Introduction
Chlorhexidine (CHX), a cationic bisbiguanide with a
broad antimicrobial spectrum, attacks the bacterial cell membrane,
causing leakage and precipitation of the cellular contents
(1). CHX is widely used clinically
to reduce inflammation, and swelling and bleeding of the gums. As a
result, it is currently recognized as one of the most effective
chemical antiplaque agents (2,3).
Listerine (LIS) is an essential oil agent, containing menthol,
thymol, methyl salicylate and eucalyptol as active agents, which
exerts a lethal effect on microbiota by disrupting the cell wall
and inhibiting enzymatic activity (1,4).
When an implant surface is exposed to the oral
cavity, it is immediately covered by the salivary pellicle and
colonized by oral microorganisms (5,6).
Mechanical instrumentation with metal curettes, plastic curettes,
ultrasonic scalers, air-powder abrasive systems and lasers, has
been widely used to remove plaque from dental implants (7,8).
However, it has been demonstrated that it is impossible to achieve
complete removal of all of the adhering microorganisms by
mechanical debridement, due to the complexity of the implant
surfaces provided with threads or roughness (9,10).
Therefore, adjunctive peri-implant therapies, including antibiotics
and antiseptics, have been proposed (11). CHX and LIS are used as alternative
or adjunctive treatments to mechanical debridement for reducing the
bacterial load in the peri-implant pocket (5,12).
CHX and LIS are reported to inhibit biofilm formation in an in
vitro model (12,13). One possible drawback to CHX use is
that CHX has been demonstrated to exhibit cytotoxic activity on
alveolar bone cells and gingival epithelial cells (14,15).
Previous studies have reported that mesenchymal stem
cells served as initial colonizers of implant surfaces (16,17).
The buccal fat pad is an readily accessible source of adult stem
cells (18). The present study
aimed to investigate the effects of the two antiseptics, CHX and
LIS, on the buccal fat pad derived stem cells grown on titanium
discs.
Materials and methods
Tissue preparation and cell
isolation/expansion
The buccal fat pad was obtained from a healthy
individual undergoing orthognathic surgery procedures in the Seoul
St. Mary’s Hospital (Seoul, Korea). The patient was in good health
and no systemic diseases were reported. This study was reviewed and
approved by the Institutional Review Board of the Catholic
University of Korea (Seoul, Korea) and informed consent was
obtained from the patient. Sample tissues were processed according
to a previously described method (19,20).
The tissues were washed several times with sterile
phosphate-buffered saline (PBS; Invitrogen Life Technologies,
Carlsbad, CA, USA), ground into small pieces and treated with 0.06
% collagenase I (Invitrogen Life Technologies) for 4 h at 37°C.
Following incubation, the tissue was centrifuged at 100 × g for 10
min to separate the adipocytes and lipid droplets from the stromal
vascular fraction. Then, the cells were passed through a 40 μm cell
strainer (BD Biosciences, Bedford, MA, USA). The cells were then
re-suspended in Dulbecco’s modified Eagle’s medium (DMEM;
Invitrogen Life Technologies), containing 10% fetal bovine serum
(FBS; Thermo Fisher Scientific, Inc., Logan, UT, USA) and
antibiotics (100 U/ml of penicillin and 100 μg/ml streptomycin;
Invitrogen Life Technologies). The cells were seeded in 100 mm
tissue culture dishes and maintained at 37°C in a humidified 5%
CO2 environment. The culture media were replenished with
fresh media every two days. The cells were sub-cultured with 0.05%
trypsin-EDTA (Invitrogen Life Technologies) at 5- to 7-day
intervals at a 1:2–4 dilution. The initial adherent cell
population, referred to as passage 0 (P0), as well as several of
the following passages (up to three passages), were analyzed by
flow cytometry (BD Biosciences).
Cell culture on titanium discs and
antiseptic application
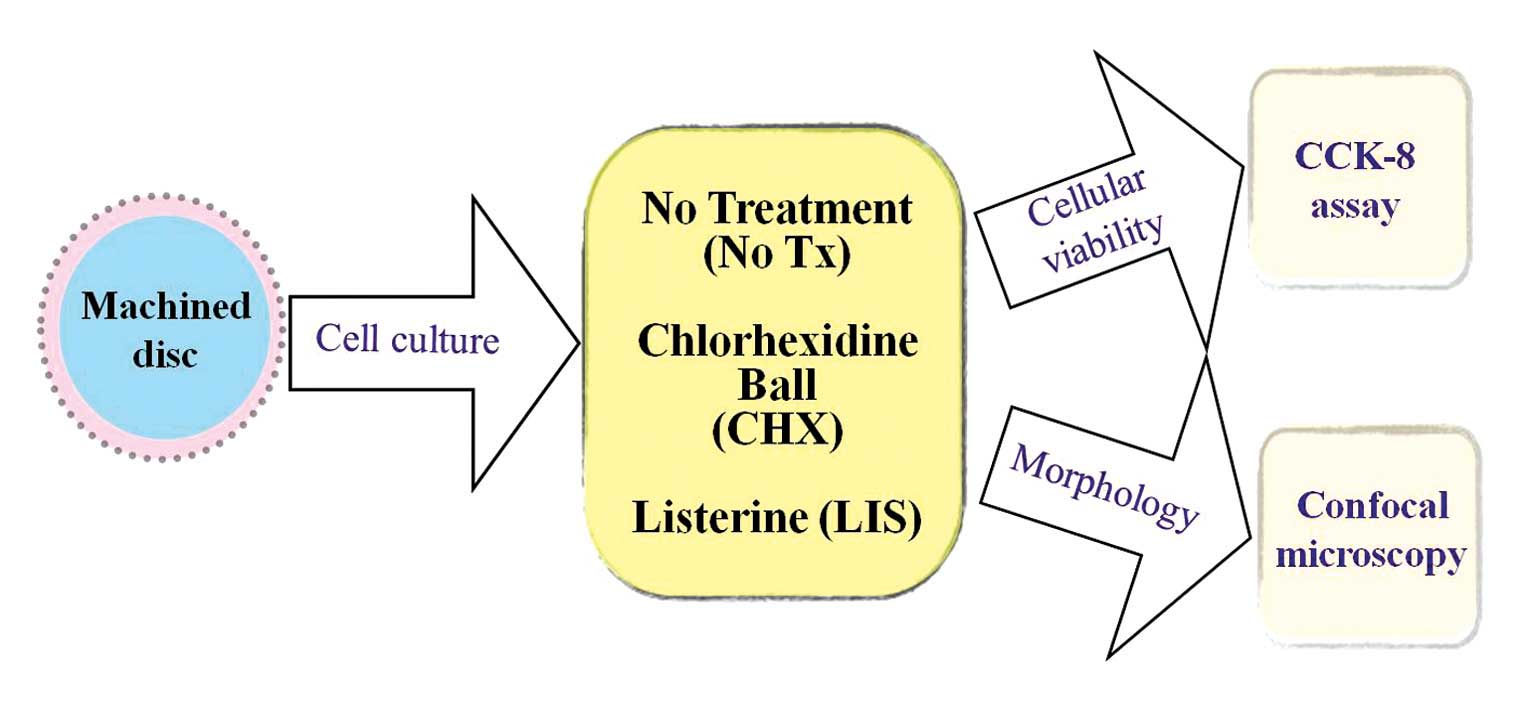
Fig. 1 illustrates
the overview of the study design. Machined titanium discs (15) measuring 10 mm in diameter and 2 mm
in thickness were used in this study (Dentium Co., Seoul, Korea).
Two mouth rinses were applied; (i) a 0.12 % chlorhexidine
digluconate solution (CHX; Hexamedine, Bukwang, Seoul, Korea) and
(ii) a solution containing essential oils (LIS,
Listerine® Coolmint; Johnson & Johnson, Bangkok,
Thailand). The stem cells were plated at a density of
3.0×104 cells/well on 24-well plates containing titanium
discs and cultured in DMEM for nine days. The media in each were
suctioned away and the discs were immersed either in CHX or LIS for
30 sec, 1.5 min or 4.5 min.
Cell viability test
The viability of the treated cells was
quantitatively analyzed by a cell counting kit-8 (CCK-8; Dojindo
Molecular Technologies Inc., Rockville, MD, USA). A water-soluble
tetrazolium salt-8 [2-(2-methoxy-
4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo
phenyl)-2H-tetrazolium, monosodium salt (WST-8)] solution was added
to the wells and the discs were incubated for 3 h. The quantity of
generated formazan at the 1 and 3 h incubation time points was
determined by reading the absorbance at a 450 nm wavelength using
the microplate spectrophotometer system (BioTek, Winooski, VT, USA)
(21).
Evaluation of cell morphology
Following the cell viability test, each implant disc
was fixed with 4% paraformaldehyde overnight. The discs were washed
in PBS three times. Actin filaments were stained with
rhodamine-conjugated-phalloidin (Molecular Probes, Eugene, OR, USA)
and the nuclei were counterstained with Hoechst 33342 blue dye
(Molecular probes). The cells were observed using a confocal laser
microscope (LSM5 Pascal; Carl Zeiss AG, Jena, Germany) at a
magnification of ×400.
Statistical analysis
The results were represented as the mean ± standard
deviation. An analysis of covariance (ANCOVA) was performed to
compare cellular viability, according to group and time, using
commercially available statistical software (SPSS 12 for Windows;
SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
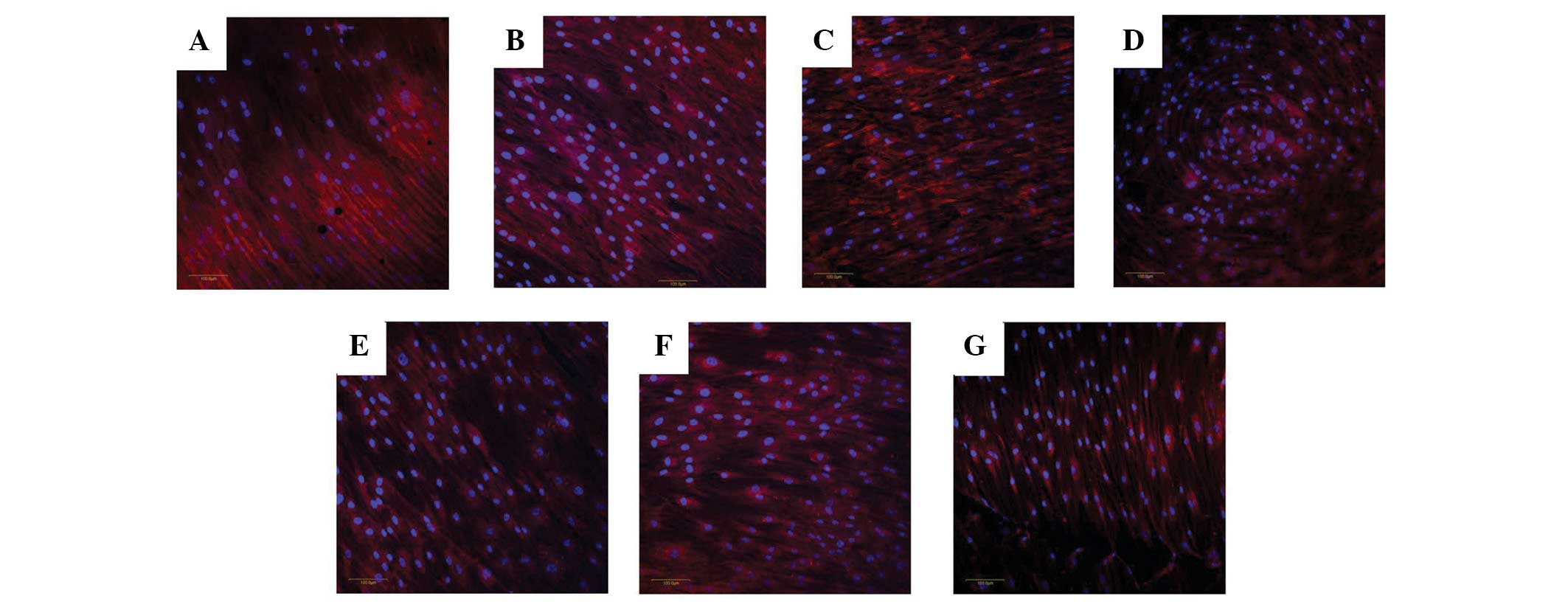
Evaluation of cell morphology
The untreated cells attached to the titanium discs
demonstrated well-organized actin cytoskeletons with blue nuclei
under a confocal microscope (Fig.
2). The treatment of the adult stem cells with 0.12% CHX for 30
sec did not cause a significant alteration compared with the
untreated group. Increasing the immersion times (1.5 and 4.5 min)
did not lead to significant changes. A similar trend was observed
in the LIS groups. No notable alteration in the cytoskeletal
organization was observed. Rounding of the cells or progressive
detachment from the substrate was not observed during the
experiments.
Cellular viability
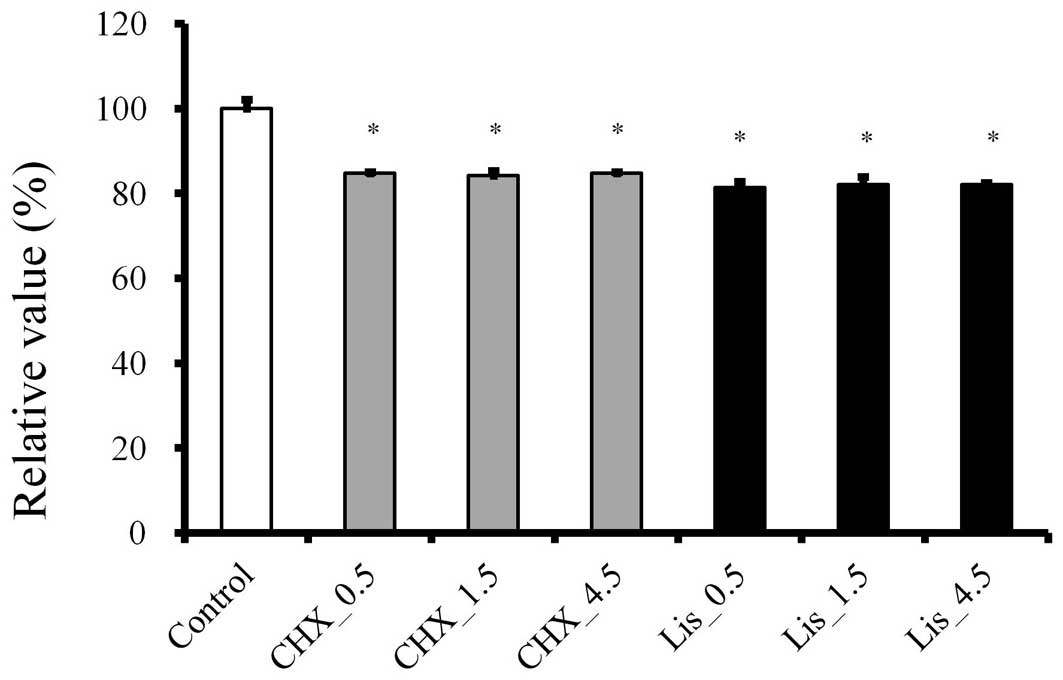
An CCK-8 assay demonstrated that the treatment with
CHX and LIS affected cell viability. The CHX and LIS demonstrated
toxic effects on adult stem cells in vitro, with a mean
viability of 84.8±0.4 and 81.3±1.5%, respectively, following
exposure for 30 sec, when the control group was considered to be
100% (100±2.1; P<0.05; Fig. 3).
The increase in the treatment time of CHX and LIS up to 4.5 min did
not induce significant decreases of viability. Mean cell viability
for the CHX group was 84.2±1.1 and 84.8±0.4% for 1.5 and 4.5 min,
and the viability of the LIS group for 1.5 and 4.5 min was 82.1±1.9
and 82.1±0.4%, respectively. The progressive increase in the
treatment time up to 4.5 min did not induce any additional
decreases of viability either in CHX and LIS at 1 h
(P>0.05).
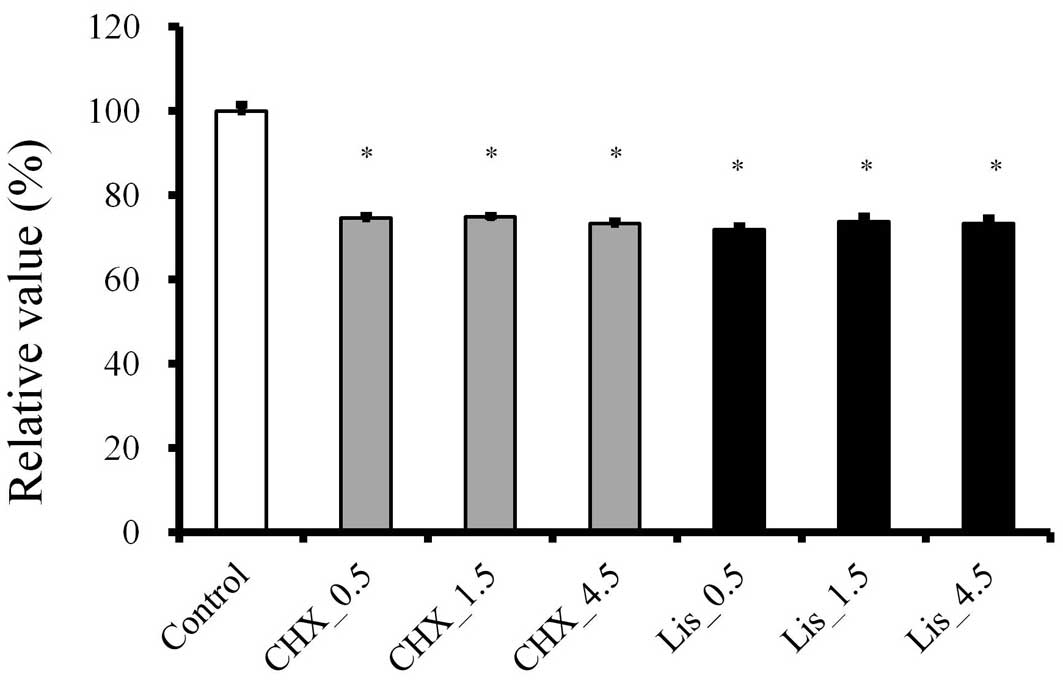
The relative viability of CHX of 30 sec, 1.5 and 4.5
min at 3 h was 74.6±0.3, 74.8±0.6 and 73.3±0.6, respectively, when
the control group was considered 100% (100±1.5; Fig. 4). The relative viability of LIS of
30 sec, 1.5 and 4.5 min was 71.8±0.9, 73.7±1.2 and 73.3±1.2%,
respectively. The 3 h results were significantly lesser than the
data from 1 h (P<0.05).
Discussion
The present study clearly demonstrated that stem
cells derived from the buccal fat pad were sensitive to CHX and
LIS, and that these cells experienced a decrease in cellular
viability with the application time of 30 sec.
A previous study demonstrated that CHX was more
cytotoxic than LIS for cultured human gingival fibroblasts, however
LIS appeared to be more cytotoxic than CHX at a diluted
concentration (1 and 2% of the given solutions) (22). In the present study, for the stem
cells from the buccal fat pad, there was no significant difference
of viability between CHX and LIS. Previous studies have identified
that CHX induced cell damage in a time-dependent manner for
osteoblastic cells and fibroblasts (23). However, this study did not
demonstrated significant decreases of viability from 30 sec to 4.5
min in application. The conflicting results regarding the different
responses to CHX and LIS may, in part, be attributed to the type of
cells, culturing period, stage of differentiation of the cells or
culturing condition (24).
The data describing the effects of CHX and LIS are
varied between different studies (5,13,25).
It was demonstrated that CHX and LIS were able to reduce the total
amount of microorganisms accumulating on titanium surfaces and that
they exhibited a significant bactericidal effect against adhering
bacteria (5). However, a
significant reduction in the amount of bacteria in the saliva was
observed after the chlorhexidine mouth rinse, but not following the
essential oils rinse (25). In one
study, CHX and LIS did not demonstrate a broad-spectrum
antimicrobial effect, so they were not recommended for the
detoxification of infected implant surfaces (13). Further investigations are required
to determine the optimal application time and concentration of the
antimicrobial agent to maximize the reduction of the bacterial load
and minimize the cytotoxicity to the surrounding cells.
CHX has the property of substantivity, allowing
prolonged adherence of the antiseptic to hard and soft oral
surfaces and its gradual release at effective doses produces the
persistence of its antimicrobial activity (26). Higher antibacterial effects of CHX
were observed in the rough titanium surface when compared with the
machined surface (3) and more
pronounced effects of CHX may be observed if the stem cells were
applied onto the rough surface.
It has been demonstrated that bacterial growth
inhibition is affected by the concentration of the antimicrobial
agent (3,27). The effects of rinsing of 0.12% CHX
was compared with irrigation with 0.06% CHX using a powered oral
irrigator and the results revealed that the irrigation group also
demonstrated a greater reduction in the bleeding index and the
calculus index than the rinsing group (28). This approach, using the powered
irrigator with a diluted solution, may be considered as an adjunct
to oral health in patients with implants and may produce less
cytotoxic effects to the surrounding cells.
The MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
assay is considered to be a more sensitive assay than the trypan
blue assay (29). Trypan blue
assay is based on the principle that live cells possess intact cell
membranes that exclude penetration of the dye, while the MTT assay
assesses cellular viability through the determination of
mitochondrial dehydrogenase activity. However, further treatment is
required to solubilize the formazan crystals and MTT may be toxic
to cells (30). In the present
study, a CCK-8 assay utilizing WST-8 was used because this is
reported to be more sensitive than the MTT assay and less toxic to
the cells (30).
From these results, we conclude that the application
of CHX and LIS on titanium discs had residual effects on the
viability of the stem cells derived from the buccal fat pad, and
that it may be suggested that the application of CHX or LIS
directly into the peri-implant pocket produces toxic effects. The
concentration and application time of the antimicrobial agent
should be meticulously controlled to obtain optimal results. These
cytotoxic effects should be considered if regenerative surgery is
planned for the treatment of peri-implantitis.
Acknowledgements
This study was supported by Seoul St. Mary’s
Hospital Clinical Medicine Research Program year of 2011 through
the Catholic University of Korea (Seoul, Korea). The authors
acknowledge Dentium (Seoul, Korea) for donating the titanium discs
for this study.
References
|
1
|
Cosyn J, Princen K, Miremadi R, Decat E,
Vaneechoutte M and De Bruyn H: A double-blind randomized
placebo-controlled study on the clinical and microbial effects of
an essential oil mouth rinse used by patients in supportive
periodontal care. Int J Dent Hyg. 11:53–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim BH, Seo HS, Jung SC, et al: Study in
bactericidal properties of chlorhexidine grafting on the modified
titanium. J Nanosci Nanotechnol. 11:1530–1533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kozlovsky A, Artzi Z, Moses O,
Kamin-Belsky N and Greenstein RB: Interaction of chlorhexidine with
smooth and rough types of titanium surfaces. J Periodontol.
77:1194–1200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kato T, Iijima H, Ishihara K, et al:
Antibacterial effects of Listerine on oral bacteria. Bull Tokyo
Dent Coll. 31:301–307. 1990.PubMed/NCBI
|
|
5
|
Gosau M, Hahnel S, Schwarz F, Gerlach T,
Reichert TE and Bürgers R: Effect of six different peri-implantitis
disinfection methods on in vivo human oral biofilm. Clin Oral
Implants Res. 21:866–872. 2010.PubMed/NCBI
|
|
6
|
Chen S and Darby I: Dental implants:
maintenance, care and treatment of peri-implant infection. Aust
Dent J. 48:212–220. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duarte PM, Reis AF, de Freitas PM and
Ota-Tsuzuki C: Bacterial adhesion on smooth and rough titanium
surfaces after treatment with different instruments. J Periodontol.
80:1824–1832. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brookshire FV, Nagy WW, Dhuru VB, Ziebert
GJ and Chada S: The qualitative effects of various types of hygiene
instrumentation on commercially pure titanium and titanium alloy
implant abutments: an in vitro and scanning electron microscope
study. J Prosthet Dent. 78:286–294. 1997. View Article : Google Scholar
|
|
9
|
Park JB, Jang YJ, Choi BK, Kim KK and Ko
Y: Treatment with various ultrasonic scaler tips affect efficiency
of brushing of SLA titanium discs. J Craniofac Surg. 24:e119–e23.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mombelli A and Lang NP: Antimicrobial
treatment of peri-implant infections. Clin Oral Implants Res.
3:162–168. 1992. View Article : Google Scholar
|
|
11
|
Renvert S, Roos-Jansåker AM and Claffey N:
Non-surgical treatment of peri-implant mucositis and
peri-implantitis: a literature review. J Clin Periodontol.
35(Suppl): 305–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baffone W, Sorgente G, Campana R, Patrone
V, Sisti D and Falcioni T: Comparative effect of chlorhexidine and
some mouthrinses on bacterial biofilm formation on titanium
surface. Curr Microbiol. 62:445–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bürgers R, Witecy C, Hahnel S and Gosau M:
The effect of various topical peri-implantitis antiseptics on
Staphylococcus epidermidis, Candida albicans, and Streptococcus
sanguinis. Arch Oral Biol. 57:940–947. 2012.PubMed/NCBI
|
|
14
|
Cabral CT and Fernandes MH: In vitro
comparison of chlorhexidine and povidone-iodine on the long-term
proliferation and functional activity of human alveolar bone cells.
Clin Oral Investig. 11:155–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Babich H and Tipton DA: In vitro response
of human gingival epithelioid S-G cells to minocycline. Toxicol In
Vitro. 16:11–21. 2002. View Article : Google Scholar
|
|
16
|
Dalby MJ, Gadegaard N, Tare R, et al: The
control of human mesenchymal cell differentiation using nanoscale
symmetry and disorder. Nat Mater. 6:997–1003. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gittens RA, Olivares-Navarrete R,
McLachlan T, et al: Differential responses of osteoblast lineage
cells to nanotopographically-modified, microroughened
titanium-aluminum-vanadium alloy surfaces. Biomaterials.
33:8986–8994. 2012. View Article : Google Scholar
|
|
18
|
Farré-Guasch E, Martí-Pagè C,
Hernádez-Alfaro F, Klein-Nulend J and Casals N: Buccal fat pad, an
oral access source of human adipose stem cells with potential for
osteochondral tissue engineering: an in vitro study. Tissue Eng
Part C Methods. 16:1083–1094. 2010.PubMed/NCBI
|
|
19
|
Kwon SG, Lee G, Kim CH and Park JU:
Microarray analysis of gene expression in osteogenic
differentiation of buccal fat pad-derived stem cells treated with
simvastatin and BMP-2. Tissue Eng Regen Med. 6:942–951. 2009.
|
|
20
|
Kim CH, Choi KS, Lee IK, Park JU and Pyo
SW: The effect of simvastin on osteogenic differentiation of human
buccal fat pad-derived stem cells. Tissue Eng Regen Med. 6:2–8.
2009.
|
|
21
|
Jung YS, Jeong JH, Yook S, et al: Surface
modification of pancreatic islets using heparin-DOPA conjugate and
anti-CD154 mAb for the prolonged survival of intrahepatic
transplanted islets in a xenograft model. Biomaterials. 33:295–303.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Flemingson, Emmadi P, Ambalavanan N,
Ramakrishnan T and Vijayalakshmi R: Effect of three commercial
mouth rinses on cultured human gingival fibroblast: an in vitro
study. Indian J Dent Res. 19:29–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giannelli M, Chellini F, Margheri M,
Tonelli P and Tani A: Effect of chlorhexidine digluconate on
different cell types: a molecular and ultrastructural
investigation. Toxicol In Vitro. 22:308–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quarles LD, Yohay DA, Lever LW, Caton R
and Wenstrup RJ: Distinct proliferative and differentiated stages
of murine MC3T3-E1 cells in culture: an in vitro model of
osteoblast development. J Bone Miner Res. 7:683–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wikén Albertsson K, Persson A and van
Dijken JW: Effect of essential oils containing and alcohol-free
chlorhexidine mouthrinses on cariogenic micro-organisms in human
saliva. Acta Odontol Scand. 71:883–891. 2012.PubMed/NCBI
|
|
26
|
Cousido MC, Tomás Carmona I,
García-Caballero L, Limeres J, Alvarez M and Diz P: In vivo
substantivity of 0.12% and 0.2% chlorhexidine mouthrinses on
salivary bacteria. Clin Oral Investig. 14:397–402. 2010.
|
|
27
|
Astasov-Frauenhoffer M, Braissant O,
Hauser-Gerspach I, et al: Quantification of vital adherent
Streptococcus sanguinis cells on protein-coated titanium after
disinfectant treatment. J Mater Sci Mater Med. 22:2045–2051. 2011.
View Article : Google Scholar
|
|
28
|
Felo A, Shibly O, Ciancio SG, Lauciello FR
and Ho A: Effects of subgingival chlorhexidine irrigation on
peri-implant maintenance. Am J Dent. 10:107–110. 1997.PubMed/NCBI
|
|
29
|
Meleti Z, Shapiro IM and Adams CS:
Inorganic phosphate induces apoptosis of osteoblast-like cells in
culture. Bone. 27:359–366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Almazin SM, Dziak R, Andreana S and
Ciancio SG: The effect of doxycycline hyclate, chlorhexidine
gluconate, and minocycline hydrochloride on osteoblastic
proliferation and differentiation in vitro. J Periodontol.
80:999–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|