Introduction
High-mobility group box 1 (HMGB1) protein, which was
previously thought to function only as a nuclear factor that
enhances transcription, was recently identified to be a crucial
cytokine that mediates the response to infection, injury and
inflammation (1). Myocardial
ischemia/reperfusion (I/R) injury has been found to involve a
complex pathophysiological process (2–3), and
HMGB1 has been reported to act as a representative and novel
proinflammatory cytokine that contributes to the pathophysiological
progress of myocardial I/R injury (4). Therefore, anti-HMGB1 release may
become a novel therapeutic target for myocardial I/R injury
(5).
Heme oxygenase-1 (HO-1), an inducible isoform of
heme oxygenase (HO) enzymes, has been reported to be
anti-inflammatory, anti-apoptotic and decrease proliferation in
several cell types, including cardiac myocytes (6,7).
Hydroxysafflor yellow A has been demonstrated to provide a
protective effect against I/R injury in H9c2 cardiac myocytes by
upregulating the expression and activity of HO-1 (8). Moreover, Hwa et al (7) further reported that
2-methoxycinnamaldehyde protects against myocardial I/R injury via
anti-oxidant and anti-inflammatory action due to HO-1
induction.
Isoproterenol (ISO) is a β-adrenergic receptor (AR)
agonist and has been reported to mediate HO-1 induction via the
phosphatidylinositol 3-kinase (PI3K) and p38 mitogen-activated
protein kinase (p38 MAPK) pathways in RAW 264.7 cells. This was
demonstrated to result in the inhibition of HMGB1 release in
lipopolysaccharide (LPS)-activated RAW 264.7 cells and increase the
survival rate of cecal ligation and puncture (CLP)-induced septic
mice (9). Therefore, it was
hypothesized that ISO may also have a pivotal role in the
protective effects on myocardial I/R injury in rats. This
protection mechanism is possibly due to the inhibition of HMGB1
release via the induction of HO-1 during rat myocardial I/R injury
in vivo.
Materials and methods
Materials
ISO hydrochloride and zinc protoporphyrin IX
(ZnPPIX) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Colorimetric lactate dehydrogenase (LDH), creatine kinase (CK),
myocardium malondialdehyde (MDA) and superoxide dismutase (SOD)
assay kits were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). The rat tumor necrosis factor-α (TNF-α)
and interleukin-6 (IL-6) enzyme-linked immunosorbent assay (ELISA)
kits were purchased from Wuhan Elabscience Biotechnology Co., Ltd.
(Wuhan, China). The antibodies used to recognize HO-1 (mouse
monoclanal anti-HO-1 antibody against HO-1) and HMGB1 (mouse
monoclonal anti-HMGB1 antibody against HMGB-1) were purchased from
Sigma-Aldrich.
Animal preparation and experimental
design
The experimental protocol conformed to the Guideline
for the Care and Use of Laboratory Animals published by the US
National Institutes of Health (NIH publication, revised 1996) and
was approved by the National Animal Care and Use Expert Committee
(China).
Male Sprague-Dawley (SD) rats (specific pathogen
free; weight, 250–300 g) were purchased from the Hunan Slac Jingda
Laboratory Animal Co., Ltd. (Hunan, China). All animals were kept
in an environmentally controlled breeding room (temperature,
23±2°C; humidity, 60±5%; 12 h dark/light cycle). Rats had access to
water and commercial pellet feed ad libitum for one week and
were randomly divided into seven groups receiving the following
treatments: Group 1: The sham operated control group (SO, n=12),
rats received saline via intraperitoneal (i.p.) injection and were
subjected to surgical manipulation without the induction of
myocardial ischemia; group 2: The I/R group (I/R, n=12), the rats
were injected with saline via i.p, after 1 h left anterior
descending coronary artery (LAD) occlusion was performed for 30 min
followed by reperfusion for 24 h; group 3: The ISO and I/R group
(ISO-I/R, n=12), rats were pretreated with ISO hydrochloride
solution (i.p., 10 mg/kg) (9),
after 1 h LAD occlusion was performed for 30 min followed by
reperfusion for 24 h; Group 4: The ZnPPIX, ISO and I/R group
(ZnPPIX-ISO-I/R; n=9), the rats were pretreated with ZnPPIX (an
inhibitor of HO-1, i.p., 10 mg/kg) (9) plus ISO hydrochloride solution (i.p.,
10 mg/kg), after 1 h, LAD occlusion was performed for 30 min
followed by reperfusion for 24 h.
Subsequent to administration of sodium pentobarbital
(45 mg/kg, i.p.; Sigma-Aldrich) anesthetic, the rats were
ventilated artificially with a volume-controlled rodent respirator
(Jinjiang Ltd., Chengdu, China) at 70 strokes/min. The rats were
placed on an electric heating pad (Jinjiang Ltd.) to maintain their
body temperature at 37.8°C. Heparin (200 IU/kg) was administered
intravenously prior to ischemia. Lead-II of the electrocardiogram
(Jinjiang Ltd.) was monitored with subcutaneous stainless steel
electrodes. The electrocardiogram was monitored using a
computer-based EP system (LEAD2000B; Jinjiang Ltd.).
A thoracotomy through the left parasternal incision
was performed. The pericardium was incised and the anterior wall of
the left ventricle was exposed. A 4-0 silk suture (Nanjing
Jiancheng Bioengineering Institute) on a small curved needle was
passed through the myocardium beneath the middle segment of the LAD
branch coursing down the middle of the anterior wall of the left
ventricle. A small vinyl flake (Nanjing Jiancheng Bioengineering
Institute) was passed into the two ends of the suture, which was
then fixed by champing the tube with a mosquito hemostat (Jinjiang
Ltd.). A successful myocardial I/R model was confirmed by changes
of ST segment elevation in Lead-II and regional cyanosis of the
myocardial surface. The rats underwent a 30 min LAD occlusion
followed by 24 h of reperfusion.
Hemodynamic measurements
After being anesthetized with sodium pentobarbital
(45 mg/kg, i.p.), the right common carotid artery of the rat was
exposed and cannulated with a 2 F Millar Catheter (Millar
Instruments, Inc., Houston, TX, USA) into the left ventricle
through the ascending aorta. Heart function was monitored and the
related hemodynamic parameters, such as the left ventricular
ejection fraction (LVEF), heart rate (HR) and mean artery pressure
(MAP) in each group were recorded as described previously (10).
Assessment of myocardial injury
To measure the LDH and CK levels, blood samples were
collected, centrifuged at 1,358.37 × g for 15 min (Jinjiang Ltd.)
and maintained at −20°C until analysis. Standard techniques using
commercial kits according to the manufacturer’s instructions
(Nanjing Jiancheng Bioengineering Institute) were applied. Values
were expressed in international units per liter.
Assessment of the infarct size
After 24 h of reperfusion, the LAD was occluded
again and 2 ml of 1.5% Evans blue dye was injected via the femoral
vein. The risk area was analyzed by negative staining with Evans
blue. The rats were then sacrificed by heart excision and frozen
overnight immediately. The atria and right ventricle were removed
and the left ventricle was cut into transverse slices (2-mm thick)
from the apex to the base. The risk area was separated from the
colored non-ischemic area (blue) and then incubated with a 1%
solution of 2,3,5-triphenyltetrazoliumchloride (TTC, in 0.2 M
Tris-buffer, pH 7.4) stain for 20 min at 37°C, viable myocardium
was stained red by TTC, whereas necrotic myocardium did not stain
red. In each slice, the infarct size and the risk area (left
ventricular areas) were determined by a computer-assisted image
analysis system (Image-Pro Plus 3.0, Media Cybernetics, Silver
Spring, MD, USA) and multiplied by the thickness of the slice to
calculate volumes of risk area. The infarct size was expressed as a
percentage of the risk area volume (%, infarct size/risk area).
Measurement of MDA and SOD activity
MDA concentration and SOD activity in myocardial
tissues were measured using colorimetric myocardium malondialdehyde
(MDA) and superoxide dismutase (SOD) assay kits according to the
manufacturer’s instructions (Nanjing Jiancheng Bioengineering
Institute) as described previously (11). The concentrations were used as
indexes of oxygen free radical and lipid superoxide levels in the
myocardium, respectively.
Measurement of inflammatory cytokine
expression (TNF-α and IL-6) in myocardial tissues of rats
The titres of TNF-α and IL-6 in cardiac muscle
samples were measured using ELISA and the detailed manipulation
process was performed according to the manufacturer’s
recommendations (Wuhan Elabscience Biotechnology Co., Ltd.).
Western blot analysis for the expression
of HO-1 and HMGB1
The protein extracts were prepared from frozen and
pulverized samples of the ischemia area of the left ventricle as
previously reported (12,13). Western blot analysis was performed
according to the manufacturer’s instructions (Sigma-Aldrich).
Briefly, 50 μg of proteins were separated on 10% sodium dodecyl
sulfate-polyacrylamide gels and transferred to nitrocellulose
membranes (Nanjing Jiancheng Bioengineering Institute). Nonspecific
binding sites were blocked with 5% non-fat dry milk in
Tris-buffered saline with 0.05% Tween. The membrane was
subsequently probed with primary antibodies (anti-HO-1 antibody,
diluted 1:400; and anti-HMGB1 antibody, diluted 1:500,
respectively) and incubated in horseradish peroxidase-conjugated
secondary antibody (diluted 1:50,000). The protein bands were
visualized by an enhanced chemiluminescence system (Jinjiang Ltd.)
and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an
internal control to correct the variations of different samples.
The expression of HO-1 and HMGB1 were normalized to GAPDH
expression.
Presentation of data and statistical
analysis
All values were expressed as the mean ± standard
deviation. Student’s t-test was used for between-group comparisons.
One-way analysis of varaince or Welch was used for comparisons
among groups and the Student-Neuman-Keuls or Dunnett’s T3 tests
were used for post hoc multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of ISO on functional recovery of
a I/R heart
Cardiac function was assessed prior to LAD occlusion
and following drug administration (ISO or ZnPPIX). As shown in
Table I, no significant difference
in LVEF, HR and MAP was identified between the groups
(P>0.05).
 | Table ISummary of hemodynamic measurements
prior to myocardial I/R and following drug treatment. |
Table I
Summary of hemodynamic measurements
prior to myocardial I/R and following drug treatment.
| LVEF (%) | HR (bpm) | MAP (mmHg) |
|---|
| SO | 79.0±2.6 | 296±10 | 83±3 |
| I/R | 77.8±3.6 | 285±11 | 81±3 |
| ISO-I/R | 82.3±2.2 | 306±8 | 84±4 |
| ZnPPIX-ISO-I/R | 81.3±1.7 | 302±9 | 82±4 |
Subesquent to 24 h of reperfusion, compared with
that of the I/R group, the LVEF was significantly improved in the
ISO-treated group (P<0.05 versus the I/R group), but no
significant difference in HR and MAP was found between the I/R and
ISO-treated groups (P>0.05 versus the I/R group). However,
compared with that of the ISO-I/R group, the effects of ISO on the
LVEF was significantly reversed by the presence of ZnPPIX
(P<0.05 versus the ISO-I/R group; Table II).
 | Table IISummary of hemodynamic measurements
following 24 h of reperfusion. |
Table II
Summary of hemodynamic measurements
following 24 h of reperfusion.
| LVEF (%) | HR (bpm) | MAP (mmHg) |
|---|
| SO | 79.8±1.7 | 291±9 | 78±3 |
| I/R | 64.8±3.4a | 274±5 | 74±5 |
| ISO-I/R | 73.8±2.1b | 269±9 | 73±5 |
| ZnPPIX-ISO-I/R | 66.5±1.3c | 270±9 | 74±3 |
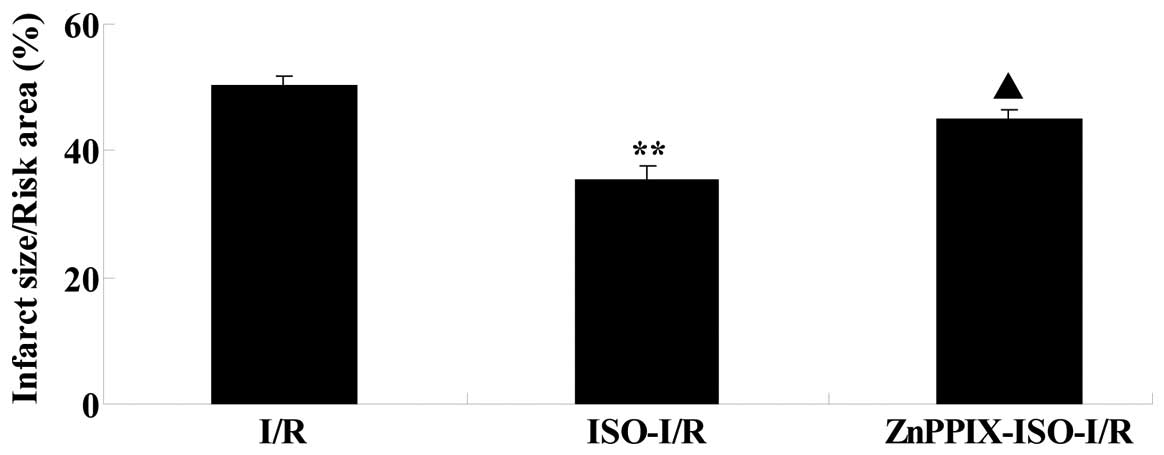
Effects of ISO on the infarct size in I/R
rats
Subsequent to 24 h of reperfusion, compared with
that of the I/R group, pretreatment with ISO significantly reduced
the infarct size (P<0.05 versus the I/R group). However, the
decreased infarct size induced by ISO was significantly reversed by
the presence of ZnPPIX (P<0.05 versus the ISO-I/R group;
Fig. 1).
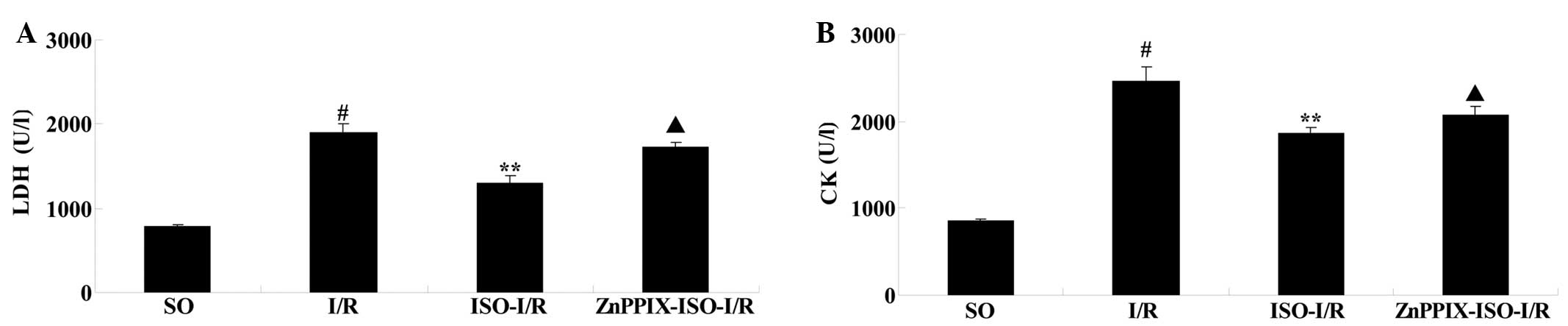
Effects of ISO on LDH and CK levels in
I/R rats
After 24 h of reperfusion, compared with that of the
SO group, the levels of LDH and CK in the I/R group were
significantly increased (P<0.05 versus the SO group). However,
compared with that of the I/R group, ISO significantly inhibited
the increase of LDH and CK levels (P<0.05 versus the I/R group).
Moreover, compared with that of the ISO-I/R group, the reduction of
LDH and CK release induced by ISO were significantly inhibited by
the presence of ZnPPIX (P<0.05 versus the ISO-I/R group;
Fig. 2).
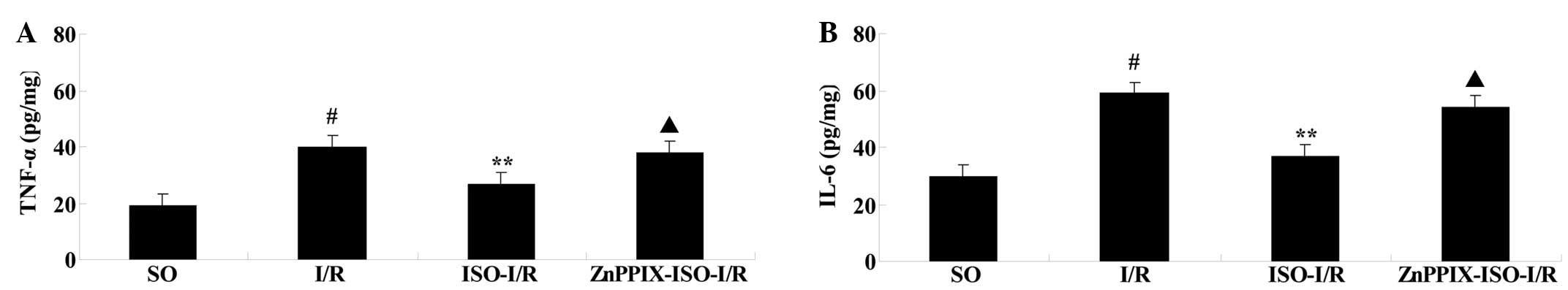
Effects of ISO on the production of TNF-α
and IL-6 in I/R rats
After 24 h of reperfusion, compared with that of the
SO group, TNF-α and IL-6 levels were significantly increased in the
I/R group (P<0.05 versus the SO group). However, compared with
that of the I/R group, ISO resulted in a statistically significant
decrease in the production of TNF-α and IL-6 (P<0.05 versus the
I/R group). Compared with that of the ISO-I/R group, the effects of
ISO on TNF-α and IL-6 production were inhibited by the presence of
ZnPPIX (P<0.05 versus the ISO-I/R group; Fig. 3).
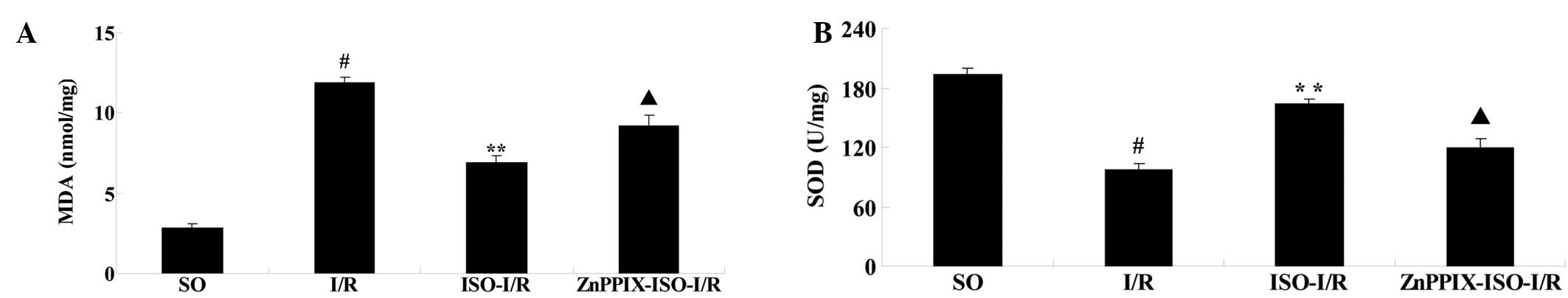
Effects of ISO on MDA and SOD activity
assay in I/R rats
After 24 h of reperfusion, compared with that of the
SO group, the levels of MDA significantly increased and the levels
of SOD significantly decreased in the I/R group (P<0.05 versus
the SO group). However, compared with that of the I/R group, ISO
significantly inhibited the increase of MDA and the decrease of SOD
(P<0.05 versus the I/R group). Conversely, compared with that of
the ISO-I/R group, the effects of ISO were significantly reversed
by the presence of ZnPPIX (P<0.05 versus the ISO-I/R group;
Fig. 4).
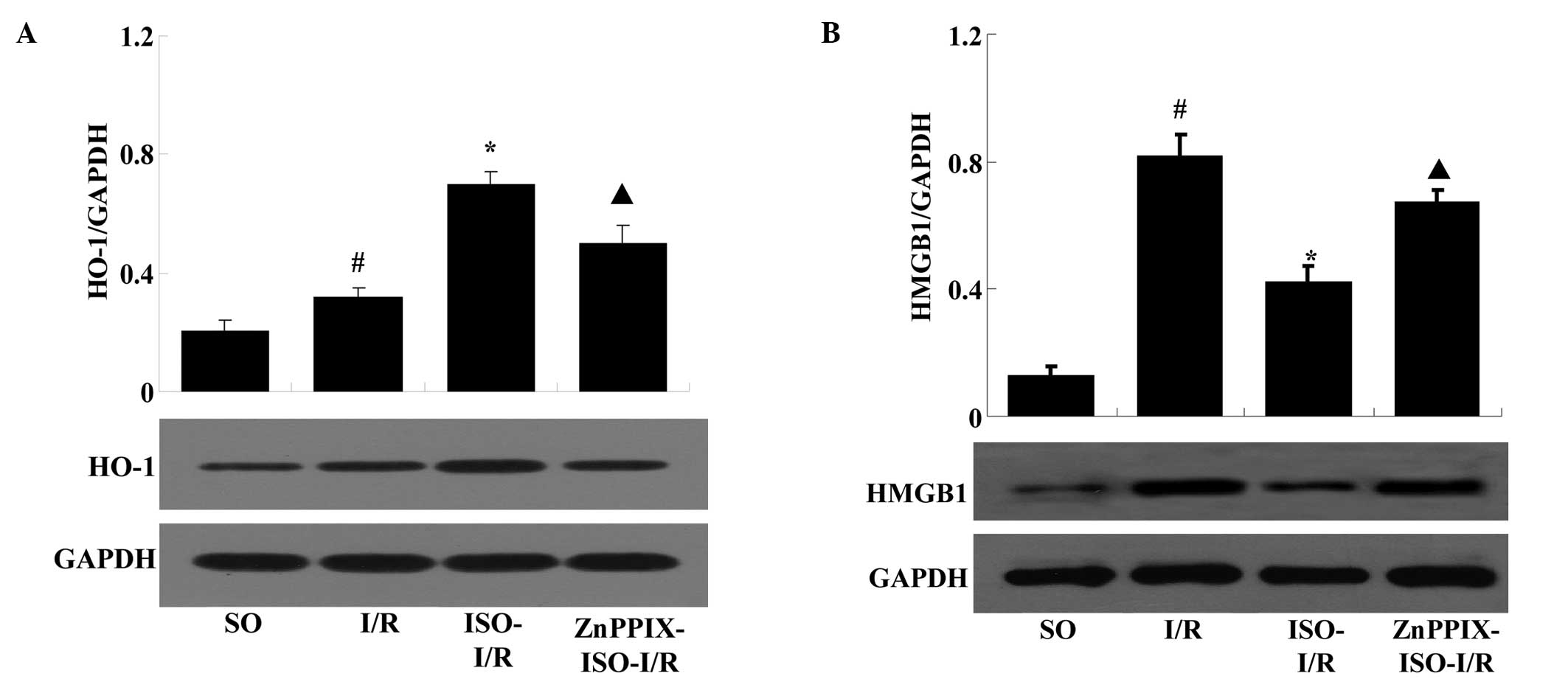
Effects of ISO on HO-1 and HMGB1
expression levels in I/R rats
Following 24 h of reperfusion, compared with that of
the SO group, HMGB1 and HO-1 expression levels were markedly
increased in the I/R group (P<0.05 versus the SO group).
However, compared with that of the I/R group, ISO significantly
mediated HO-1 induction and HMGB1 inhibition (P<0.05 versus the
I/R group). However, the effects of ISO were significantly reversed
by the presence of ZnPPIX (P<0.05 versus the ISO-I/R group)
(Fig. 5).
Discussion
The present study demonstrated that ISO
significantly attenuated myocardial I/R injury and oxidative
stress. Furthermore, the induction of HO-1 and inhibition of HMGB1
were shown to be involved in the effects of ISO on attenuating
myocardial I/R injury in rats.
HMGB1, once released from necrotic cells and
macrophages, may significantly upregulate IL-1, IL-6, TNF-α,
C-reactive protein and macrophage inflammatory proteins (MIP-1α and
MIP-1β) (14–16). Furthermore, it has been reported to
act as a novel proinflammatory mediator that contributes to the
progression of myocardial I/R injury (3). Moreover, in addition to its
anti-inflammatory and anti-apoptotic effects and its ability to
decrease proliferation, HO-1 is induced and responds to a variety
of stimuli and injury, such as oxidative stress and myocardial I/R
injury in several organs and cell types (5,6).
Furthermore, Takamiya et al (17) demonstrated that the expression
levels of HMGB1 were higher in HO-1−/−mice than that of HO-1+/+
mice. Additionally, the induction of HO-1 has been shown to prevent
the release of HMGB1 in endotoxin-activated macrophages in
vitro and septic animals in vivo (18), which is further supported in the
rat myocardial I/R injury model in the present study.
Furthermore, there are a number of reports on
β-AR-mediated modulation of inflammatory effects (TNF-α and IL-6)
(19–21). ISO, as a β-AR agonist, has been
reported to increase cluster of differentiation (CD)14 expression
and live E. coli phagocytosis in macrophages, which may
result from the increase in cAMP/PKA signaling (22,23).
Macrophages have been shown to exhibit accelerated LPS
internalization and detoxification through increased surface CD14
expression or synthesis and release of higher levels of soluble
CD14 at inflammatory foci, thus limiting the biological toxicity of
LPS (24). These studies suggest
the potential impact of ISO on innate immune functions.
Furthermore, as ISO has been reported to mediate HO-1 induction in
RAW 264.7 cells, which results in the inhibition of HMGB1 release
in LPS-activated RAW 264.7 cells and increase in the survival rate
of CLP-induced septic mice (9),
ISO-mediated HO-1 induction during rat myocardial I/R injury in
vivo was investigated. In the present study, it was
demonstrated that ISO significantly attenuated myocardial I/R
injury and inhibited HMGB1 release. These effects were
significantly reversed by ZnPPIX, an inhibitor of HO-1, which
further confirmed that HO-1 activity is important for ISO to
significantly inhibit HMGB1 release and attenuate myocardial I/R
injury in rats.
Notably, in the present study, it was also observed
that pretreatment with ISO significantly decreased the levels of
MDA, a reactive oxygen species (ROS), and increased the levels of
SOD, a key antioxidant enzyme. Hydrogen peroxide has been
demonstrated to stimulate cardiac myocytes to cause the release of
HMGB1, which suggested that ROS may be involved in the release of
HMGB1 (25,26). In addition, HO-1 has been reported
to occur in the lung in response to oxidative stress associated
with infection, altered oxygen tension and inflammatory diseases.
HO-1 remains widely regarded as protective against oxidative tissue
injury and as a beneficial molecule in protecting against the
oxidative stress induced by numerous stimuli (27,28).
Therefore, ISO reduced oxidative stress during myocardial I/R
injury in rats in the present study, which may also be associated
with the actions of HO-1 induction and inhibition of HMGB1
release.
The present study demonstrated that ISO attenuated
myocardial I/R injury in rats, which may be due to its inhibitory
effects on HMGB1 release via the induction of HO-1 in cardiac
myocytes.
There are a number of limitations to the present
study. This study only demonstrated that ISO mediated the induction
of HO-1, inhibited HMGB1 release and attenuated rat myocardial I/R
injury in vivo. However, the associated signaling pathways,
such as PI3K/p38 MAPK and other factors, such as nuclear factor
erythroid 2-related factor 2 translocation, may have contributed to
these cardioprotective effects in cardiac myocytes and should be
considered in a future study (29–32).
Additionally, the present study only observed that ISO reduced
oxidative stress during myocardial I/R injury in rats; the precise
mechanisms require further elucidation.
In conclusion, the present study demonstrated that
ISO mediated the inhibition of HMGB1 release via the induction of
HO-1 in cardiac myocytes and attenuated rat myocardial I/R injury
in vivo, which may provide an important therapeutic approach
for protection against myocardial ischemia and reperfusion
injury.
Acknowledgements
This study was partially supported by grants from
the National Natural Science foundation of China (grant nos.
81100146 and 81370308), the Fundamental Research Funds for the
Central Universities (grant no. 111023), the Specialized Research
Fund for the Doctoral Program of Higher Education of China (grant
no. 20110141120060) and the Fundamental Research Funds of Wuhan
City (No. 2013070104010044).
References
|
1
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vakeva AP, Agah A, Rollins SA, Matis LA,
Li L and Stahl GL: Myocardial infarction and apoptosis after
myocardial ischemia and reperfusion: role of the terminal
complement components and inhibition by anti-C5 therapy.
Circulation. 97:2259–2267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andrassy M, Volz HC, Igwe JC, et al:
High-mobility group box-1 in ischemia-reperfusion injury of the
heart. Circulation. 117:3216–3226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu X, Fu W and Jiang H: HMGB1: a potential
therapeutic target for myocardial ischemia and reperfusion injury.
Int J Cardiol. 155:4892012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Otterbein LE, Soares MP, Yamashita K and
Bach FH: Heme oxygenase-1: unleashing the protective properties of
heme. Trends Immunol. 24:449–455. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hwa JS, Jin YC, Lee YS, et al:
2-methoxycinnamaldehyde from Cinnamomum cassia reduces rat
myocardial ischemia and reperfusion injury in vivo due to HO-1
induction. J Ethnopharmacol. 139:605–615. 2012.
|
|
8
|
Liu SX, Zhang Y, Wang YF, et al:
Upregulation of heme oxygenase-1 expression by hydroxysafflor
yellow A conferring protection from anoxia/reoxygenation-induced
apoptosis in H9c2 cardiomyocytes. Int J Cardiol. 160:95–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ha YM, Ham SA, Kim YM, et al:
β1-adrenergic receptor-mediated HO-1 induction, via PI3K
and p38 MAPK, by isoproterenol in RAW 264.7 cells leads to
inhibition of HMGB1 release in LPS-activated RAW 264.7 cells and
increases in survival rate of CLP-induced septic mice. Biochem
Pharmacol. 82:769–777. 2011.
|
|
10
|
Jin YC, Kim CW, Kim YM, et al:
Cryptotanshinone, a lipophilic compound of Salvia
miltiorrriza root, inhibits TNF-α-induced expression of
adhesion molecules in HUVEC and attenuates rat myocardial
ischemia/reperfusion injury in vivo. Eur J Pharmacol. 614:91–97.
2009.PubMed/NCBI
|
|
11
|
Wang J, Yang H, Hu X, et al:
Dobutamine-mediated heme oxygenase-1 induction via PI3K and p38
MAPK inhibits high mobility group box 1 protein release and
attenuates rat myocardial ischemia/reperfusion injury in vivo. J
Surg Res. 183:509–516. 2013. View Article : Google Scholar
|
|
12
|
Yan L, Tang Q, Shen D, et al: SOCS-1
inhibits TNF-α-induced cardiomyocyte apoptosis via ERK1/2 pathway
activation. Inflammation. 31:180–188. 2008.
|
|
13
|
Yang J, Jiang H, Yang J, Ding JW, Chen LH,
Li S and Zhang XD: Valsartan preconditioning protects against
myocardial ischemia-reperfusion injury through TLR4/NF-κB signaling
pathway. Mol Cell Biochem. 330:39–46. 2009.PubMed/NCBI
|
|
14
|
Inoue K, Kawahara K, Biswas KK, et al:
HMGB1 expression by activated vascular smooth muscle cells in
advanced human atherosclerosis plaques. Cardiovasc Pathol.
16:136–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arroyo-Espliguero R, Avanzas P, Quiles J
and Kaski JC: Predictive value of coronary artery stenoses and
C-reactive protein levels in patients with stable coronary artery
disease. Atherosclerosis. 204:239–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andersson U, Wang H, Palmblad K, et al:
High mobility group 1 protein (HMG-1) stimulates proinflammatory
cytokine synthesis in human monocytes. J Exp Med. 192:565–570.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takamiya R, Hung CC, Hall SR, et al:
High-mobility group box 1 contributes to lethality of endotoxemia
in heme oxygenase-1-deficient mice. Am J Respir Cell Mol Biol.
41:129–135. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsoyi K, Lee TY, Lee YS, et al:
Heme-oxygenase-1 induction and carbon monoxide-releasing molecule
inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1
release in vitro and improve survival of mice in LPS- and cecal
ligation and puncture-induced sepsis model in vivo. Mol Pharmacol.
76:173–182. 2009. View Article : Google Scholar
|
|
19
|
Guirao X, Kumar A, Katz J, et al:
Catecholamines increase monocyte TNF receptors and inhibit TNF
through β2-adrenoreceptor activation. Am J Physiol.
273:E1203–E1208. 1997.PubMed/NCBI
|
|
20
|
Kappel M, Poulsen TD, Galbo H and Pedersen
BK: Effects of elevated plasma noradrenaline concentration on the
immune system in humans. Eur J Appl Physiol Occup Physiol.
79:93–98. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sanders VM, Baker RA, Ramer-Quinn DS,
Kasprowicz DJ, Fuchs BA and Street NE: Differential expression of
the beta2-adrenergic receptor by Th1 and Th2 clones: implications
for cytokine production and B cell help. J Immunol. 158:4200–4210.
1997.PubMed/NCBI
|
|
22
|
Muthu K, He LK, Szilagyi A, Strotmon P,
Gamelli RL and Shankar R: β-adrenergic stimulation increases
macrophage CD14 expression and E. coli phagocytosis through
PKA signaling mechanisms. J Leukoc Biol. 88:715–724. 2010.
|
|
23
|
Liu S, Morris SM Jr, Nie S, Shapiro RA and
Billiar TR: cAMP induces CD14 expression in murine macrophages via
increased transcription. J Leukoc Biol. 67:894–901. 2000.PubMed/NCBI
|
|
24
|
Gegner JA, Ulevitch RJ and Tobias PS:
Lipopolysaccharide (LPS) signal transduction and clearance. Dual
roles for LPS binding protein and membrane CD14. J Biol Chem.
270:5320–5325. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsung A, Klune JR, Zhang X, et al: HMGB1
release induced by liver ischemia involves Toll-like receptor 4
dependent reactive oxygen species production and calcium-mediated
signaling. J Exp Med. 204:2913–2923. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu Y, Liu M, Zhang L, et al: Heat shock
transcription factor 1 inhibits H2O2-induced
cardiomyocyte death through suppression of high-mobility group box
1. Mol Cell Biochem. 364:263–269. 2012.PubMed/NCBI
|
|
27
|
Iles KE, Dickinson DA, Wigley AF, Welty
NE, Blank V and Forman HJ: HNE increases HO-1 through activation of
the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med.
39:355–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ryter SW and Choi AM: Heme oxygenase-1:
redox regulation of a stress protein in lung and cell culture
models. Antioxid Redox Signal. 7:80–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eom HW, Park SY, Kim YH, et al: Bambusae
Caulis in Taeniam modulates neuroprotective and
anti-neuroinflammatory effects in hippocampal and microglial cells
via HO-1- and Nrf-2-mediated pathways. Int J Mol Med. 30:1512–1520.
2012.PubMed/NCBI
|
|
30
|
Jin GH, Park SY, Kim E, et al:
Anti-inflammatory activity of Bambusae Caulis in Taeniam through
heme oxygenase-1 expression via Nrf-2 and p38 MAPK signaling in
macrophages. Environ Toxicol Pharmacol. 34:315–323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Senthil Kumar KJ, Liao JW, Xiao JH, Gokila
Vani M and Wang SY: Hepatoprotective effect of lucidone against
alcohol-induced oxidative stress in human hepatic HepG2 cells
through the up-regulation of HO-1/Nrf-2 antioxidant genes. Toxicol
In Vitro. 26:700–708. 2012.PubMed/NCBI
|
|
32
|
Ryu EY, Park AJ, Park SY, et al:
Inhibitory effects of Ginkgo biloba extract on inflammatory
mediator production by Porphyromonas gingivalis
lipopolysaccharide in murine macrophages via Nrf-2 mediated heme
oxygenase-1 signaling pathways. Inflammation. 35:1477–1486. 2012.
View Article : Google Scholar : PubMed/NCBI
|