±0.1990a |
| Paraquat (60
mg/kg) | 8 | 1.6003±0.1976a |
| Paraquat (120
mg/kg) | 8 | 1.8376±0.2563a |
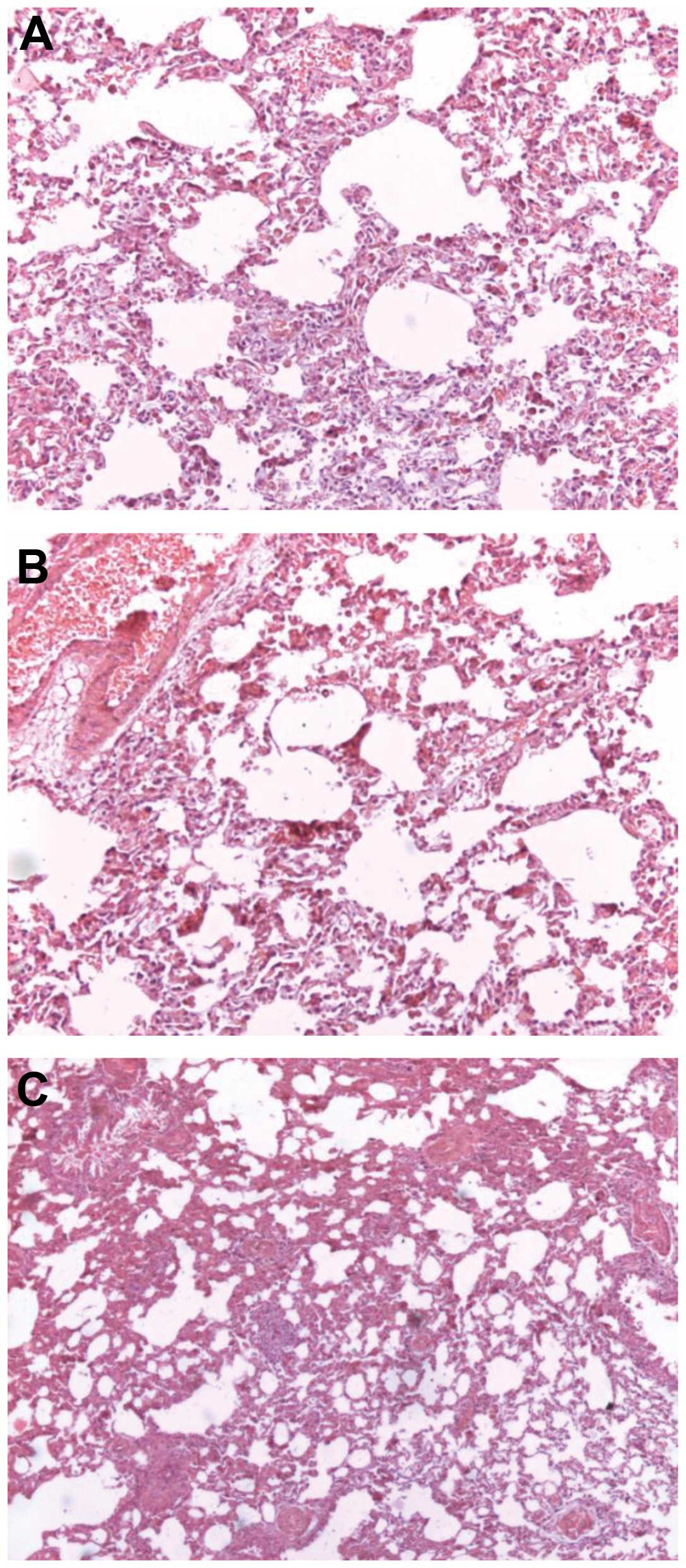
Pathology observation
Histological examination indicated that lung tissue
appeared broad and congested with numerous infiltrating
inflammatory cells, and the emergence of early interstitial
fibrosis. Partial lung alveoli tissues were disorganized. Vascular
endothelial cells demonstrated cloudy swelling and granular
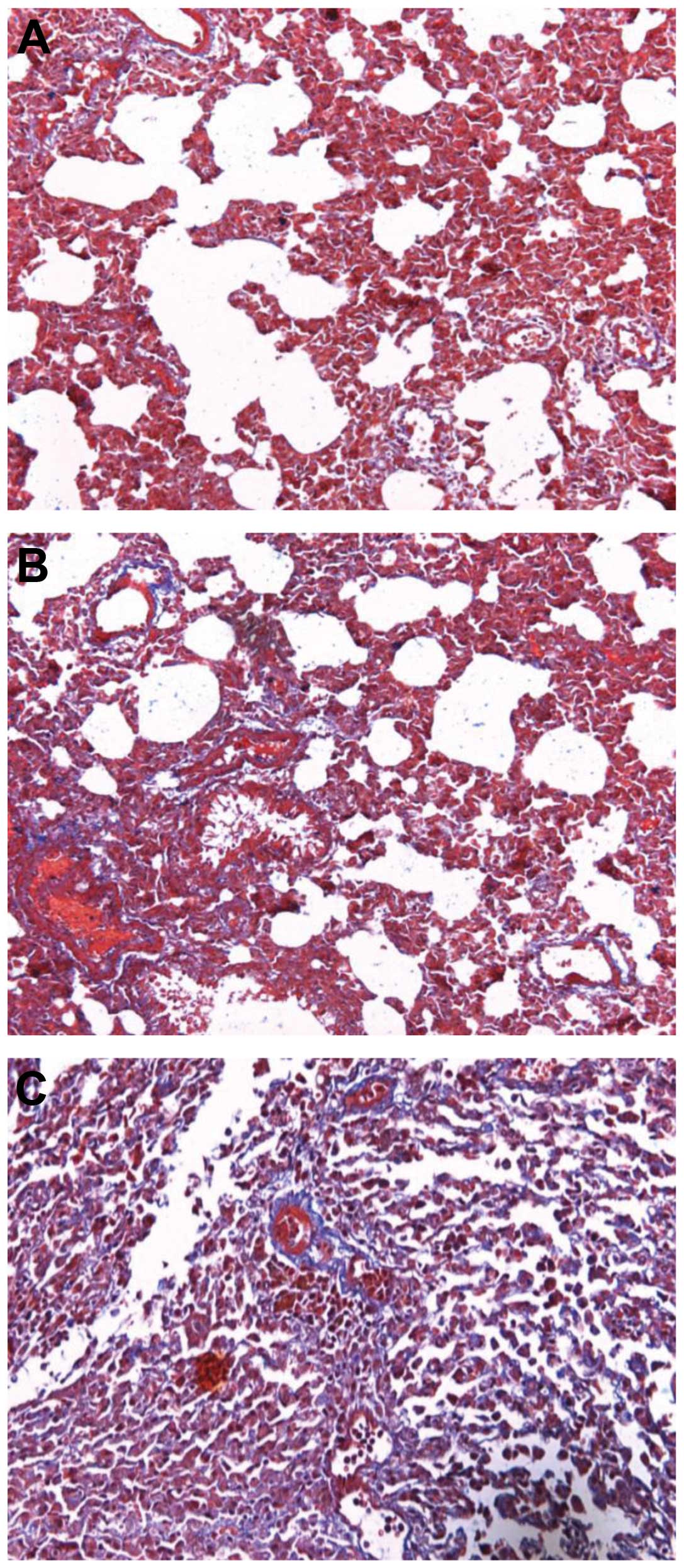
degeneration (Fig. 3). Masson’s
trichrome staining for collagen revealed lung tissue fibrosis
following paraquat poisoning (Fig.
4).
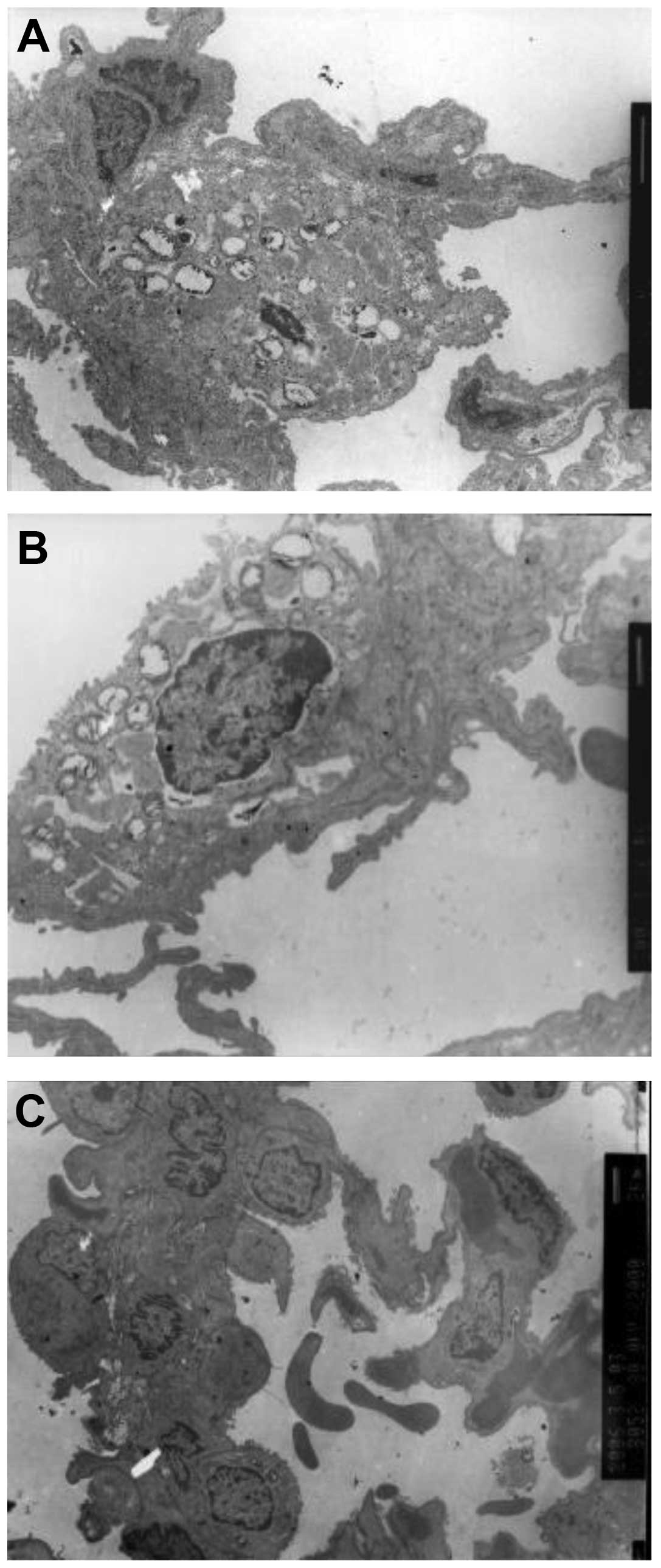
Ultramicrostructure observation
Ultramicrostructure observation revealed numerous
macrophages, red blood cells, lymphocytes and granulocytes in the
alveolar space and numerous cytolysosomes in the macrophages. The
shape of the type II alveolar epithelial cell nuclei was irregular,
heterochromatin migrated to the cell edge and lamellar body
vacuolization was also observed. Type II alveolar epithelial cell
karyolemma exhibited marked swelling and karyopyknosis. Type I
alveolar epithelial cells underwent caryon pyknosis and shrank
(Fig. 5).
Discussion
Recently studies have shown that cytokines have an
important role in the occurrence of pulmonary fibrosis. Cytokine
secretion has been implicated as a fundamental component in the
process of lung fibrosis, observed in response to bleomycin
(18). Although paraquat is known
to induce pulmonary injury, the mechanism by which it does so is
unclear (19). Paraquat
accumulates in the lung through a characteristic polyamine uptake
system. Several studies have been undertaken with regard to
paraquat as a useful tool for the exploration of the early
pathogenesis of pulmonary injury (20).
Paraquat is a well-known pneumotoxicant and provides
an established model of oxidative stress. Respiratory failure is a
frequent cause of mortality in cases of moderate to severe paraquat
poisoning. Certain results indicate that the acclimation to
oxidative stress is a highly complex process at the onset of
paraquat-induced damage (1,8,21).
Mainwaring et al (22)
reported that a number of the transcriptional responses to paraquat
were rapid, and that the predominant molecular functions and
biological processes associated with these genes included membrane
transport, oxidative stress, lung development, epithelial cell
differentiation and TGF-β1 signaling (22). In the present study, it was
demonstrated that paraquat was capable of increasing rat serum
TGF-β1 levels early. TGF-β1 mRNA expression of the rat lung was
also increased significantly by paraquat and followed by acute lung
injury. We also found that numerous inflammatory cells infiltrated
the injured rat lung alveoli. The abnormal expression of TGF-β1 is
hypothesized to be important in the pathogenesis of a number of
chronic inflammatory and immune lung diseases, including asthma,
chronic obstructive pulmonary disease and pulmonary fibrosis
(23). In the present study it was
demonstrated that TGF-β1 levels increased significantly following
paraquat poisoning, simultaneous to the development of lung injury
developed. Thus, it was conclude that TGF-β1 may contribute to
acute lung injury.
Paraquat-induced pulmonary toxicity is characterized
by the initial development of pulmonary edema, the infiltration of
inflammatory cells and damage to the alveolar epithelium, which may
progress to severe fibrosis. However, the exact role of paraquat in
the progression of pathogenesis has not been clearly established.
The pathological progression of lung pathology in the rat model was
similar to that found in paraquat-poisoned patients. Certain
cytokines, such as TGF-β1, which potentially regulates fibrosis
have yet to be identified. In the future the use of cytokines and
their inhibitors may provide novel therapies for the treatment of
acute lung injury and pulmonary fibrosis.
References
|
1
|
Bismuth C, Hall AH, Baud FJ and Borron S:
Pulmonary dysfunction in survivors of acute paraquat poisoning. Vet
Hum Toxicol. 38:220–222. 1996.PubMed/NCBI
|
|
2
|
Kurniawan AN: Product stewardship of
paraquat in Indonesia. Int Arch Occup Environ Health. 68:516–518.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hart TB: Paraquat - a review of safety in
agricultural and horticultural use. Hum Toxicol. 6:13–18. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kan BT, Liu HM, Jian XD, et al: Clinical
studies of dynamic changes on the renal injury indicators of acute
paraquat poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi.
30:839–841. 2012.(In Chinese).
|
|
5
|
Zhang Q, Wu WZ, Lu YQ, et al: Successful
treatment of patients with paraquat intoxication: three case
reports and review of the literature. J Zhejiang Univ Sci B.
13:413–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim HR, Park BK, Oh YM, et al: Green tea
extract inhibits paraquat-induced pulmonary fibrosis by suppression
of oxidative stress and endothelin-I expression. Lung. 184:287–295.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mohammadi-Karakani A, Ghazi-Khansari M and
Sotoudeh M: Lisinopril ameliorates paraquat-induced lung fibrosis.
Clin Chim Acta. 367:170–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamashita M, Yamashita M and Ando Y: A
long-term follow-up of lung function in survivors of paraquat
poisoning. Hum Exp Toxicol. 19:99–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghaffari AR, Noshad H, Ostadi A and
Hasanzadeh N: Effect of pulse therapy with glucocorticoid and
cyclophosphamide in lung fibrosis due to paraquat poisoning in
rats. Saudi Med J. 32:249–253. 2011.PubMed/NCBI
|
|
10
|
Gray JP, Heck DE, Mishin V, et al:
Paraquat increases cyanide-insensitive respiration in murine lung
epithelial cells by activating an NAD(P)H: paraquat oxidoreductase:
identification of the enzyme as thioredoxin reductase. J Biol Chem.
282:7939–7949. 2007. View Article : Google Scholar
|
|
11
|
Takizawa M, Komori K, Tampo Y and Yonaha
M: Paraquat-induced oxidative stress and dysfunction of cellular
redox systems including antioxidative defense enzymes glutathione
peroxidase and thioredoxin reductase. Toxicol In Vitro. 21:355–363.
2007. View Article : Google Scholar
|
|
12
|
Griffith KL, Shah IM, Myers TE, et al:
Evidence for ‘pre-recruitment’ as a new mechanism of transcription
activation in Escherichia coli: the large excess of SoxS
binding sites per cell relative to the number of SoxS molecules per
cell. Biochem Biophys Res Commun. 291:979–986. 2002.
|
|
13
|
Huh JW, Hong SB, Lim CM, et al: Sequential
radiologic and functional pulmonary changes in patients with
paraquat intoxication. Int J Occup Environ Health. 12:203–208.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomita M, Okuyama T, Katsuyama H, et al:
Mouse model of paraquat-poisoned lungs and its gene expression
profile. Toxicology. 231:200–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghazi-Khansari M, Mohammadi-Karakani A,
Sotoudeh M, et al: Antifibrotic effect of captopril and enalapril
on paraquat-induced lung fibrosis in rats. J Appl Toxicol.
27:342–349. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen CM, Chou HC, Hsu HH and Wang LF:
Transforming growth factor-β1 upregulation is independent of
angiotensin in paraquat-induced lung fibrosis. Toxicology.
216:181–187. 2005.
|
|
17
|
Son JY, Kim SY, Cho SH, et al: TGF-β1
T869C polymorphism may affect susceptibility to idiopathic
pulmonary fibrosis and disease severity. Lung. 191:199–205.
2013.
|
|
18
|
Ortiz LA, Lasky J, Hamilton RF Jr, et al:
Expression of TNF and the necessity of TNF receptors in
bleomycin-induced lung injury in mice. Exp Lung Res. 24:721–743.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satomi Y, Tsuchiya W, Miura D, et al: DNA
microarray analysis of pulmonary fibrosis three months after
exposure to paraquat in rats. J Toxicol Sci. 31:345–355. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dinis-Oliveira RJ, De Jesus Valle MJ,
Bastos ML, et al: Kinetics of paraquat in the isolated rat lung:
Influence of sodium depletion. Xenobiotica. 36:724–737. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomita M, Okuyama T, Katsuyama H, et al:
Gene expression in rat lungs during early response to
paraquat-induced oxidative stress. Int J Mol Med. 17:37–44.
2006.PubMed/NCBI
|
|
22
|
Mainwaring G, Lim FL, Antrobus K, et al:
Identification of early molecular pathways affected by paraquat in
rat lung. Toxicology. 225:157–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kopiński P, Sladek K, Szczeklik J, et al:
Expression of insulin-like growth factor-I (IGF-I) in alveolar
macrophages and lymphocytes obtained by bronchoalveolar lavage
(BAL) in interstitial lung diseases (ILD). Assessment of IGF-I as a
potential local mitogen and antiapoptotic cytokine. Folia Histochem
Cytobiol. 44:249–258. 2006.
|