Introduction
Myocardial infarction (MI) leads to the permanent
loss of cardiomyocytes, scar formation and the absence of the
endogenous repair mechanism which leads to heart failure (1). Bone marrow-derived mesenchymal stem
cells (MSCs) are self-renewing, multipotent adult cells (2), which are able to differentiate into
osteoblasts, chondrocytes (3),
astrocytes (4), neurons (5), endothelial cells (6), skeletal muscle cells (7), vascular smooth muscle cells (6) and cardiomyocytes in vitro
(8) and in vivo (9–10).
Animal and clinical studies have provided evidence that cell
therapy with MSCs is able to improve cardiac function potentially
via angiogenesis and myogenesis following MI (11–12).
However, the clinical exploitation of MSC transplantation is
hampered by their poor viability in the diseased myocardium
post-transplantation. For instance, the majority of MSCs injected
into the left ventricle of the adult murine heart died within 1
week during the injection period (9). This reflects that the ischemic
microenvironment of the infarcted myocardium was not conducive of
MSC survival. Furthermore, a large number of studies have
demonstrated that increasing the differentiation of implanted MSCs
into cardiomyocytes was able to promote heart function during the
acute phase of MI (13–15). Therefore, promoting the survival of
implanted MSCs and improving the differentiation of implanted MSCs
into cardiomyocytes following transplantation is critical for
successful cellular therapy.
The extract of Ginkgo biloba (EGb) leaves has
been applied as a traditional Chinese medicine for numerous years.
At present, the extract of the standardized G. biloba leaf
with well-defined components, labeled EGb 761, which contains 24%
ginkgo-flavone glycosides (e.g. kaempferol, quercetin and
isorhamnetin derivatives) and 6% terpenoid (e.g. ginkgolides A, B,
C, J and bilobalide), has been developed and is extensively
consumed as a dietary supplement and a herbal remedy (16). Numerous studies have verified that
EGb 761 protects the cell from a variety of extrinsic toxic
stimuli, which induce damage in different experimental disease
models (17–20). Previous studies have reported that
EGb 761 mediates its protective effects against doxorubicin-induced
cardiac injury through antioxidant, anti-inflammatory and
antiapoptotic mechanisms. Furthermore, it may induce neuronal
differentiation of cultured PC12 cells and neuroblast
differentiation in the mouse hippocampal dentate gyrus, and promote
the differentiation of human umbilical cord-derived mesenchymal
stem cells (21–24). However, it is unclear whether EGb
761 is able to improve the cardiac performance of infarcted hearts
through increasing the viability of implanted MSCs and improving
the differentiation of implanted MSCs into cardiomyocytes following
cellular therapy. The present study aimed to investigate the roles
and the potential mechanisms of EGb 761 on implanted MSCs in the
infarcted myocardium, and to provide experimental evidence for the
potential application of EGb 761 for cellular therapy during
ischemic heart disease.
Materials and methods
Reagents
The reagents and detection kits used were as
follows: Low-Dulbecco’s modified Eagle’s medium (L-DMEM),
trypsin-EDTA, fetal bovine serum, penicillin and streptomycin were
purchased from Gibco-BRL (Carlsbad, CA, USA); malondialdehyde (MDA)
and superoxide dismutase (SOD)assay kits were obtained from Nanjing
Jiancheng Biochemical Institute (Nanjing, Jiangsu, China); EGb 761
was obtained from Invitrogen Life Technologies (Karlsruhe,
Germany); the In situ Cell Death Detection kit was purchased
from Nanjing KeyGen Biochemical Institute (Nanjing, China); Fas and
cTnI antibodies were obtained from Antibody Design Labs (San Diego,
CA, USA); and IRDye 800 CW Donkey anti-rabbit IgG, IRDye 800 CW
Donkey anti-mouse IgG and IRDye 680 Donkey anti-goat IgG were
purchased from Li-Cor Biosciences, (Lincoln, NE, USA). Unless
stated otherwise, all other chemicals were purchased from Sigma
(St. Louis, MO, USA).
Animals
Sprague-Dawley rats weighing 250±15 g were obtained
from the Experimental Animal Center of Shanghai Medical College
(Shanghai, China). All animals were housed in a light-controlled
room with a 12 h light/dark cycle and were allowed access to food
and water ad libitum. Experimental protocols and animal care
methods were approved by the Experimental Animal Research Committee
of Fudan University (Shanghai, China).
Cell culture
The MSCs were obtained from bone marrow aspirates of
femurs and tibias from rats using a modified method originally
described by Xie et al (25). The marrow pellet was washed in
phosphate-buffered saline (PBS), centrifuged at 2,000 × g for 18
min and then resuspended in L-DMEM. Nucleated cells were isolated
with a density gradient centrifugation (Ficoll or Paque, GE
Healthcare Life Sciences, Schenectady, NY, USA), then introduced
into a 25-cm2 flask and cultured (at a density of
5×107 cell/ml) at 37°C in humidified air with 5%
CO2 in L-DMEM containing 15% fetal calf serum (FCS),
penicillin (100 U/ml) and streptomycin (100 mg/ml). The medium was
changed to remove non-adherent cells 72 h after seeding and every 3
days for ~10 days.
The primary cultured cells were replated into two
new flasks when the MSCs had grown to ~80% confluence. For
subculture, the cells were resuspended with 0.25% trypsin + 0.02%
EDTA and passaged at a ratio of 1:2 plates. For the four passages,
homogeneous MSCs devoid of hematopoietic cells were used for the
experiments.
The cultured cells were initially identified by
fluorescence-activated cell sorting (FACS) analysis prior to the
experiments. Briefly, MSCs were trypsinized and washed with 4% FCS
buffer in PBS. Then, ~106/100 μl cells were incubated at
4°C for 30 min with 20 μl antibodies, i.e phycoerythrin-conjugated
anti-rat CD14, CD90 and CD45 (AbD Serotec, Düsseldorf, Germany),
fluorescein isothiocyanate (FITC)-conjugated anti-rat CD105 and
CD44 (BioLegend, San Diego, CA, USA) and FITC-conjugated anti-rat
CD34 antibodies (Santa Cruz Biotechnology, Inc, Santa Cruz, CA,
USA). A normal mouse IgG was considered as the negative isotype
control. The cells were washed twice with PBS and were then labeled
with rabbit or mouse-FITC conjugated IgG for 20 min in a dark
environment at room temperature. Flow cytometric analyses were
performed on a FACS Calibur system using Cell Quest TM software
(Becton-Dickinson, Franklin Lakes, NJ, USA). In total, 10,000
events were recorded for each sample.
MI animal model and MSC
transplantation
The rats underwent myocardial ischemia by occlusion
of the left coronary artery. Briefly, the rats were anesthetized by
intraperitoneal injection of sodium pentobarbital (45 mg/kg). The
animal hearts were exposed by a left thoracotomy. Ligation of the
left anterior descending (LAD) artery was performed 1–2 mm distal
to the line between the left border of the pulmonary conus and the
right border of the left atrial appendage. MI was confirmed by
electrocardiogram (ECG; ADI Instruments, Houston, TX, USA). A final
200 μl suspension containing a total of 1×105 MSCs
labeled with PKH-26 red fluorescent cell tracker dye (Red
Fluorescent Cell Linker Kit; Sigma) was then transplanted into four
sites around the ischemic myocardium marginal zone of the left
ventricle via intramyocardial injection. The animal chest cavity
was closed following transplantation of MSCs. The rats were left to
recover in a temperature-controlled chamber until they resumed full
alertness and mobility, at which point they were returned to their
cages.
The animals were randomly divided into two groups.
The control group and the EGb 761-treated groups. In the control
group, following MSC transplantation, 3 ml normal sodium (0.9%
NaCl) was injected intraperitoneally (n=18) and in the EGb
761-treated groups, following MSC (1×105)
transplantation, 100 mg/kg/day EGb 761 (3 ml) was injected
intraperitoneally (n=18). For each animal, the total quantity of
normal sodium and EGb 761 was divided into two intraperitoneal
injections per day, respectively. Following 1, 2 and 7 days of MSC
transplantation and EGb 761 treatment, the animal hearts from each
group (six animals per group) were harvested under inhalation
anesthesia, respectively, and fixed with 4% neutral formaldehyde
for further analysis.
Measurement of cardiac function
Following 1, 2 and 7 days of normal sodium and EGb
761 treatment, the cardiac function of rats was assessed by a
multichannel physiological recorder (Jinjiang Tongyong Industry
Co., Ltd., Sichuan, China). Briefly, after obtaining the body
weight of each animal, animals were sedated (100 mg/kg ketamine and
1.5 mg/kg xylazine) and a transducer (Jinjiang Tongyong Industry
Co., Ltd., Chengdu, China) was applied to the left hemithorax. The
heart was visualized using a 2-dimensional mode with the axial view
of the left ventricle. Next, through the right carotid artery, a
cannula was inserted into the left ventricle. The ejection fraction
(EF), left ventricular end-diastolic volumes (LVEDV), left
ventricular end-diastolic pressure (LVEDP), left ventricular ±
dp/dt (maximum rate of pressure rise), left ventricular end
diastolic diameters (LVDd), fractional shortening (FS) and left
ventricular end-systolic pressure (LVESP) were calculated by the
standard formulas of ECToolbox software (Emory University, Atlanta,
GA, USA).
Histopathological examination
Following 1, 2 and 7 days of normal sodium and EGb
761 treatment, each group of animals were sacrificed by high dose
anesthesia (45 mg/kg, respectively, and perfused with 0.9% saline
followed by 4% paraformaldehyde through a needle inserted into the
left ventricle of the rat through the left atrium, for in
situ perfusion fixation. Myocardial tissues in the left
ventricle of sacrificed rats were collected and fixed in 4%
pre-cooled paraformaldehyde for 72 h and embedded in paraffin for
histological studies. Paraffin-embedded tissues were sectioned into
5-μm thick slices. The sections were stained for hematoxylin and
eosin (H&E) according to the manufacturer’s instructions
(Beyotime Biochemical Institute, Shanghai, China). Images were
visualized under an optical microscope at ×100 and ×400
magnifications.
Determination of reactive oxygen species
production
Following 1, 2 and 7 days of normal sodium and EGb
761 treatment, each group of animals was sacrificed using high-dose
anesthesia (sodium pentobarbital, 45 mg/kg), respectively. The
hearts of the animals were then removed and opened along the
greater curvature and washed with PBS. The homogenate was
centrifuged (10,000 × g for 15 min) and then the supernatant was
subjected to glutathione peroxidase (GSH-Px), catalase (CAT) and
superoxide dismutase (SOD) activity, and malondialdehyde (MDA)
content assays, according to the manufacturer’s instructions.
Terminal dUTP nick-end labeling (TUNEL)
assay
The cryosections (40-μm thick) from animal
myocardial samples were stained with an in situ cell death
detection kit (Kaiji Biological Technology development Co., Ltd.,
Nanjing, China) according to the manufacturer’s instructions, and
then the stained cryosections were observed with a confocal
microscope (Leica TCS SP8 MP, Leica, Wetzlar, Germany). The
apoptotic index (AI) was determined using the following formula: AI
= number of TUNEL-positive cells/total transplanted cells
pre-labeled with PKH-26 × 100.
Western blot analysis
The infarcted heart tissues were lysed in Tris-HCl
buffer (0.05 mol/l) containing 0.15 mol/l NaCl, 0.02%
NaN3, 0.1% SDS, 1% nonidet P (NP-40), 11 μg/ml aprotinin
and 0.1 mol/l phenylmethyl sulfonylfluoride (pH 8.0). The
expression of the Fas protein was determined by immunoblotting.
Briefly, the protein content of the supernatant prepared from
cultured cells was quantified by Lowry’s method. A 100 μg sample of
total protein was separated by 10% SDS-PAGE and then transferred
onto a polyvinylidene fluoride membrane (pore size, 0.45 μm; 3.0
mA/cm2 for 40 min). Following inhibition with 5% non-fat
milk powder/PBS containing 5% Tween-20 (PBS-T) for 3 h at room
temperature, the membranes were incubated with the following
antibodies: Mouse anti-fas and rabbit anti-GAPDH. The membranes
were probed with the primary antibodies overnight at 4°C. Then,
IRDye-conjugated secondary antibodies (IRDye donkey anti-mouse IgG
and IRDye donkey anti-rabbit IgG; 1:100) were added for 2 h and
subsequently scanned by the Odyssey Infrared Imaging system (Li-Cor
Biosciences). Specific densitometry values for the bands were
calculated by normalization to GAPDH.
Differentiation of implanted cells
Animals were sacrificed using high-dose anesthesia
(sodium pentobarbital, 45 mg/kg). The hearts were quickly harvested
and tissues from the free wall of the left ventricle, including the
infarct and peri-infarct regions were then embedded in optimal
cutting temperature tissue-freezing medium. The frozen sections
(40-μm thick) of the left ventricular samples were prepared for
identification of implanted cells. Briefly, the mounted frozen
sections on glass slides were fixed with methanol at −20°C for 20
min. Following washing three times with PBS, the sections were
incubated with blocking buffer (1% donkey serum, 1% bovine serum
albumin and 0.2% Tween-20 in PBS) for 30 min at room temperature
and then with the mouse anti-rat cTnI antibodies in blocking buffer
for 1 h at room temperature. The sections were then washed three
times in PBS, followed by incubation with FITC-conjugated goat
anti-mouse IgG second antibody for 1 h at room temperature.
Following washing three times with PBS, the stained cryosections
were observed under a confocal microscope (Leica).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean and analyzed by analysis of variance and a
Student-Newman-Keuls test using SPSS 18.0 software (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of surface markers of
MSCs
Consistent with previous studies (25,26),
the cultured MSCs expressed typical mesenchymal markers, including
CD44, CD90 and CD105. However, the surface markers of hematopoietic
stem cells (CD45), macrophages (CD14) and lymphocytes (CD34) were
not expressed (data not shown).
Establishment of the MI model
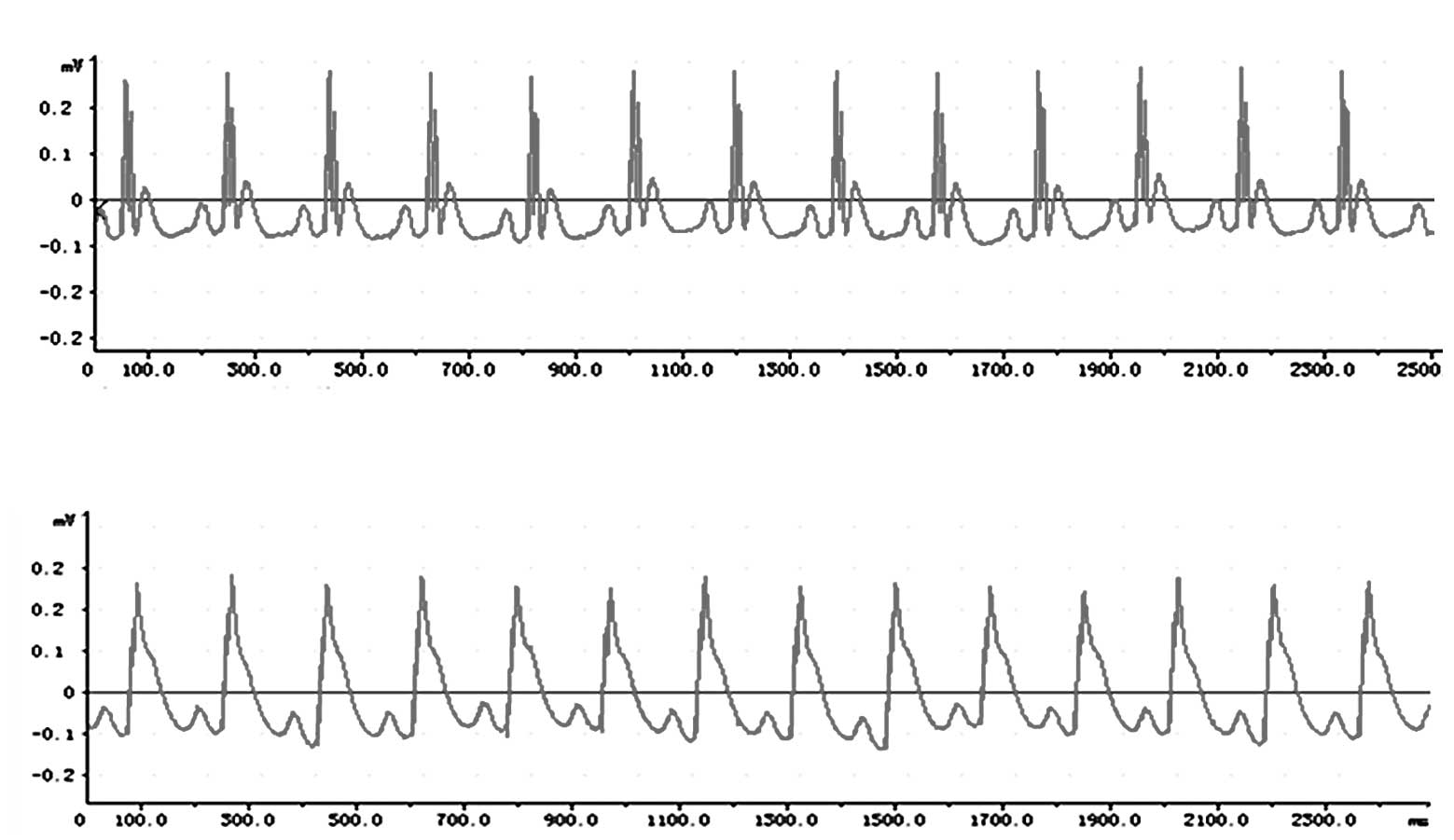
Compared with the ECG trace from a normal rat that
had not undergone ligation of the LAD coronary artery (Fig. 1A), a deep S wave, R-wave
elimination ST segment elevation was observed on the ECG from a rat
that had undergone ligation of LAD coronary artery (Fig. 1B).
Amelioration of cardiac function by EGb
761 treatment
The parameters of echocardiography and hemodynamics
were analyzed. Compared with the respective control group, FS, EF,
LVESP and dp/dtmax significantly increased, and LVDd, LVEDV and
LVEDP significantly decreased following EGb 761 treatment
(P<0.05 vs. the control group; Table I).
 | Table IEGb 761 ameliorates cardiac
function. |
Table I
EGb 761 ameliorates cardiac
function.
| 1 day | 2 days | 7 days |
|---|
|
|
|
|
|---|
| Group | Control | EGb 761 | Control | EGb 761 | Control | EGb 761 |
|---|
| EF (%) | 72.32±1.46 | 75.29±2.43a | 70.34±0.51b | 78.53±0.95a,b | 68.57±0.43c | 83.66±1.57a,c |
| FS (%) | 40.20±0.56 | 43.32±1.82a | 35.43±1.43b | 47.64±1.55a,b | 30.12±2.00c | 53.00± 2.51a,c |
| LVDd (cm) | 0.62±0.04 | 0.57±0.05a | 0.67±0.02b | 0.53±0.08a,b | 0.73±0.06c | 0.48±0.03a,c |
| LVEDV (ml) | 0.310±0.021 | 0.300±0.034a | 0.315±0.038b | 0.290±0.033a,b | 0.320±0.030c | 0.280±0.040a,c |
| LVESP (mmHg) | 105.40±3.28 | 115.40±3.38a | 98.40±3.16b | 125.40±3.68a,b | 92.10±2.32c |
135.400±3.41a,c |
| LVEDP (mmHg) | 9.300±0.31 | 7.300±0.22a | 11.300±0.28b | 6.306±0.21a,b | 12.320±0.31c | 5.300±0.18a,c |
| dp/dtmax
(mmHg/sec) | 6560.5±133.5 |
7260.5±135.9a |
5860.5±115.5b |
7950.4±136.6a,b |
4860.3±210.3c |
8500.0±234.3a,c |
EGb 761 reduces MI-induced heart
inflammation
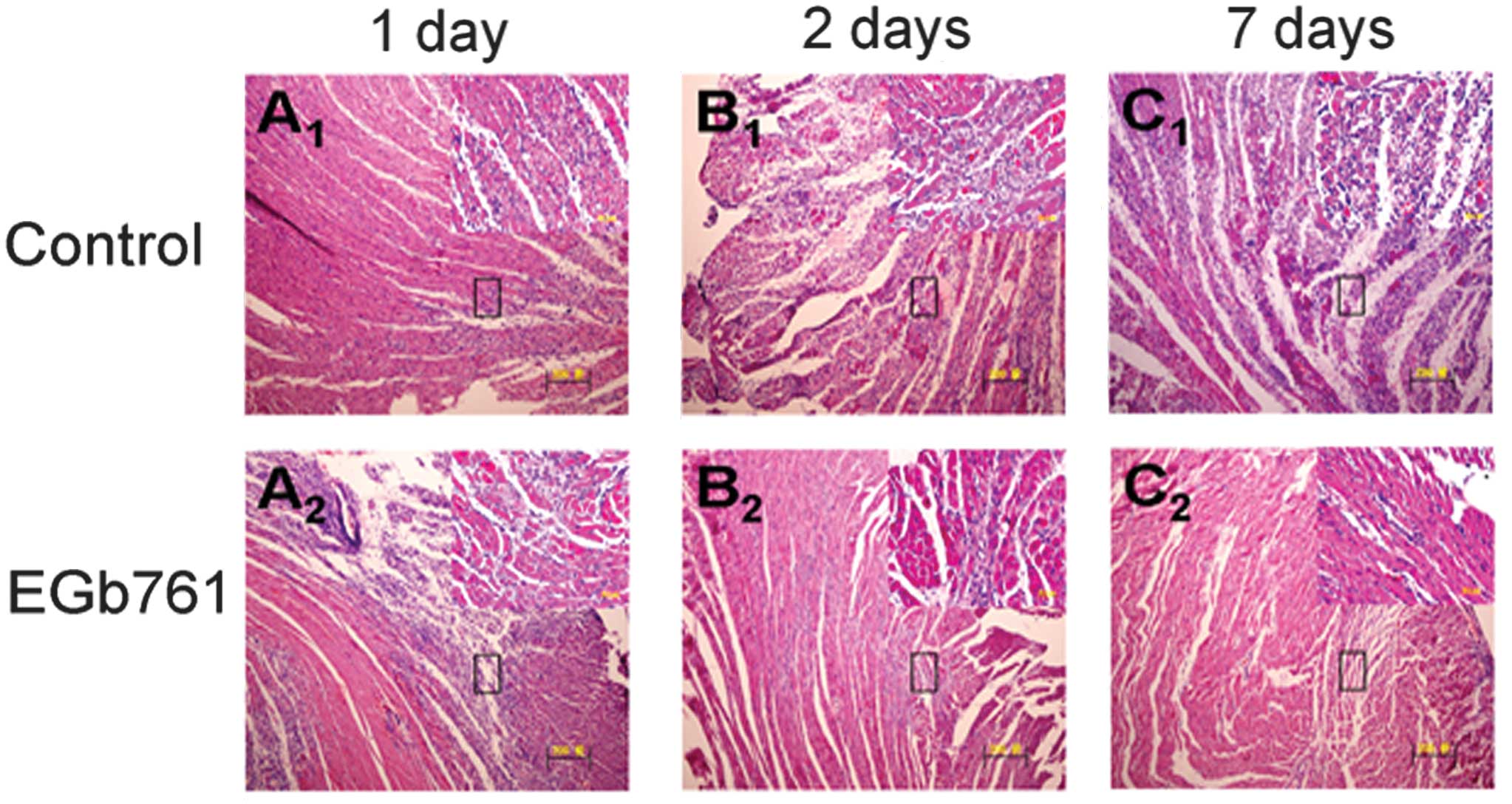
H&E staining images demonstrated that MI
elicited typical cardiac histopathological changes in the
myocardium, which were manifested as the presence of mononuclear
cells, fragments of necrotic myocardial fibers, eosinophils,
polymorphonuclear neutrophils and macrophagocytes. Compared with
the respective control group, EGb 761 significantly reduced the
number of infiltrated inflammatory cells in the infarcted
myocardium following 1, 2 and 7 days of MSC transplantation
(Fig. 2).
Anti-oxidative effects of EGb 761 on the
heart
The effects of EGb 761 on the activity of the
antioxidant enzymes SOD, CAT and GSH-Px, and the level of MDA in
the heart tissues of rats are shown in Fig. 3. The results indicated that MDA
content (a product of lipid peroxidation) significantly decreased
and the SOD, CAT and GSH-Px activity increased following EGb 761
treatment. With the extension of time of MI, MDA content gradually
increased and the activity of SOD, CAT and GSH-Px gradually
decreased in the control group. Compared with the respective
control group, the activity of SOD, CAT and GSH-Px gradually
increased and MDA content gradually decreased in the EGb 761
treatment group (P<0.05).
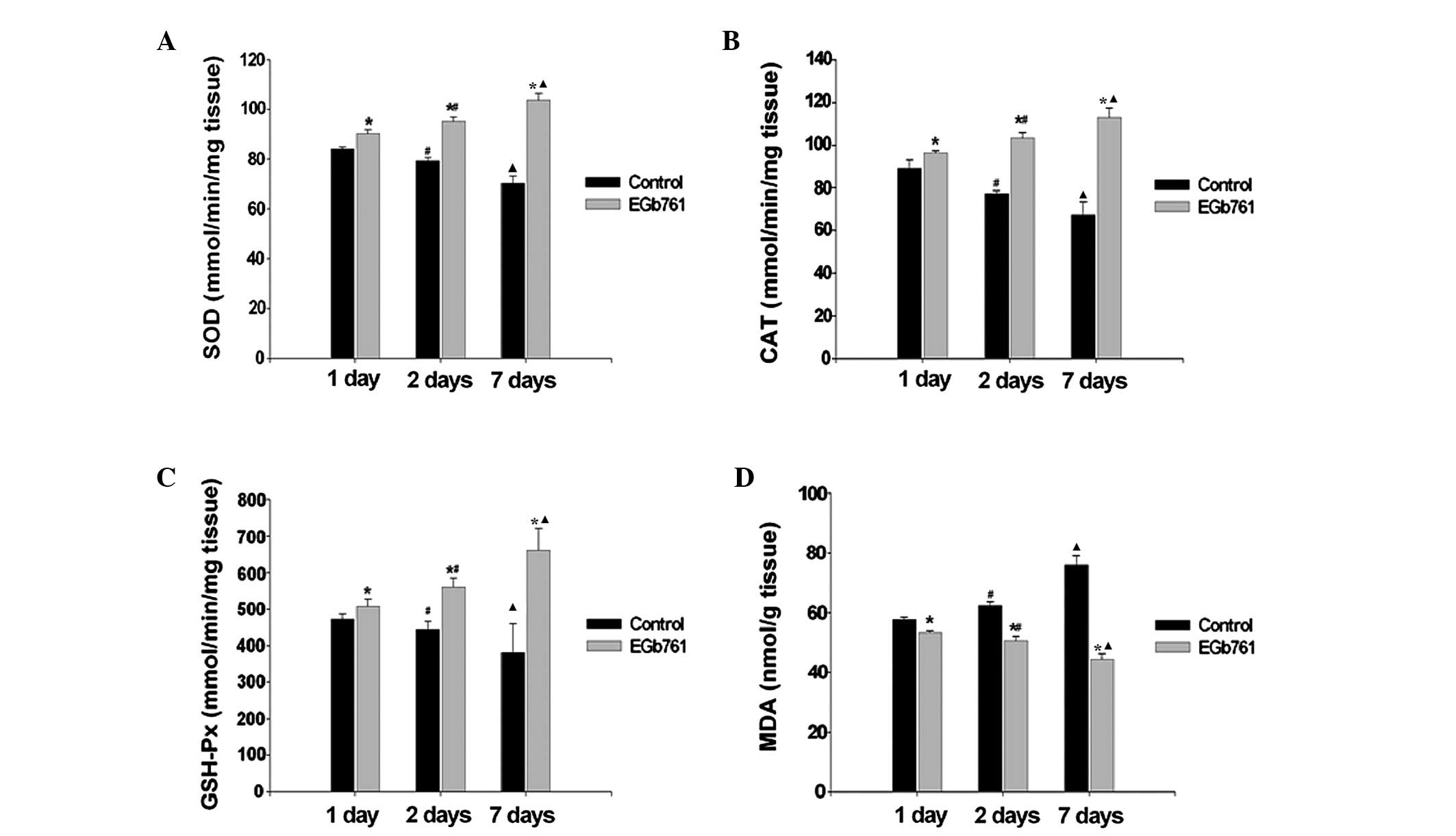 | Figure 3Effects of EGb 761 on MI-induced
oxidative stress. Activity of (A) SOD, (B) CAT, (C) GSH-Px and (D)
MDA are presented as histograms. *P<0.05, vs. the
respective control group, #P<0.05, vs. the 1 day
group. ▲P<0.05, vs. the 2 days group.(n=6). MI,
myocardial infarction; SOD, superoxide dismutase; CAT, catalase;
GSH-Px, glutathione peroxidase; MDA, malonyldialdehyde; EGb,
extract of Ginkgo biloba. |
Antagonism of EGb 761 on MI-induced MSC
apoptosis
Compared with the respective control group, the AI
in the EGb 761 treatment group was significantly decreased
(P<0.05). With the extension of MI time, the AI gradually
increased in the control groups and the EGb761 treatment groups
(P<0.05; Fig. 4A and B).
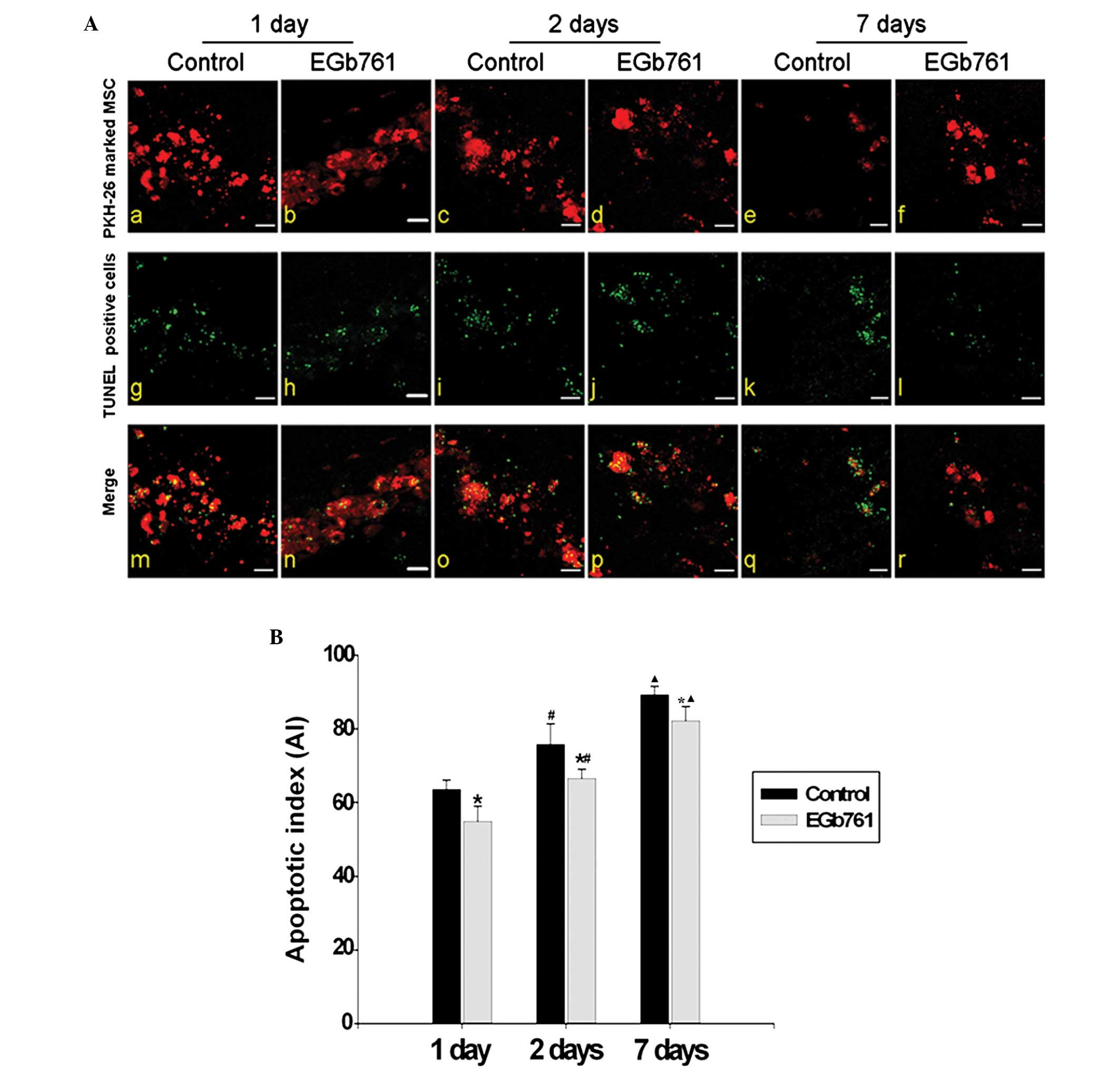 | Figure 4Effect of EGb 761 on the apoptosis of
transplanted MSCs. (A) MSCs labeled with PKH-26 (red) were
transplanted into the rat ischemic myocardium. Cellular apoptosis
was determined using TUNEL-positive staining (green). The
co-labeling of PKH-26 (red) and TUNEL (green) indicated cell
apoptosis following MSC transplantation (n=6). (Aa-f) The
transplanted cells identified by PKH-26-positive staining (red).
(Ag-i) The apoptotic cells identified with TUNEL-positive staining
(green). (Am-r) Merged figures show the apoptosis of the grafted
MSCs co-labeled with PKH-26 (red) and TUNEL (green; scale bar, 10
μm). (Aa, c, e, g, i, k, m, o and q) Control group; (Ab, d, f, h,
j, l, n, p and r) EGb 761-treated groups; (Aa, b, g, h, m and n)
Apoptotic images of transplanted MSCs following 1 day of MI; (Ac,
d, i, j, o and p) Apoptotic images of transplanted MSCs following 2
days of MI; (Ae, f, k, l, q and r) Apoptotic images of transplanted
MSCs following 7 days of MI. (B) Apoptotic indexes are presented as
histograms. *P<0.05, vs. the respective control
group. There were clear differences between the 1 day group and the
2 day group (#P<0.05), and between the 2 day group
and the 7 day group (▲P<0.05). n=6. MSCs, bone
marrow-derived mesenchymal stem cells; TUNEL, terminal dUTP
nick-end labeling; MI, myocardial infarction; EGb, extract of
Ginkgo biloba. |
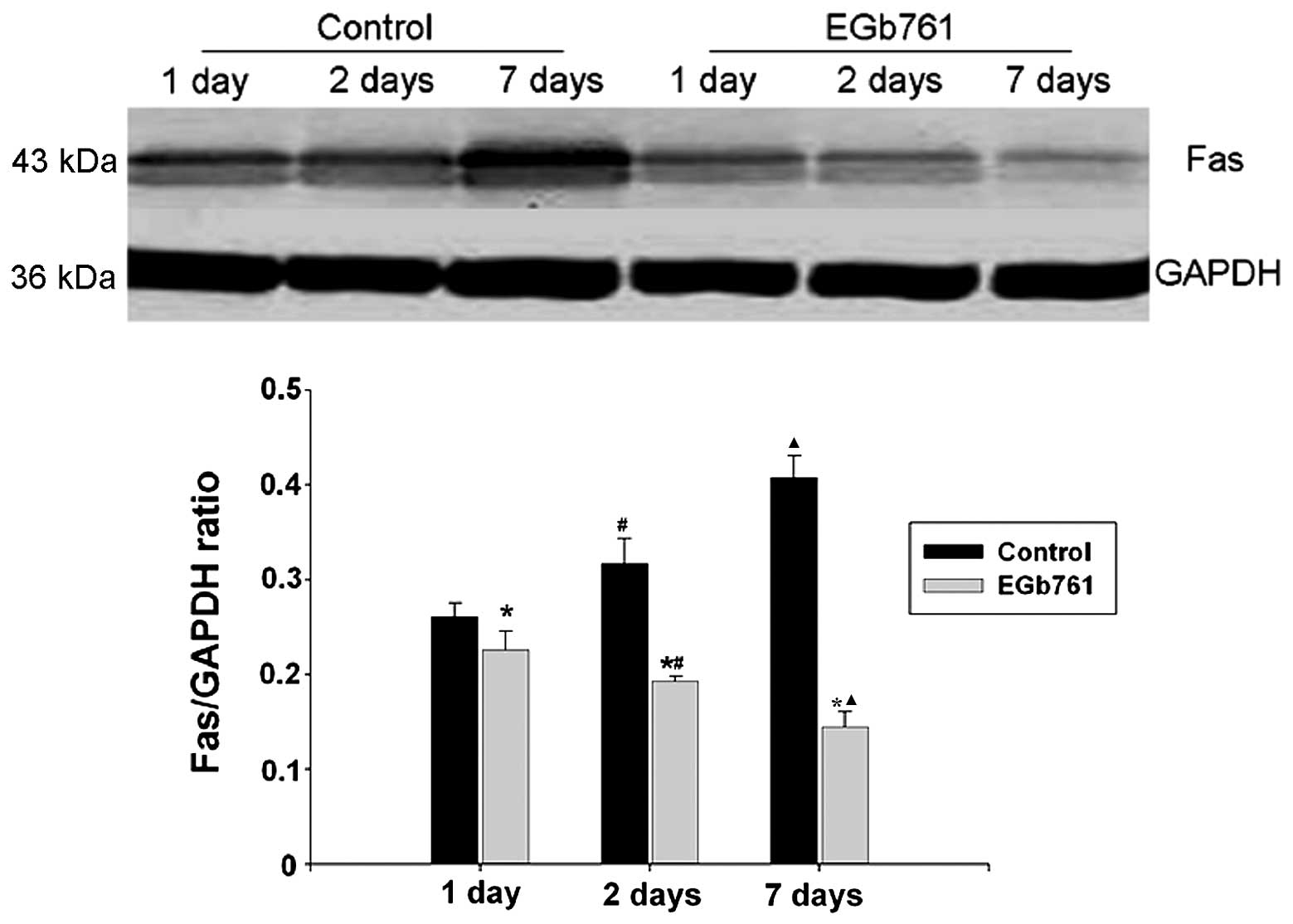
Expression of Fas protein
As shown in Fig. 5,
western blotting indicated that Fas protein was upregulated
gradually and reached a high level following 7 days of normal
sodium treatment in the control group, whereas the Fas protein was
downregulated gradually in the EGb 761 treatment group. Compared
with the respective control group, the level of Fas expression was
significantly decreased (P<0.05).
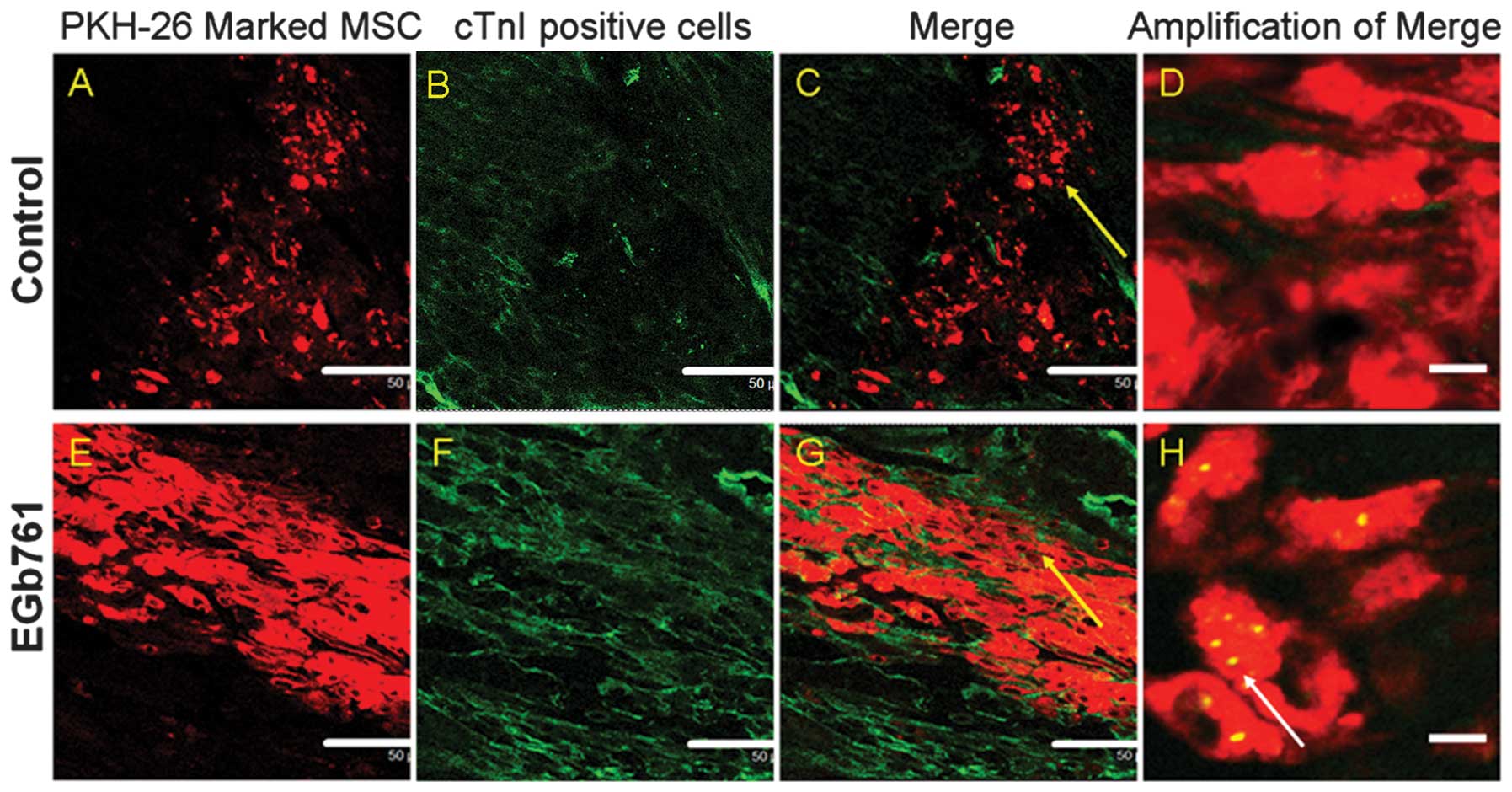
Differentiation of MSCs following
transplantation
To detect whether implanted MSCs differentiated into
cardiomyocytes, the cTnI-expression in the PKH26-labeled MSCs of
the transplanted myocardium was assessed using immunohistological
staining following 7 days of MSC transplantation. The results
demonstrated that, in the control group, the muscle-specific
markers, including cTnI staining were not expressed on the
PKH26-positive cells as observed by confocal microscopy (Fig. 6A–D). Conversely, co-localization
expression of cTnI and PKH26 was observed in the MSCs of the EGb
761 treatment group (Fig.
6E–H).
Discussion
MSC therapy prevents disadvantaged remodeling of
heart function after MI and improves recovery when introduced into
the infarcted heart; however, further promotion of the
differentiation of MSCs in the cardiac scar tissue is required. The
present study examined a novel strategy to promote the therapeutic
effects of MSCs within the ischemic heart and improve cardiac
function under pathological conditions. The present study indicated
that EGb 761 is able to improve deteriorated cardiac function,
which partially contributes to the protective effect of EGb 761 on
the grafted MSCs against MI-induced apoptosis and its promotion for
the differentiation of MSCs into myocardial cells. Several previous
studies have demonstrated that EGb 761 reduces extrinsic damage and
anti-oxidative stress, which induced the apoptosis of
cardiomyocytes (26,27). The present study demonstrated new
evidence that EGb 761 promotes heart function recovery through
enhancing implanted cell survival and differentiation in the
ischemic myocardium.
Cardiac inflammation is associated with left
ventricular remodeling in the MI model. Previous studies have
demonstrated that autologous or allogeneic MSCs markedly suppress
T-lymphocyte proliferation in the infarcted myocardium (28,29).
Furthermore, previous evidence suggests that the transplantation of
MSCs attenuated histiocytic infiltration in a rat model of acute
myocarditis (30). However,
inflammation remains one of the main reasons for the low survival
rate of transplanted cells in the infarcted myocardium. The present
study revealed that EGb 761 significantly reduced the number of
infiltrated inflammatory cells in the infarcted myocardium, which
was consistent with other studies demonstrating the
anti-inflammatory property of EGb 761 in various animal models
(31,32).
Consistent with other studies of the anti-oxidative
effect of EGb 761 in vivo and in vitro (33,34),
the present study also demonstrated that EGb 761 had an
anti-oxidative effect on the infarcted myocardium. In the present
study, its effects on the reduction of MDA content and the
enhancement of the activity of antioxidant enzymes SOD, CAT and
GSH-Px, were demonstrated. In addition, the anti-oxidative effect
of EGb 761 was positively correlated with the treatment time of EGb
761.
The present study also demonstrated that EGb 761 has
a protective effect on MI-induced MSC apoptosis during MSC
transplantation. The TUNEL-positive rate in the transplanted MSCs
was significantly decreased following EGb 761 treatment, and the
TUNEL-positive rate in the transplanted MSCs was negatively
correlated with the treatment time of EGb 761.
Apoptosis, or programmed cell death, can be
triggered via the extrinsic (death receptor-mediated) signaling
pathways. FasL interacts with its receptor, Fas (CD95/APO-1), and
then triggers a cascade of subcellular events involved in the
extrinsic signaling pathways, which exhibit a role in cell
apoptosis (17). In the present
study, the level of Fas expression was positively correlated with
the time of MI in the control groups. EGb 761 significantly
downregulated the expression of the Fas protein and it was
negatively correlated with the treatment time of EGb 761.
Therefore, the present study hypothesized that the attenuating
effect of EGb 761 on cell apoptosis may be mediated by the Fas
death receptor signaling pathways. However, the detailed mechanism
of the extrinsic apoptotic pathway requires further
investigation.
Previously, it has been demonstrated by Daigneault
et al (35) that the
transplanted MSCs did not express myocyte-specific markers after 2
weeks of transplantation. Our data also demonstrated that MSCs did
not express cTnI, a myocyte-specific marker, 7 days after exogenous
MSCs were engrafted into infarcted hearts. Therefore, enhancing the
differentiation of MSCs into myocytes is a crucial problem in the
regeneration of myocardium via stem cell transplantation.
To identify the enhancement of the differentiation
of MSCs into cardiac cells, the rats were treated with 100
mg/kg/day EGb 761 following MSC transplantation. Notably, the
present study demonstrated the expression of cTnI in implanted MSCs
following 7 days of EGb 761 treatment. However, the detailed
mechanisms of the differentiation of MSCs into cardiomyocyte-like
cells requires further investigation.
The present study demonstrated the protective effect
of EGb 761 on MI following the transplantation of MSCs. These data
markedly support the theory that EGb 761 protects implanted-MSCs
against MI-induced apoptosis through the activation of the
Fas-mediated death receptor signaling pathways. Furthermore,
prevention of inflammation, apoptosis and oxidative stress enhanced
the differentiation of the transplanted MSCs. The present study
provides a promising approach for promoting cardiac repair with a
widely available and acceptable therapeutic agent. Therefore, EGb
761 may have considerable significance in improving the efficiency
of stem cell therapy for MI. However, due to the complexity of
G. biloba composition, further investigation of the detailed
mechanisms of specific components involved, particularly in animal
ischemic hearts, need to be conducted in order to improve our
understanding of the effects of G. biloba products.
In conclusion, the present study presents unique
evidence that EGb 761 is able to ameliorate the decrease in heart
function in a rat model with transplanted MSCs via enhancing the
survival and differentiation of implanted MSCs, and attenuating
inflammation and oxidative stress in the infarcted myocardial
microenvironment.
Acknowledgements
This study was supported by grants from the National
Nature Science Foundation of China (grant no. 31100838; http://www.nsfc.gov.cn).
References
|
1
|
Braunwald E and Bristow MR: Congestive
heart failure: fifty years of progress. Circulation. 102:IV14–IV23.
2000.PubMed/NCBI
|
|
2
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pereira RF, Halford KW, O’Hara MD, Leeper
DB, Sokolov BP, Pollard MD, Bagasra O and Prockop DJ: Cultured
adherent cells from marrow can serve as long-lasting precursor
cells for bone, cartilage, and lung in irradiated mice. Proc Natl
Acad Sci USA. 92:4857–4861. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schäfer S, Calas AG, Vergouts M and
Hermans E: Immunomodulatory influence of bone marrow-derived
mesenchymal stem cells on neuroinflammation in astrocyte cultures.
J Neuroimmunol. 249:40–48. 2012.PubMed/NCBI
|
|
5
|
Kohyama J, Abe H, Shimazaki T, Koizumi A,
Nakashima K, Gojo S, Taga T, Okano H, Hata J and Umezawa A: Brain
from bone: efficient ‘meta-differentiation’ of marrow
stroma-derived mature osteoblasts to neurons with Noggin or a
demethylating agent. Differentiation. 68:235–244. 2001.
|
|
6
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
Leri A and Anversa P: Bone marrow cells regenerate infracted
myocardium. Nature. 410:701–705. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Yan X, Sun Z, Chen B, Han Q, Li J
and Zhao RC: Flk-1+ adipose-derived mesenchymal stem cells
differentiate into skeletal muscle satellite cells and ameliorate
muscular dystrophy in mdx mice. Stem Cells Dev. 16:695–706.
2007.
|
|
8
|
Makino S, Fukuda K, Miyoshi S, Konishi F,
Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J,
Umezawa A and Ogawa S: Cardiomyocytes can be generated from marrow
stromal cells in vitro. J Clin Invest. 103:697–705. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toma C, Pittenger MF, Cahill KS, Byme BJ
and Kessler PD: Human mesenchymal stem cells differentiate to a
cardiomyocyte phenotype in the adult murine heart. Circulation.
105:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shake JG, Gruber PJ, Baumgartner WA,
Senechal G, Meyers J, Redmond JM, Pittenger MF and Martin BJ:
Mesenchymal stem cell implantation in a swine myocardial infarct
model: Engraftment and functional effects. Ann Thorac Surg.
73:1919–1925. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wollert KC, Meyer GP, Lotz J,
Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte
T, Hornig B, Messinger D, et al: Intracoronary autologous
bone-marrow cell transfer after myocardial infarction: the BOOST
randomised controlled clinical trial. Lancet. 364:141–148. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Zhang G, Hou Y, Chen J, Wang J,
Zou C, Li D, Li H, Zhang Q, Wang A and Fan Q: Transplantation of
microencapsulated Schwann cells and mesenchymal stem cells augment
angiogenesis and improve heart function. Mol Cell Biochem.
366:139–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong HY, Zhang ZM and Zhou ZX: Effects of
endothelin-1 on differentiation of cardiac myocyte induced from
rabbit bone marrow stromal cells. Chin Med J (Engl). 119:832–839.
2006.PubMed/NCBI
|
|
14
|
Piao H, Youn TJ, Kwon JS, Kim YH, Bae JW,
Bora-Sohn, Kim DW, Cho MC, Lee MM and Park YB: Effects of bone
marrow derived mesenchymal stem cells transplantation in acutely
infarcting myocardium. Eur J Heart Fail. 7:730–738. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamihata H, Matsubara H, Nishiue T,
Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi
E, et al: Implantation of bone marrow mononuclear cells into
ischemic myocardium enhances collateral perfusion and regional
function via side supply of angioblasts, angiogenic ligands, and
cytokines. Circulation. 104:1046–1052. 2001. View Article : Google Scholar
|
|
16
|
Jacobs BP and Browner WS: Ginkgo
biloba: a living fossil. Am J Med. 108:341–342. 2000.
View Article : Google Scholar
|
|
17
|
Yeh YC, Liu TJ, Wang LC, Lee HW, Ting CT,
Lee WL, Hung CJ, Wang KY and Lai HC and Lai HC: A standardized
extract of Ginkgo biloba suppresses doxorubicin-induced
oxidative stress and p53-mediated mitochondrial apoptosis in rat
testes. Br J Pharmacol. 156:48–61. 2009.
|
|
18
|
Kang X, Chen J, Xu Z, Li H and Wang B:
Protective effects of Ginkgo biloba extract on
paraquat-induced apoptosis of PC12 cells. Toxicology in Vitro.
21:1003–1009. 2007.
|
|
19
|
Zhao Z, Liu N, Huang J, Lu PH and Xu XM:
Inhibition of cPLA2 activation by Ginkgo biloba extract
protects spinal cord neurons from glutamate excitotoxicity and
oxidative stress-induced cell death. J Neurochem. 116:1057–1065.
2011.PubMed/NCBI
|
|
20
|
Yang SF, Wu Q, Sun AS, Huang XN and Shi
JS: Protective effect and mechanism of Ginkgo biloba leaf
extracts for Parkinson disease induced by
1-methyl-4-pheny1–1,2,3,6-tetra-hydropyridine. Acta Pharmacologic
Sin. 22:1089–1093. 2001.PubMed/NCBI
|
|
21
|
Cavuşoğlu K, Yapar K, Oruç E and Yalçın E:
Protective effect of Ginkgo biloba L. leaf extract against
glyphosate toxicity in Swiss albino mice. J Med Food. 14:1263–1272.
2011.
|
|
22
|
Wang YC, Zhang Y, Duan AL, Lin WX, Zheng
QD and Xu WL: Rapid differentiation of human umbilical cord-derived
mesenchymal stem cells into insulin-secreting cells under the sole
induction of biological products. Sheng Li Xue Bao. 62:73–78.
2010.(In Chinese).
|
|
23
|
Yoo DY, Nam Y, Kim W, Yoo KY, Park J, Lee
CH, Choi JH, Yoon YS, Kim DW, Won MH and Hwang IK: Effects of
Ginkgo biloba extract on promotion of neurogenesis in the
hippocampal dentate gyrus in C57BL/6 mice. J Vet Med Sci. 73:71–76.
2011.
|
|
24
|
Didier A and Jourdan F: The Ginkgo
biloba extract modulates the balance between proliferation and
differentiation in the olfactory epithelium of adult mice following
bulbectomy. Cell Mol Biol (Noisy-le-grand). 48:717–723. 2002.
|
|
25
|
Xie XJ, Wang JA, Cao J and Zhang X:
Differentiation of bone marrow mesenchymal stem cells induced by
myocardial medium under hypoxic conditions. Acta Pharmacol Sin.
27:1153–1158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu SL, Choi RC and Zhu KY: Isorhamnetin, A
flavonol aglycone from Ginkgo biloba L, induces neuronal
differentiation of cultured PC12 cells: potentiating the effect of
nerve growth factor. Evid Based Complement Alternat Med.
2012:2782732012.PubMed/NCBI
|
|
27
|
Liu TJ, Yeh YC, Ting CT, Lee WL, Wang LC,
Lee HW, Wang KY and Lai HC and Lai HC: Ginkgo biloba extract
761 reduces doxorubicin-induced apoptotic damage in rat hearts and
neonatal cardiomyocytes. Cardiovasc Res. 80:227–235. 2008.
View Article : Google Scholar
|
|
28
|
Liu J, Wang J, Chen X, Guo C, Guo Y and
Wang H: Ginkgo biloba extract EGb 761 protects against
aging-associated diastolic dysfunction in cardiomyocytes of
D-galactose-induced aging rat. Oxid Med Cell Longev.
2012:4187482012.
|
|
29
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, Grisanti S and Gianni AM:
Human bone marrow stromal cells suppress T-lymphocyte proliferation
induced by cellular or nonspecific mitogenic stimuli. Blood.
99:3838–3843. 2002.
|
|
30
|
Tse WT, Pendleton JD, Beyer WM, Egalka MC
and Guinan EC: Suppression of allogeneic T-cell proliferation by
human marrow stromal cells: implications in transplantation.
Transplantation. 75:389–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohnishi S, Yanagawa B, Tanaka K, Miyahara
Y, Obata H, Kataoka M, Kodama M, Ishibashi-Ueda H, Kangawa K,
Kitamura S and Nagaya N: Transplantation of mesenchymal stem cells
attenuates myocardial injury and dysfunction in a rat model of
acute myocarditis. J Mol Cell Cardiol. 42:88–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haines DD, Varga B, Bak I, Juhasz B,
Mahmoud FF, Kalantari H, Gesztelyi R, Lekli I, Czompa A and Tosaki
A: Summative interaction between astaxanthin, Ginkgo biloba
extract (EGb761) and vitamin C in suppression of respiratory
inflammation: a comparison with ibuprofen. Phytother Res.
1:128–136. 2011.PubMed/NCBI
|
|
33
|
Shi C, Xiao S, Liu J, Guo K, Wu F, Yew DT
and Xu J: Ginkgo biloba extract EGb761 protects against
aging-associated mitochondrial dysfunction in platelets and
hippocampi of SAMP8 mice. Platelets. 21:373–379. 2010. View Article : Google Scholar
|
|
34
|
Jiang X, Nie B, Fu S, Hu J, Yin L, Lin L,
Wang X, Lu P and Xu XM: EGb761 protects hydrogen peroxide-induced
death of spinal cord neurons through inhibition of intracellular
ROS production and modulation of apoptotic regulating genes. J Mol
Neurosci. 38:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Daigneault M, De Silva TI, Bewley MA,
Preston JA, Marriott HM, Mitchell AM, Mitchell TJ, Read RC, Whyte
MK and Dockrell DH: Monocytes regulate the mechanism of T-cell
death by inducing Fas-mediated apoptosis during bacterial
infection. PLoS Pathog. 8:e10028142012. View Article : Google Scholar : PubMed/NCBI
|