Introduction
The human endometrium consists of complex tissue
composed of various cell components, and changes during the
proliferative and secretory phase of the menstrual cycle (1). It is capable of blastocyst
implantation, immunological tolerance, regulation of trophoblast
invasion and infectious agent control (2). A number of factors participate in the
remodeling of the endometrium, including steroid hormone,
cytokines, tumor suppressor gene and growth factors.
The von Hippel-Lindau (VHL) gene was isolated by
positional cloning and identified as a tumor suppressor gene in
1993 (3). The VHL gene, which is a
classical tumor suppressor gene and regulates a number of target
genes involved in mRNA stabilisation through the selective
degradation of RNA bound proteins, including the endothelial growth
factor, vascular endothelial growth factor, transforming growth
factor α and carbonic anhydrase 9 (4). It is widely expressed in a variety of
tissues, demonstrating that the expression of the VHL gene
transcript is not restricted to organs affected by the VHL disease
(5). Mutations of the VHL gene
were associated with carcinogenesis in various organs, including
the kidney, lung, breast, eye, ovary and cervix (6). Additionally, a deficiency or
inactivation of the VHL gene is able to cause ovarian tumors and
uterine cervix carcinoma (7,8). The
VHL protein (pVHL), the expression product of the VHL gene, has
been ascribed several distinct biochemical activities and
involvement in the regulation of the cell cycle (9). The pVHL is a multifunctional protein,
which is associated with the inhibition of angiogenesis, cell cycle
arrest, fibronectin matrix assembly, activation of p53 and
proteolysis (10). However, it has
yet to be elucidated whether the VHL gene is expressed in the human
endometrium during the menstrual cycle.
The aim of the present study was to investigate
whether the VHL gene is expressed in the human endometrium, and to
assess its expression levels in the human endometrium during the
different phases of the menstrual cycle.
Materials and methods
Tissue samples
Samples of human normal endometrial tissue were
obtained by hysterectomy from patients with benign diseases. A
total of 35 fresh tissue samples consisting of proliferative (n=17)
and secretory endometrium (n=18) were immediately frozen in liquid
nitrogen and subsequently stored at −80°C until further processing
for reverse transcription polymerase chain reaction (RT-PCR) and
Western blot analysis. The exclusion criterion of patients was
treatment with exogenous hormones within six months prior to
surgery. All patients signed informed consent letters, and the
protocol for the present study was approved by the Local Ethics
Committee (Guangzhou, China). Patients with normal endometrial
tissue were subjected to surgery for benign reasons not associated
with endometrial dysfunction, and their age was 43±2.5 years (mean
± standard deviation; range, 30–48 years). The menstrual day of the
patients was correlated with the histologic stage of the
endometrium according to the criteria established by Noyes et
al (11).
RNA isolation and RT-PCR
Total mRNA was extracted from 35 fresh human
endometrial tissue samples in the proliferative phase (n=17) and
secretory phase (n=18) using a commercial kit (Omega Bio-Tek,
Norcross, GA, USA) according to the manufacturer’s instructions.
Concentration and purity of the mRNA were assessed using
electrophoresis on a 1.0% agarose gel with an OD260/280 absorption
ratio >1.8. Aliquots of mRNA (20 μg) from each sample were
reverse transcribed using Oligo (dT) 18 primer and moloney murine
leukemia virus reverse transcriptase (M-MLV RT) (Promega Corp.,
Madison, WI, USA). The housekeeping gene glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as an internal control to normalize
the results in terms of variations in the amount of input RNA and
efficiency of reverse transcription. The primers used were: GAPDH,
forward: 5′-CTGGCGCTGAGTACGTCG-3′ and reverse:
5′-TTGACAAAGTGGTCGTTGA-3′ (657 bp), and VHL gene, forward:
5′-GTCGAAGAGTACCGCCCTGAAG-3′ and reverse:
5′-GTGTCCCTGCATCTCTGAAGAG-3′ (300 bp). PCR amplification was
performed under the following conditions: Initial denaturation at
94°C for 2 min, followed by 32 cycles of denaturation at 94°C for
30 sec, annealing at 59.5°C for 30 sec (60°C for GAPDH), extension
at 72°C for 1 min and a final extension at 72°C for 7 min. The PCR
products were verified by electrophoresis on a 1.5% agarose gel and
densitometric analysis was performed using the Bio-Rad Gel Doc 2000
Imaging System (Bio-Rad, Hercules, CA, USA). Densitometrical values
were used to calculate the ratios between target and GAPDH
bands.
Western blot analysis
Western blot analysis was performed with the same
endometrial tissue samples as those used for PCR. The fresh
endometrium was homogenized and lysed on ice using cell lysis
buffer and protease inhibitor cocktail. Following centrifugation at
12,800 × g for 5 min at 4°C, protein concentrations were assessed
using the Bicinchoninic Acid Protein Assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). Total protein was
denatured in Laemmli buffer, fractionated using a 10%
one-dimensional SDS-PAGE and transferred onto a nitrocellulose
membrane (Amersham Biosciences, Piscataway, NJ, USA). The gels were
blocked for 2 h in TBST solution (20 mmol/l Tris (pH 7.6), 137
mmol/l sodium chloride, 0.1% Tween 20) containing 10% non-fat dry
milk and incubated with antibodies against human VHL (1:1000; Cell
Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C and
against β-actin (1:3,000; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) for 1 h at room temperature with agitating. The gels
were then washed three times for 10 min each in TBST followed by
incubation for 1 h at room temperature with anti-rabbit
immunoglobulin G (IgG; 1:1,000; Boster Biological Engineering Co.,
Ltd., Wuhan, China) and anti-mouse IgG horseradish
peroxidase-linked species-specific antibodies (1:500; Boster
Biological Engineering Co., Ltd.). The bound antibodies were
detected with the enhanced chemiluminescence system BeyoECL Plus
(Beyotime, Shanghai, China). Band intensities were quantified by
scanning densitometry using the Bio-Rad Quantity One software
(Bio-Rad, Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Values were presented as the mean ±
standard deviation, and the one-way analysis of variance test
(ANOVA) was used. Differences were considered as statistically
significant for P<0.05.
Results
RT-PCR analysis
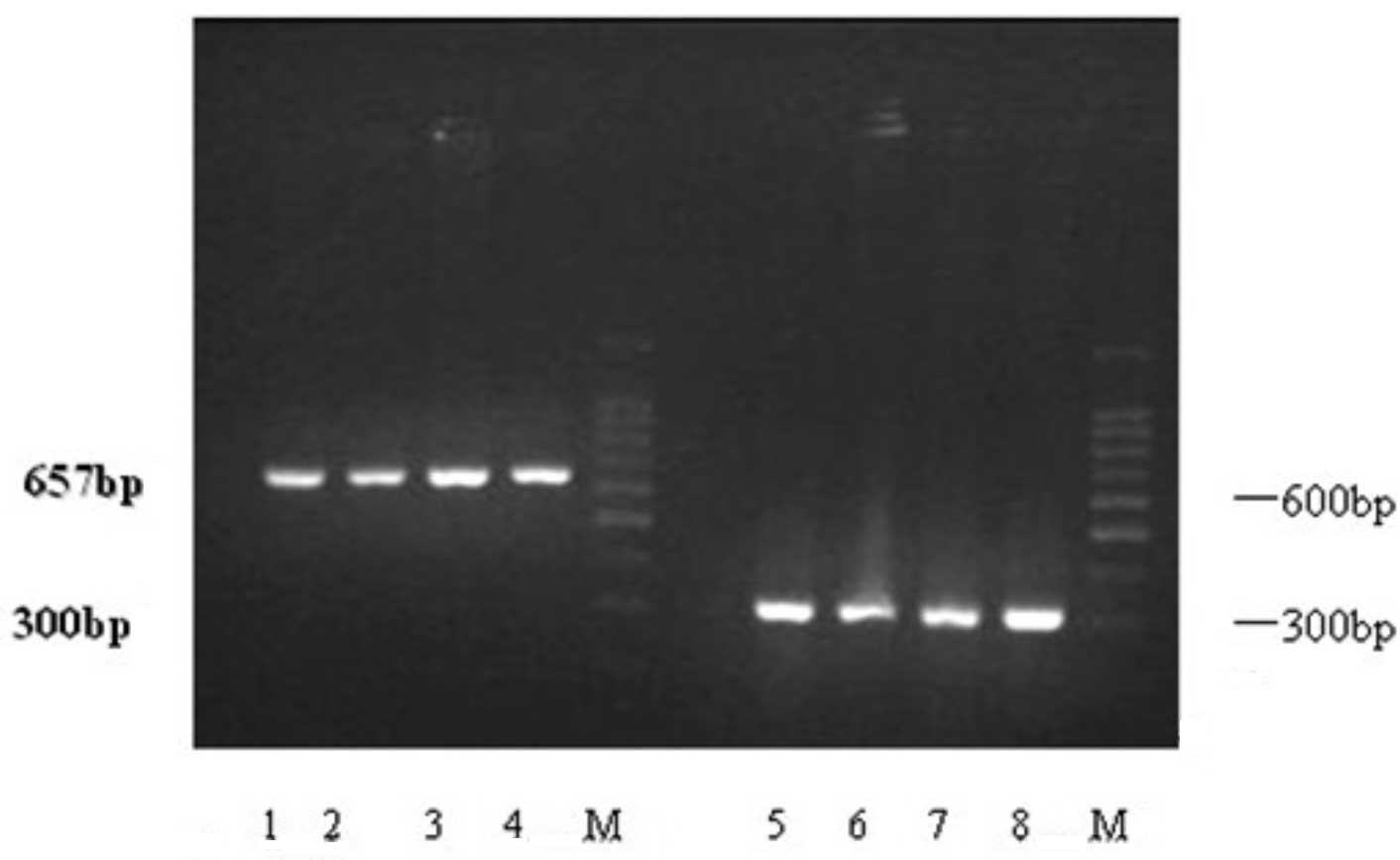
Expression of VHL mRNA in the human endometrium was
analyzed by RT-PCR. A total of 35 fresh endometrial samples were
analysed in regard to GAPDH mRNA expression, and when they were
positive, they were considered for further assessment of VHL mRNA
expression levels (Fig. 1). VHL
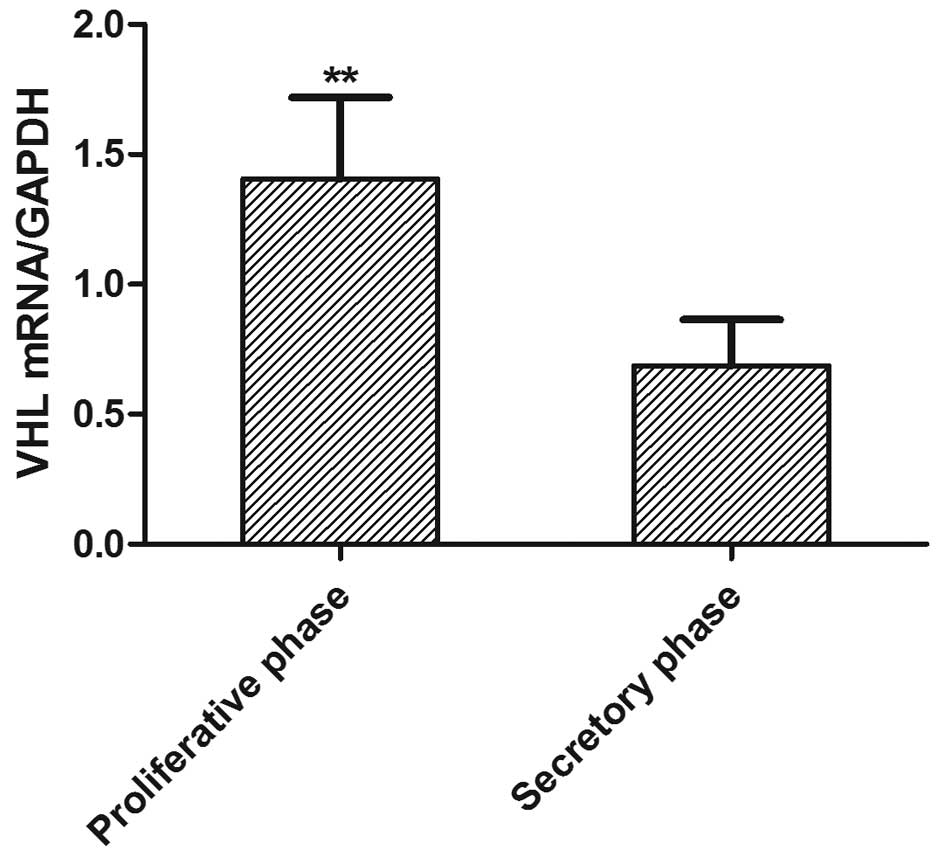
mRNA expression levels are shown in Fig. 2. The expression of VHL mRNA in the
proliferative phase was higher than that in the secretory phase
(1.26±0.46 versus 0.69±0.28)(P<0.05). A decrease in the
expression of VHL mRNA in the endometrium was observed from the
proliferative to the secretory phase of the menstrual cycle.
Western blot analysis
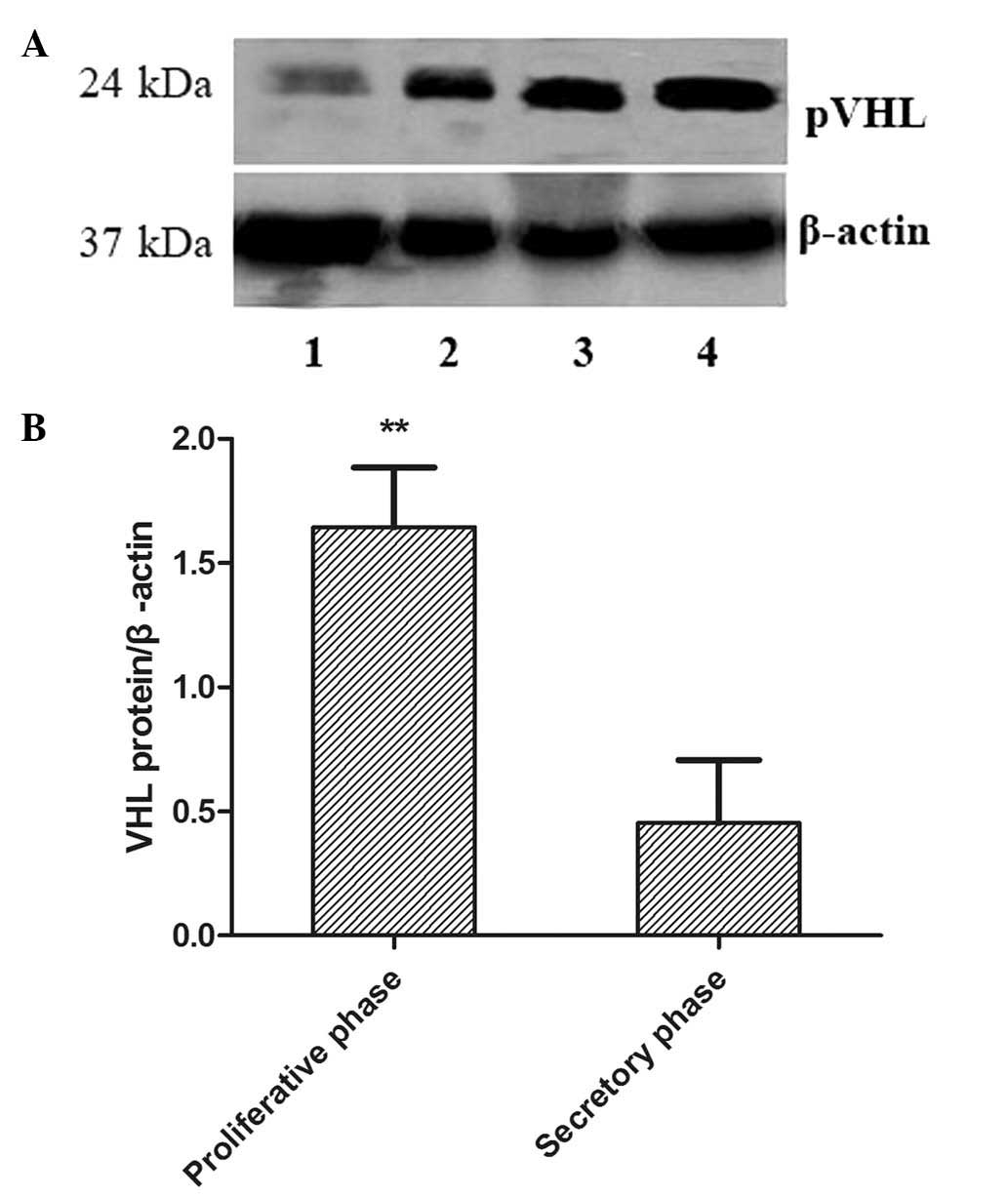
pVHL levels in the fresh endometrial tissue were
assessed by western blot analysis and normalized to β-actin, and a
single band with a molecular mass of 24 kDa was observed in all the
samples (Fig. 3A). The quantified
results are shown in Fig. 3B. pVHL
expression levels were significantly increased in the proliferative
phase compared with the secretory phase (1.83±0.67 versus
0.43±0.37) (P<0.05).
Discussion
The VHL gene is ubiquitously expressed in a variety
of organs, with particularly high levels of expression in the
urogenital system, brain, spinal cord, sensory ganglia, eyes and
bronchial epithelium (12). Its
deactivation in patients is able to induce the development of VHL
disease, central nervous system haemangioblastoma, renal carcinoma
and cysts. pVHL is a multifunctional protein, which is essential
for endothelial extracellular matrix deposition and inhibits cell
motility (13). pVHL is also able
to induce cell differentiation and growth arrest through
integration of cell-cell and cell-extracellular matrix signaling
(14).
The endometrium undergoes changes in the cyclic
blood vessels during the menstrual cycle, influenced by steroid
hormones and other angiogenesis genes. A previous study confirmed
that the expression levels of the VHL gene in human placental
villous tissue were associated with the vascular endothelial growth
factor (VEGF), and that it was a positive regulator of VEGF
production (15). However, the
expression of the VHL gene has yet to be assessed in the human
endometrium during the menstrual cycle. Angiogenesis is regulated
by a number of cytokines, including VEGF, transforming growth
factor and tumor necrosis factor. VEGF is a key mediator of
angiogenesis in physiological and pathological conditions, and also
regulates endometrial vascular development (16). In addition, VEGF expression at the
transcriptional level is able to promote endometrial angiogenesis
during the menstrual cycle. The VHL gene regulates VEGF expression
at both the transcriptional and post-transcriptional levels, and
its inactivation in target cells leads to loss of VEGF suppression
(17). The present study has
demonstrated that VHL gene expression levels in the endometrium in
the proliferative phase are higher than those in the secretory
phase (P<0.05). This result suggests that the VHL gene inhibits
the expression of VEGF in the endometrium during the proliferative
phase to prevent an excess of vascular proliferation.
The hypoxia inducible factor-1 (HIF1) has a crucial
role in the cell responses to the availablity of oxygen and
vasculogenesis through the transcriptional activation of specific
genes. Expression of HIF1-α protein in the human endometrial
glandular epithelium may be responsible for the upregulation of
VEGF (18). In addition, HIF1-α
protein was increasingly expressed from the proliferative to the
secretory phase in the human endometrium (19). pVHL is regarded as a key factor for
the oxygen-dependent proteolysis of α-subunits of HIF1-α, and in
pVHL-defective cells, HIF1-α subunits were not downregulated.
Therefore, the HIF1-α subunit was identified and targeted for rapid
proteasome-dependent degradation by the VHL E3 ubiquitin ligase
complex at normal oxygen concentrations (20). Additionally, hypoxic cells were
able to regulate VHL gene expression levels through HIF1-α
(21). Thus, the deficiency and
inactivation of the VHL gene is able to promote cellular HIF1-α
expression. In the present study, the expression of VHL mRNA and
protein in the endometrium were decreased from the proliferative to
the secretory phase. This result indicates that the VHL gene may
prevent excessive growth of blood vessels in the endometrium during
the proliferative phase.
The pVHL as a tumor suppressor protein regulates
extracellular fibronectin matrix assembly and cell cycle.
Fibronectin is a regulator of various cell activities including the
promotion of cell migration, spreading, and extracellular matrix
assembly or tissue turnover. Fibronectin has a crucial role in the
control of trophoblast invasion, angiogenesis and determination of
cell shape, and thus regulates endometrial receptivity and
pregnancy (22). In the human
endometrium, fibronectin was detected by immunocytochemical
localization in the epithelial and stromal cells of the
endometrium. Additionally, fibronectin levels decrease in the
endometrium from the proliferative to the secretory phase and
modulate the progression of endometrium (23). Overexpression of pVHL may increase
fibronectin expression post-transcriptionally and the secretion of
extracullar fibronectin (24).
pVHL mutations lead to diseases associated with fibronectin
assembly defects, and pVHL-deficient cells fail to assemble the
extracellular fibronectin matrix. In the present study, pVHL
expressed in the endometrium during the proliferative phase was
higher than that in the secretory phase. This result suggested that
pVHL may take an active role in the human endometrium during
menstrual cycle through the interaction with fibronectin.
In conclusion, to the best of our knowledge, the
present study is the first to assess VHL mRNA and protein
expression levels in the human endometrium during the menstrual
cycle. Expression of VHL mRNA and protein were decreased in the
human endometrium from the proliferative to the secretory phase.
This result may provide a novel view on the mechanism of
endometrial disease. There is a requirement for further elucidation
of whether VHL mRNA and protein expression levels may be a target
for novel therapies of endometrial diseases.
References
|
1
|
Maas JW, Groothuis PG, Dunselman GA, de
Goeij AF, Struyker Boudier HA and Evers JL: Endometrial
angiogenesis throughout the human menstrual cycle. Hum Reprod.
16:1557–1561. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uz YH, Murk W, Yetkin CE, Kayisli UA and
Arici A: Expression and role of interleukin-23 in human endometrium
throughout the menstrual cycle and early pregnancy. J Reprod
Immunol. 87:21–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Latif F, Tory K, Gnarra J, Yao M, Duh FM,
Orcutt ML, Stackhouse T, Kuzmin I, Modi W and Geil L:
Identification of the von Hippel-Lindau disease tumor suppressor
gene. Science. 260:1317–1320. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamada M, Suzuki K, Kato Y, Okuda H and
Shuin T: von Hippel-Lindau protein promotes the assembly of actin
and vinculin and inhibits cell motility. Cancer Res. 61:4184–4189.
2001.PubMed/NCBI
|
|
5
|
Lonser RR, Glenn GM, Walther M, Chew EY,
Libutti SK, Linehan WM and Oldfield EH: von Hippel-Lindau disease.
Lancet. 361:2059–2067. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pavlova TV, Kashuba VI, Muravenko OV,
Yenamandra SP, Ivanova TA, Zabarovskaia VI, Rakhmanaliev ER,
Petrenko LA, Pronina IV, Loginov VI, Iurkevich OIu, Kiselev LL,
Zelenin AV and Zabarovskiĭ ER: Technology of analysis of
epigenetic and structural changes of epithelial tumors genome with
NotI-microarrays by the example of human chromosome. Mol Biol
(Mosk). 43:339–347. 2009.(In Russian).
|
|
7
|
Osada R, Horiuchi A, Kikuchi N, Yoshida J,
Hayashi A, Ota M, Katsuyama Y, Mellilo G and Konishi I: Expression
of hypoxia-inducible factor 1α, hypoxia-inducible factor 2α, and
von Hippel-Lindau protein in epithelial ovarian neoplasms and
allelic loss of von Hippel-Lindau gene: nuclear expression of
hypoxia-inducible factor 1α is an independent prognostic factor in
ovarian carcinoma. Hum Pathol. 38:1310–1320. 2007.
|
|
8
|
Choi CH, Lee KM, Choi JJ, Kim TJ, Kim WY,
Lee JW, Lee SJ, Lee JH, Bae DS and Kim BG: Hypermethylation and
loss of heterozygosity of tumor suppressor genes on chromosome 3p
in cervical cancer. Cancer Lett. 255:26–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaelin WG Jr: The von Hippel-Lindau tumor
suppressor protein and clear cell renal carcinoma. Clin Cancer Res.
13:680s–684s. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamada M, Suzuki K, Kato Y, Okuda H and
Shuin T: von Hippel-Lindau protein promotes the assembly of actin
and vinculin and inhibits cell motility. Cancer Res. 61:4184–4189.
2001.PubMed/NCBI
|
|
11
|
Noyes RW, Herting A and Rock J: Dating the
endometrial biopsy. Am J Obstet Gynecol. 122:262–263.
1975.PubMed/NCBI
|
|
12
|
Richards FM, Schofield PN, Fleming S and
Maher ER: Expression of the von Hippel-Lindau disease tumour
suppressor gene during human embryogenesis. Hum Mol Genet.
5:639–644. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang N, Mack F, Haase VH, Simon MC and
Johnson RS: pVHL function is essential for endothelial
extracellular matrix deposition. Mol Cell Biol. 26:2519–2530. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu JY, Zhu WJ, Cao XZ, Li XF and Wu J:
Aberrant expression of the von Hippel-Lindau gene in human
endometrial hyperplasia and endometrial carcinoma. Int J Gynecol
Cancer. 21:430–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rajakumar A, Doty K, Daftary A, Markovic N
and Conrad KP: Expression of von Hippel Lindau (pVHL) protein in
placentae from normal pregnant women and women with preeclampsia.
Placenta. 27:411–421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Datta K, Mondal S, Sinha S, Li J, Wang E,
Knebelmann B, Karumanchi SA and Mukhopadhyay D: Role of
elongin-binding domain of von Hippel Lindau gene product on
HuR-mediated VPF/VEGF mRNA stability in renal cell carcinoma.
Oncogene. 24:7850–7858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choueiri TK, Vaziri SA, Jaeger E, Elson P,
Wood L, Bhalla IP, Small EJ, Weinberg V, Sein N, Simko J, Golshayan
AR, Sercia L, Zhou M, Waldman FM, Rini BI, Bukowski RM and
Ganapathi R: von Hippel-Lindau gene status and response to vascular
endothelial growth factor targeted therapy for metastatic clear
cell renal cell carcinoma. J Urol. 180:860–865. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nayak NR and Brenner RM: Vascular
proliferation and vascular endothelial growth factor expression in
the rhesus macaque endometrium. J Clin Endocrinol Metab.
87:1845–1855. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Critchley HO, Osei J, Henderson TA,
Boswell L, Sales KJ, Jabbour HN and Hirani N: Hypoxia-inducible
factor-1α expression in human endometrium and its regulation by
prostaglandin E-series prostanoid receptor 2 (EP2). Endocrinology.
147:744–753. 2006.
|
|
20
|
André H and Pereira TS: Identification of
an alternative mechanism of degradation of the hypoxia-inducible
factor-1α. J Biol Chem. 283:29375–29384. 2008.
|
|
21
|
Genbacev O, Krtolica A, Kaelin W and
Fisher SJ: Human cytotrophoblast expression of the von
Hippel-Lindau protein is downregulated during uterine invasion in
situ and upregulated by hypoxia in vitro. Dev Biol. 233:526–536.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaloglu C and Onarlioglu B: Extracellular
matrix remodelling in rat endometrium during early pregnancy: the
role of fibronectin and laminin. Tissue Cell. 42:301–306. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Kreiner M, van der Walle CF and
Mardon HJ: Clustered integrin α5β1 ligand displays model
fibronectin-mediated adhesion of human endometrial stromal cells.
Biochem Biophys Res Commun. 407:777–782. 2011.
|
|
24
|
Zhou Q, Pardo A, Königshoff M, Eickelberg
O, Budinger GR, Thavarajah K, Gottardi CJ, Jones J, Varga J, Selman
M, Sznajder JI, Raj JU and Zhou G: Role of von Hippel-Lindau
protein in fibroblast proliferation and fibrosis. FASEB J.
25:3032–3044. 2011. View Article : Google Scholar : PubMed/NCBI
|