Introduction
Tissue injury caused by ischemia or hypoxia is a
major cause of fatal diseases, including coronary atherosclerosis
caused by myocardial infarction and stroke (1,2). The
main causes of myocardial ischemia injury include myocardial cell
apoptosis, necrosis or temporarily impaired function, which are
induced by myocardial hypoxia or/and lack of nutrients (3). In the treatment of ischemia, however,
the restoration of blood supply may damage tissue, which is known
as ischemia-reperfusion (I/R) injury.
The characteristics of I/R injury include apoptosis
and necrosis of myocardial cells, the dysfunction of mitochondria,
increase of lipid peroxides and the generation of free radicals
(4,5). For instance, free oxygen radicals,
generated from the reaction between the oxygen carried by
oxyhemoglobin in the blood and substances dissolved by impaired or
necrotic myocardial cells caused by I/R, cause myocardial injury
(6). Additionally, I/R injury also
results in the inhibition of myocardial function, including the
occurrence of malignant arrhythmia, the decrease of the left
ventricular contractility and the decline of intraventricular
pressure (7). As a result,
developing an effective strategy for preventing and/or treating I/R
injury is urgently required.
microRNAs (miRNAs) are a group of endogenous,
non-coding, single-strand, small RNAs of 22–25 nucleotides, which
downregulate the expression of multiple target genes via
degradation or translational inhibition of their mRNAs (8). According to statistics, miRNAs
directly regulate >30% of genes, which are associated with
almost all major cellular functions, including cell growth,
proliferation, differentiation, migration and apoptosis (9). It has been reported that several
miRNAs have a crucial role in the protection against myocardial I/R
injury (10). miR-21 has been
found to be consistently upregulated in cardiac hypertrophy and to
be relevant in the inhibition of cellular apoptosis (11). In fact, several targets of miR-21
have been demonstrated to be involved in the regulation of
myocardial I/R injury, including phosphatase and tensin homolog
(PTEN), programmed cell death 4 (PDCD4) and sprouty 1 and 2
(12).
PTEN is a negative regulator of Akt, which has a
crucial role in cellular survival (13). B-cell lymphoma 2 (Bcl-2) and
Bcl-2-associated X protein (Bax), the representatives of the Bcl-2
family, are considered to be the primary regulators of apoptosis.
Caspase-3 is a downstream regulator of the Bcl-2 family and acts as
a key effector of cellular apoptosis (14,15).
However, the anti-apoptotic mechanism of miR-21 in myocardial I/R
injury has yet to be fully elucidated.
In the present study, the anti-apoptotic role of
miR-21 in a rat model of myocardial I/R injury and in H9C2 cells
with injury induced by hypoxia reoxygenation (H/R), was assessed by
determining the expression of Bcl-2/Bax, caspase-3, PTEN and
p-AKT.
Materials and methods
Reagents and materials
Dulbecco’s modified Eagle medium (DMEM) was
purchased from Gibco Laboratories (Grand Island, NY, USA).
OPTI-minimal essential medium (MEM®), fetal bovine serum
(FBS), TRIzol, TaqMan quantitative reverse transcription polymerase
chain reaction (qRT-PCR) miRNA assay kit, RT-PCR kit, Lipofectamine
2000, miR-21 mimics and miR-21 inhibitor were purchased from Thermo
Fisher Scientific (Waltham, MA, USA). SYBR® Green qRCR
mix was purchased from Toyobo Co., Ltd. (Osaka, Japan). All the
antibodies were purchased from Abcam (Cambridge, UK).
Rat model of I/R injury
All the protocols in the present study’s experiment
were approved by the Animal Ethics Committee of Central South
University (Changsha, China). All Sprague-Dawley female rats (age,
10 weeks; weight, 250–300 g) were purchased from the Animal Center
of Central South University. These rats were divided into four
groups, including sham (served as controls), I/R 2, 4 and 6 h.
Under sterile conditions, intraperitoneal injection of 10% chloral
hydrate (350 mg/kg) was performed. Following endotracheal
intubation, a ventilator was used to support their lives. The heart
was then exposed and the aorta was clamped with a non-invasive
vascular clamp for 10 sec. Subsequent to that, the aorta was
reperfused for 2, 4 and 6 h, respectively. In the sham group, the
heart was exposed without clamping the aorta.
Adenovirus-mediated miR-21 gene transfer
in vivo
To further investigate the role of miR-21 in
myocardial I/R injury, the rAAV9-ZsGreen-pre-miR-21 adenovirus was
constructed using the rAAV9-ZsGreen expression system (Clontech
Laboratories, Mountain View, CA, USA) according to the
manufacturer’s instructions. The rAAV9-ZsGreen adenovirus was used
as a negative control. The titer was 5.0×1012 vg/ml. In
total, 30 rats were divided into five groups. In the control group,
rats were injected with rAAV9-ZsGreen adenovirus through the
coronary artery. In the miR-21 group, rats were injected with
rAAV9-ZsGreen-pre-miR-21 adenovirus through coronary artery. In the
sham group, rats were injected with rAAV9-ZsGreen adenovirus
through the coronary artery, and 14 days following that, the
sham-surgery was performed as described above. In the I/R group,
rats were injected with rAAV9-ZsGreen adenovirus through the
coronary artery, and 14 days after that, the I/R was performed as
described above. In the I/R+miR-21 group, rats were injected with
rAAV9-ZsGreen-pre-miR-21 adenovirus through the coronary artery,
and 14 days after that, I/R was performed. At 2 h after I/R, the
animals were sacrificed.
Cell culture
The human H9C2 cell line was purchased from The
Institute of Cell Biology at the Chinese Academy of Sciences
(Shanghai, China). H9C2 cells were cultured in DMEM containing 10%
FBS and incubated at 37°C in a humidified incubator with 5%
CO2.
Apoptosis analysis
Flow cytometry was used to determine the cell
apoptosis with the Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit (Abcam). At 24 h post-transfection, the
cells were harvested and washed with cold phosphate-buffered saline
(PBS) twice. Following that, 106 cells were resuspended
in 200 μl binding buffer, 10 μl Annexin V-FITC and 5 μl propidium
iodide phycoerythrin were added, and cells were incubated in the
dark for 30 min. Next, 300 μl binding buffer was added followed by
flow cytometric analysis.
H/R treatment of H9C2 cells
H9C2 cells were cultured in DMEM with neither serum
nor antibiotics at 37°C with 5% CO2 for 12 h, which were
then cultured at 37°C with 1% O2-94% N2-5%
CO2 for 4 h. Subsequent to that, the cells were cultured
in DMEM containing 10% FBS, incubated at 37°C with 5%
CO2 for 3 h and used in the following experiments.
In the in vitro experiment, H9C2 cells were
divided into the following groups: In the vehicle control (VC)
group, the H9C2 cells were without any treatment. In the inhibitor
negative control (INC) group, the cells were transfected with 50 nM
miR-21 inhibitor. In the mimics NC (MNC) group, the cells were
transfected with 50 nM NC mimics. In the VC+H/R group, the cells
were treated with H/R. In the INC+H/R group, the cells transfected
with 50 nM NC inhibitor were then treated with H/R. In the MNC+H/R
group, the cells transfected with 50 nM NC mimics were then treated
with H/R. In the miR-21 inhibitors+H/R group, the cells transfected
with 50 nM miR-21 inhibitor were then treated with H/R. In the
miR-21 mimics+H/R group, cells transfected with 50 nM miR-21 mimics
were then treated with H/R.
qPCR analysis
Total RNA was extracted with TRIzol according to the
manufacturer’s instructions. For miR-21 expression analysis, 2 μg
RNA was transcribed to cDNA using a stem-loop RT primer (Invitrogen
Life Technologies, Carlsbad, CA, USA) and a miRNA reverse
transcription kit (Applied Biosystems, Foster City, CA, USA) was
used. The U6 gene was used as a normalization control. The amount
of miR-21 to U6 was calculated using the equation 2−ΔCT,
with ΔCT = CT, miR-21 - CT,
U6.
For the detection of PTEN mRNA expression, qPCR
analysis was performed using SYBR Green qPCR Mix and specific
primers synthesized from Sangon Company (Shanghai, China). The
following primers were used for amplification of PTEN: sense,
5′-GACGACAATCATGTTGCAGCA-3′ and antisense,
5′-GCCTTTAAAAACTTGCCCCG-3′. GAPDH was used as an internal control
with sense, 5′-ACAACTTTGGTATCGTGGAAGG-3′ and antisense,
5′-GCCATCACGCCACAGTTTC-3′. The relative expression levels of genes
were analyzed by the 2−ΔΔCT method.
Western blot analysis
Tissue samples were snap-frozen using liquid
nitrogen in a mortar and vigorously ground. Cell samples were
rinsed twice with cold PBS. Next, cold radioimmunoprecipitation
assay buffer was used to lyse the protein from the tissue or cell
samples. The concentration of protein was determined using a
bicinchoninic acid assay kit. Following that, proteins of 20
μg/lane were loaded on a 10% SDS-PAGE to be separated, and then
electrophoretically transferred to polyvinylidene fluoride
membranes. Proteins on the membranes were then probed using primary
antibodies, including mouse anti-Bcl-2, Bax, caspase-3, PTEN, p-Akt
and β-actin, according to the manufacturer’s instructions.
Following incubation with secondary antibodies, including rabbit
anti-mouse secondary antibody, the results were visualized with
horseradish peroxidase and an enhanced chemiluminescence system,
and quantified by the Quantity One software (Bio-Rad, Hercules, CA,
USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation of three independent experiments. A statistical analysis
was performed by the SPSS 19.0 software (SPSS, Inc., Chicago, IL,
USA). One-way analysis of variance and Student’s t-test were used
to analyze all the data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-21 in the myocardial
tissue of the rat model of I/R injury
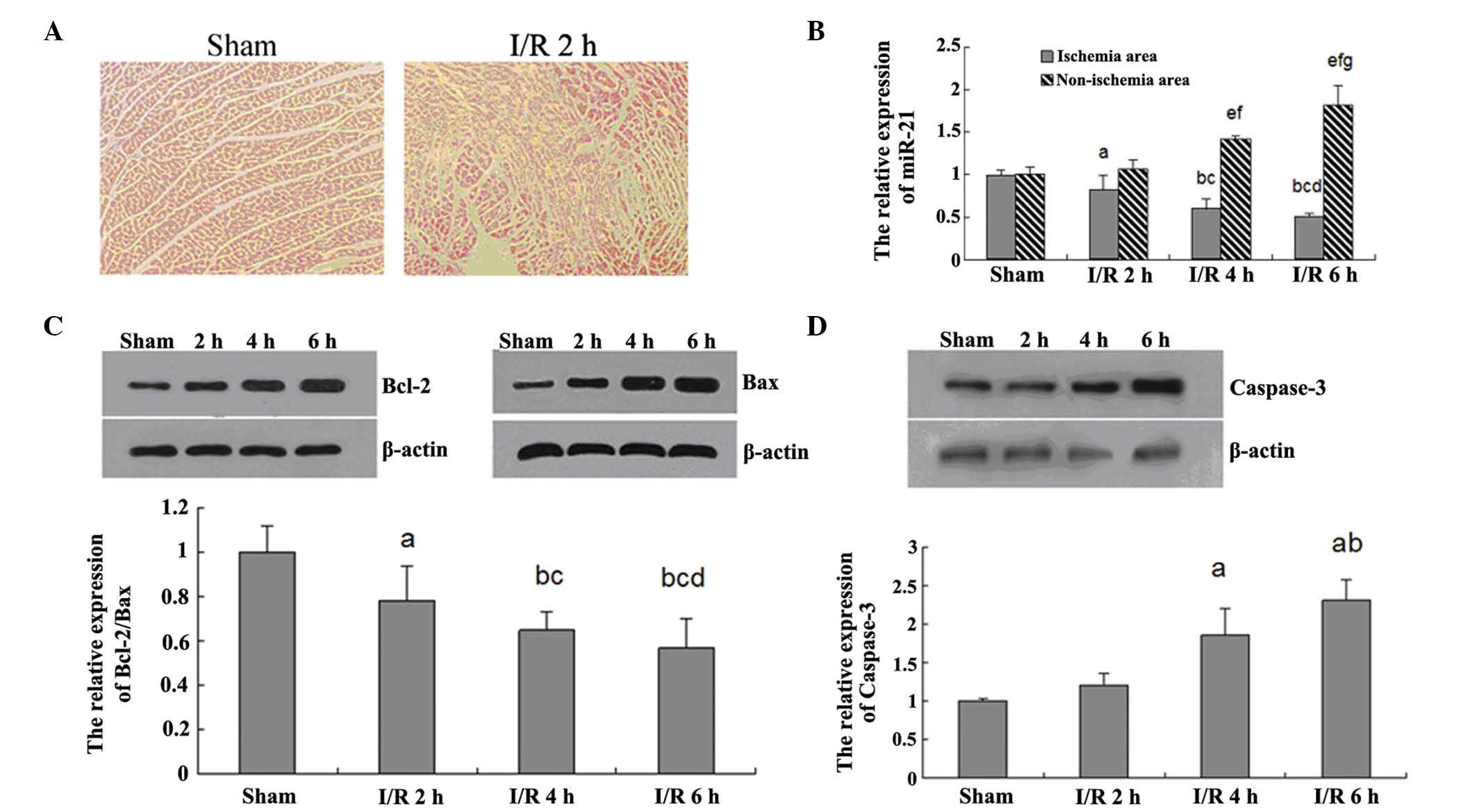
As shown in Fig.
1A, the myocardial tissue in the sham group demonstrated normal
morphology, and its structure was clear without any edema or
inflammatory cell infiltration. However, in the I/R 2 h group, the
myocardial tissue exhibited structural disorder, irregular nuclei
and edema. This result indicates that the rat model of I/R injury
was successfully established.
miR-21 has been implicated to be involved in the
myocardial I/R injury. Thus, qPCR was further applied in order to
determine the expression levels of miR-21 in the ischemic and
non-ischemic area of the myocardial tissue in each group. As
demonstrated in Fig. 1B, in the
ischemic area of the myocardial tissue, the expression levels of
miR-21 exhibited a decreasing tendency with the extension of I/R
time. However, in the non-ischemic area of the myocardial tissue,
the miR-21 expression was gradually upregulated with the extension
of I/R time. These data indicate that miR-21 may have an inhibitory
role in the myocardial tissue injured by I/R.
Apoptotic pathways in response to I/R injury. In
order to gain a better understanding of the molecular mechanism
underlying myocardial I/R injury, the present study focused on
cellular apoptosis. The Bcl-2 family has a crucial role in the
regulation of apoptosis. Thus, western blot analysis was applied to
examine the protein expression levels of Bcl-2 and Bax, two key
members in the Bcl-2 family. As shown in Fig. 1C, I/R injury increased the Bcl-2
and Bax protein expression levels in a time-dependent manner.
However, the relative ratio of Bcl-2 to Bax was gradually
decreased, indicating that apoptotic signaling was activated.
Additionally, the expression levels of caspase-3, a downstream
effector of Bcl-2, were further determined in each group. As shown
in Fig. 1D, I/R increased the
expression levels of caspase-3 in a time-dependent manner, further
indicating that, with increasing I/R time, apoptosis was gradually
upregulated.
The role of miR-21 in the early phase of
myocardial I/R injury in rats
To further investigate the role of miR-21 in
myocardial I/R injury, the rAAV9-ZsGreen-pre-miR-21 or
rAAV9-ZsGreen adenovirus was injected into the coronary artery of
the rats in each group, respectively. The expression levels of
miR-21 were initially determined, and it was found that in the
miR-21 group, the expression of miR-21 in the myocardial region was
significantly upregulated as compared with that in the control
group (Fig. 2A), indicating that
the rAAV9-ZsGreen-pre-miR-21 adenovirus was able to effectively
express pre-miR-21 in vivo.
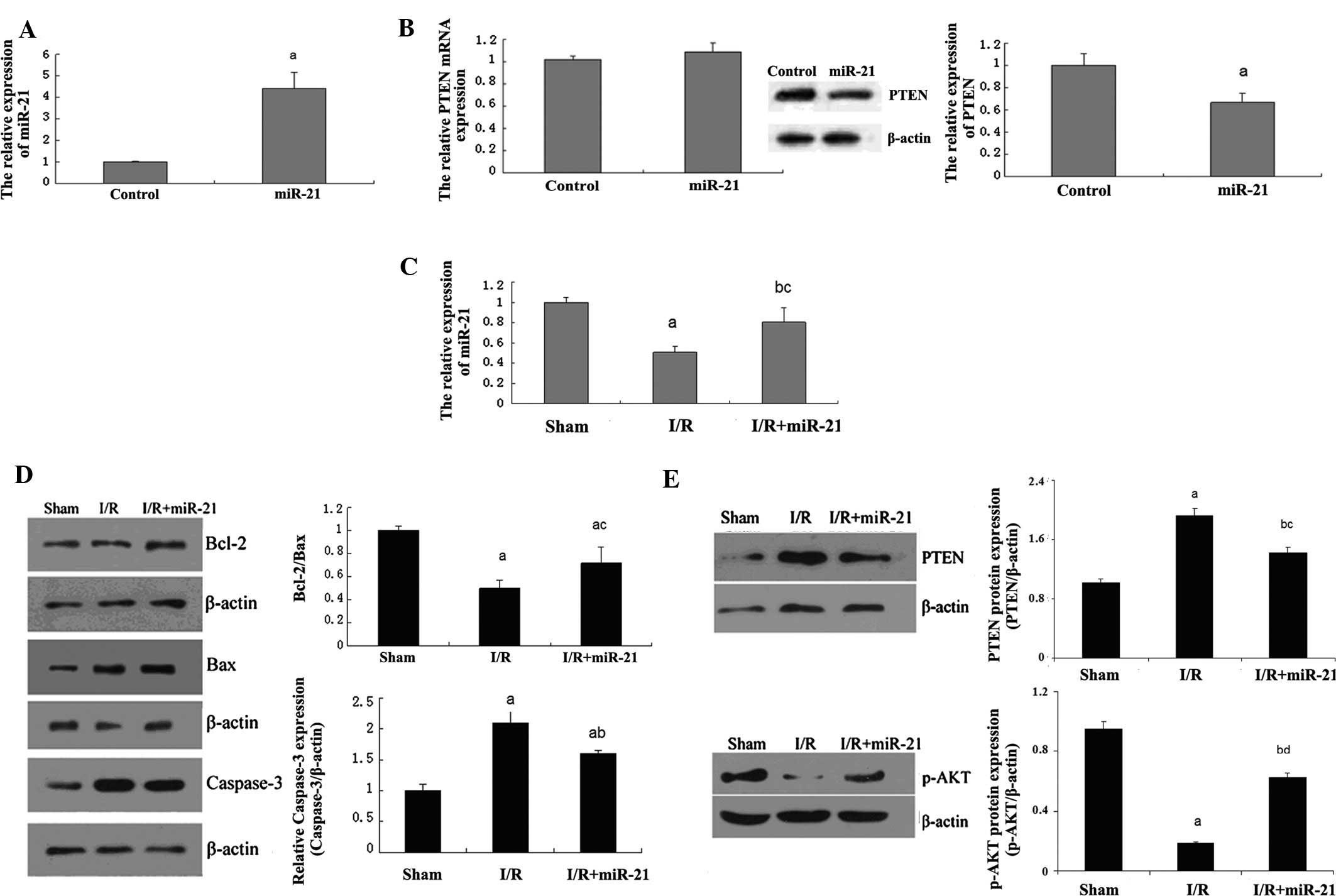 | Figure 2Role of miR-21 in the early phase of
myocardial I/R injury in rats. (A) The rAAV9-ZsGreen-pre-miR-21
(miR-21 group) or rAAV9-ZsGreen adenovirus (control group) was
injected into the coronary artery of the rats, respectively. qPCR
assay determined the relative expression of miR-21 following
transfection. (aP<0.05 vs. control group). (B)
Following injection mentioned above, the mRNA and protein
expression of PTEN was examined by qPCR and western blot analysis,
respectively. (aP<0.05 vs. control group). (C) The
expression of miR-21 was determined in each group. In the sham
group, rats were injected with rAAV9-ZsGreen adenovirus through the
coronary artery, and 14 days after that, the sham-operation was
performed as described above. In the I/R group, rats were injected
with rAAV9-ZsGreen adenovirus through the coronary artery, and 14
days after that, the I/R was performed as described above. In the
I/R+miR-21 group, rats were injected with rAAV9-ZsGreen-pre-miR-21
adenovirus through the coronary artery, and 14 days after that, I/R
was performed. (aP<0.01 and bP<0.05 vs.
sham group; cP<0.05 vs. I/R group). (D) Western blot
analysis was then performed to determine the protein expression of
Bcl-2, Bax and caspase-3 in each group. β-actin was used as an
internal reference. The relative expression of Bcl2/Bax was
calculated. (aP<0.01 vs. sham group;
bP<0.01 and cP<0.05 vs. I/R group). (E)
Western blot analysis was then performed to determine the protein
expression of PTEN and p-Akt in each group. β-actin was used as an
internal reference. (aP<0.01 and
bP<0.05 vs. sham group; cP<0.05 and
dP<0.01 vs. I/R group). miR, microRNA; I/R,
ischemia-reperfusion; PTEN, phosphatase and tensin homolog; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2-associated X protein; qPRC,
quantitative polymerase chain reaction. |
Since PTEN has been demonstrated to be a target of
miR-21 and to have a crucial role in the regulation of cellular
biological processes, the mRNA and protein expression levels of
PTEN were determined next. As shown in Fig. 2B, the mRNA expression of PTEN
demonstrated no difference between the control group and miR-21
group; however, the protein expression levels in the miR-21 group
were significantly downregulated as compared with those in the
control group, indicating that miR-21 has a post-transcriptional
inhibitory effect on PTEN expression. Additionally, in the I/R
group, the expression of miR-21 was notably decreased as compared
with that in the sham group, while following injection with
rAAV9-ZsGreen-pre-miR-21 adenovirus, the expression of miR-21 was
restored (Fig. 2C).
Based on these data, expression levels of certain
significant factors associated with apoptosis, including Bcl-2, Bax
and caspase-3, were determined further. As shown in Fig. 2D, the protein expression levels of
Bcl-2 and Bax were increased in the I/R group compared with the
sham group; however, the Bcl-2/Bax ratio was decreased, which were
reverted in the I/R+miR-21 group. Additionally, the protein levels
of caspase-3 were also upregulated in the I/R group as compared
with those in the sham group, which were reverted in the I/R+miR-21
group. These results indicate that miR-21 has an anti-apoptotic
role in I/R-induced myocardial injury.
PTEN has an inhibitory role in the regulation of the
Akt signaling pathway, which acts as a key regulator in cellular
survival. Thus, to further investigate the involved regulatory
mechanism, the expression of PTEN and the phosphorylation levels of
Akt were determined, which directly reflect the activity of the Akt
signaling pathway. As demonstrated in Fig. 2E, the expression of PTEN was
significantly increased in the I/R group compared with the control
group, which was, to a certain degree, reverted in the I/R+miR-21
group. Furthermore, as expected, the phosphorylation levels of Akt
were evidently decreased in the I/R group compared with the control
group, which could be restored in the I/R+miR-21 group. These
findings indicate that miR-21 protects against I/R-induced
myocardial cell apoptosis, most likely by inhibiting PTEN and
therefore upregulating the activity of the Akt signaling pathway,
which further suppresses pro-apoptotic factors such as caspase-3,
while increasing anti-apoptotic factors, including Bcl-2/Bax.
The role of miR-21 in H/R-induced
apoptosis of H9C2 cells
The cardiac myoblast cell line H9C2 was used to
investigate the role of miR-21 in H/R-induced cellular apoptosis.
The expression of miR-21 in each group was initially determined,
and H/R treatment and an miR-21 inhibitor were found to be capable
of significantly downregulating the expression of miR-21, which was
restored by miR-21 mimics, as expected (Fig. 3A). Subsequent to that, the
apoptotic levels in each group were examined. As shown in Fig. 3B, induction of H/R and presence of
the miR-21 inhibitor significantly enhanced cellular apoptosis,
which was restored by miR-21 mimics.
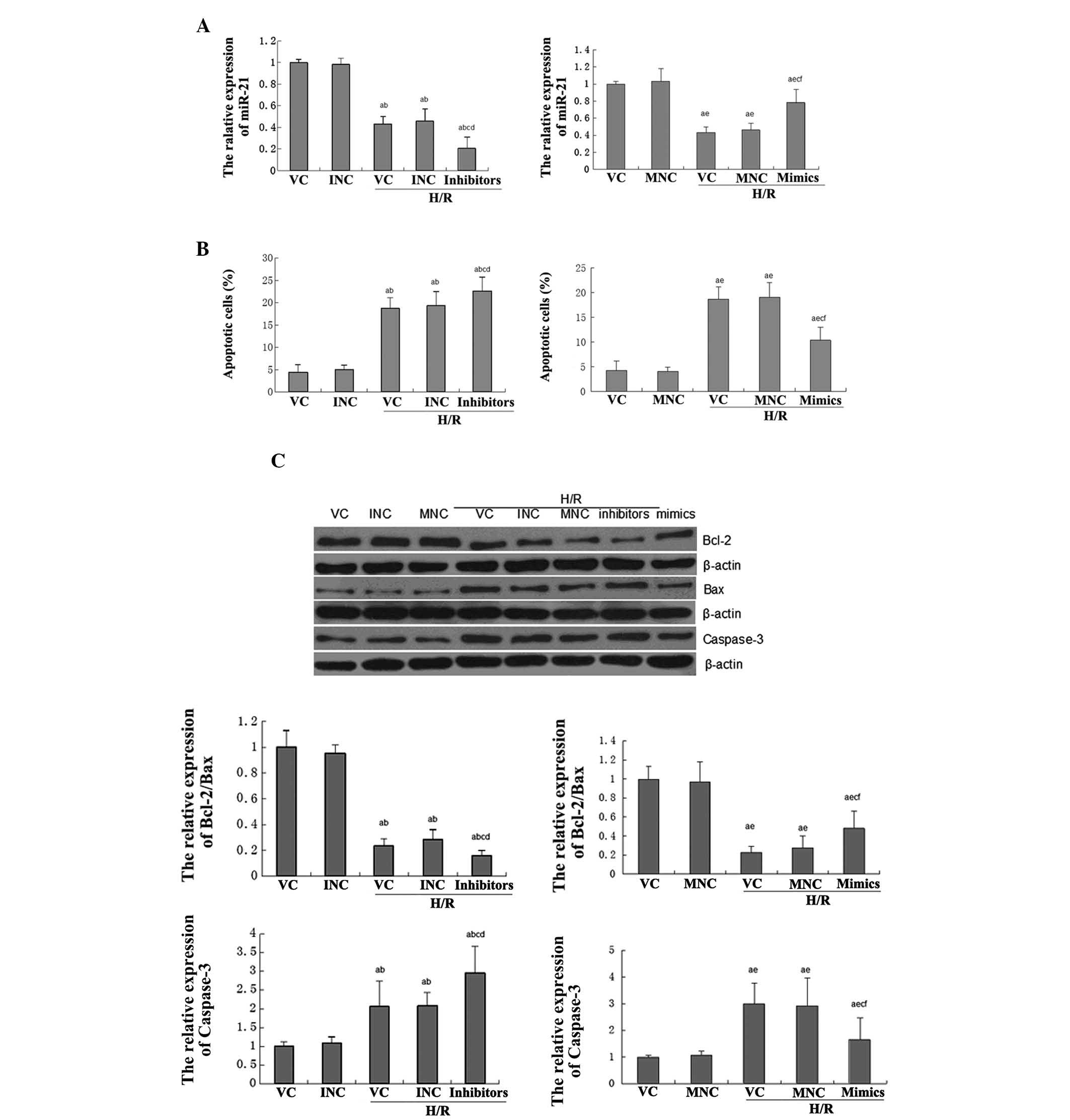 | Figure 3Role of miR-21 in H/R-induced
apoptosis of H9C2 cells. In the VC group, H9C2 cells were without
any treatment. In the INC group, cells were transfected with 50 nM
miR-21 inhibitor. In the MNC group, cells were transfected with 50
nM NC mimics. In the VC + H/R group, cells were treated with H/R.
In the INC+H/R group, cells transfected with 50 nM NC inhibitor
were then treated with H/R. In the MNC+H/R group, cells transfected
with 50 nM NC mimics were then treated with H/R. In the
inhibitors+H/R group, cells transfected with 50 nM miR-21 inhibitor
were then treated with H/R. In the mimics+H/R group, cells
transfected with 50 nM miR-21 mimics were then treated with H/R.
(A) Quantitative polymerase chain reaction was performed to
determine the expression of miR-21 in each group. (B) A cell
apoptosis assay was performed in each group. (C) Western blot
analysis was then performed to determine the protein expression of
Bcl-2, Bax and caspase-3 in each group. β-actin was used as an
internal reference. The relative expression of Bcl2/Bax was
calculated. H/R, hypoxia-reperfusion; miR, microRNA; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein; NC, negative control;
VC, vehicle control; INC, inhibitor negative control; MNC, minics
NC; PTEN, phosphatase and tensin homolog. (D) Western blot analysis
was then performed to determine the protein expression of PTEN and
p-Akt in each group. β-actin was used as an internal reference. For
Fig. 3, aP<0.01 vs. VC group; bP<0.01
vs. INC group; cP<0.05 vs. VC+H/R group;
dP<0.05 vs. INC+H/R group; eP<0.05 vs.
MNC group; and fP<0.05 vs. MNC+H/R group. H/R,
hypoxia-reperfusion; miR, microRNA; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; NC, negative control; VC, vehicle
control; INC, inhibitor negative control; MNC, minics NC; PTEN,
phosphatase and tensin homolog. |
To further investigate the molecular mechanisms
involved, the protein levels of Bcl-2, Bax and caspase-3 were
examined in each group. As shown in Fig. 3C, H/R treatment and presence of the
miR-21 inhibitor significantly downregulated the ratio of Bcl-2/Bax
while increasing the expression of caspase-3, which was also
reverted by the miR-21 mimics.
The role of miR-21 in H/R-induced
activation of PTEN/Akt signaling
Consistent with the aforementioned results in
I/R-induced myocardial injury experiments, it was also identified
that H/R treatment and miR-21 inhibitor evidently upregulated the
PTEN expression while reducing the phosphorylation levels of Akt,
which was also reverted by miR-21 mimics (Fig. 3D).
In summary, the results indicate that miR-21 has an
inhibitory role in H/R-induced cellular apoptosis, partially by
inhibiting PTEN expression and thus promoting the activity of the
Akt signaling pathway, which further suppresses the expression
levels of caspase-3 while increasing the protein ratio of
Bcl-2/Bax.
Discussion
The present study found that miR-21 had an
anti-apoptotic role in I/R-induced myocardial damage in
vivo, and in H/R-induced H9C2 cell death in vitro. Of
note, the present study indicated that a common molecular mechanism
is likely to exist in I/R- and H/R-induced cardiocyte apoptosis,
and that during I/R and H/R, miR-21 can upregulate the Akt
signaling activity via suppressing the expression of PTEN. This
increased activity of Akt signaling further inhibits cell
apoptosis, partially by upregulating the ratio of Bcl-2/Bax, which
can reduce the expression of caspase-3.
Thus far, the biological role of miR-21 in
cardiocytes has not been fully elucidated. Cheng et al
(16) have reported that miR-21 is
highly expressed in the adult heart, indicating that it may have a
crucial role in the regulation of normal biological functions of
the myocardial tissue. Previously, accumulating evidence has shown
that miR-21 has a protective effect on cardiocyte apoptosis via its
target genes. For example, Qin et al (17) revealed that miR-21 inhibited left
ventricular remodeling in the early phase of I/R injury by
suppressing cell apoptosis in rats. Sayed et al (18) reported that Akt mediated the
anti-apoptotic effects of miR-21 via suppression of the Fas ligand.
Besides, miR-21 has been found to protect against the
H2O2-induced myocardial cell injury via
targeting PDCD4 (19). In the
present study, it was revealed that the PTEN/Akt dependent
mechanism involved in I/R- and H/R-induced cardiocyte apoptosis
in vivo or in vitro, respectively. In fact, PTEN has
previously been shown to be a direct target of miR-21 (20,21),
and downregulate the regulation of Akt signaling, which has a
crucial effect on the cell survival rate (22). Recently, it has also been reported
that miR-21 protects cardiomyocytes from tumor necrosis
factor-α-induced apoptosis in vitro via modulating the
PTEN/Akt/forkhead box O3A pathway (23).
It was further revealed that the protein expression
levels of several key apoptotic effectors, including Bcl-2, Bax and
caspase-3, were mediated by miR-21 in rat and cell models of I/R or
H/R injury, respectively. Bcl-2 is a highly conserved
anti-apoptotic protein in the Bcl-2 family and has a low expression
or no expression in apoptotic cells. A number of studies have
indicated that Bcl-2, together with several mitochondrial
membrane-associated proteins, can suppress the production of free
radicals and thus inhibit apoptosis via downregulating cell
endoplasmic reticulum Ca2+ release or the formation of
lipid peroxides. Of note, Bcl-2 has been suggested to have a
central role in the promotion of cardiomyocyte survival by
suppressing apoptosis (24). It
has been well established that Bax, generally expressed in the
majority of tissues and organs, has a pro-apoptotic role in the
mitochondrial-dependent apoptotic pathway (25). In fact, Bax exerts its
pro-apoptotic role by inhibiting the function of Bcl-2 through a
related protein homologue to Bcl-2. Thus, Bax is a major endogenous
antagonist of Bcl-2. On the contrary, however, Bcl-2 and B-cell
lymphoma-extra large can form a heterodimer and cause Bax to lose
its pro-apoptotic effect (26,27).
Thus, under physiological conditions, the expression of Bcl-2 and
Bax are maintained on a balanced level, which, once broken, may
induce cellular apoptosis.
Caspases are a significant
cysteine-aspartate-specific protease family, ubiquitously expressed
in various mammalian cells. It has been demonstrated that the
activation of the caspase family acts as a key effector as well as
the ultimate enforcer of cell apoptosis (28). Caspase-3 is a significant member of
this protease family and its activation has been found in multiple
types of cells undergoing apoptosis (29). Of note, Bcl-2 and caspase-3 have an
interaction mechanism. Bcl-2 was previously found to be upstream of
caspase-3, and to have an inhibitory role in the regulation of
caspase-3 expression. Bcl-2 was then found to be a direct substrate
of caspase-3, and thus, to be inversely regulated by caspase-3.
Once hydrolyzed by caspase-3, the fragment of Bcl-2 is not likely
to have any more anti-apoptotic function, but it was demonstrated
to have pro-apoptotic activity (15,30,31).
As a result, there also exists a balance between Bcl-2 and
caspase-3.
In conclusion, to the best of our knowledge, the
present study was the first to reveal that miR-21 had a protective
role in I/R- and H/R-induced cardiocyte apoptosis, most likely
depending on a common mechanism, which is involved in the PTEN/Akt
signaling activity, Bcl-2 protein family and caspase-3. As a
result, it is speculated that miR-21 may be a promising agent for
the treatment of I/R- and H/R-induced myocardial injury.
References
|
1
|
Ganguly R, Lytwyn MS and Pierce GN:
Differential effects of trans and polyunsaturated fatty acids on
ischemia/reperfusion injury and its associated cardiovascular
disease states. Curr Pharm Des. Apr 10–2013.(Epub ahead of
print).
|
|
2
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu T, Li D and Jiang D: Targeting cell
signaling and apoptotic pathways by luteolin: cardioprotective role
in rat cardiomyocytes following ischemia/reperfusion. Nutrients.
4:2008–2019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo W, Kan JT, Cheng ZY, et al: Hydrogen
sulfide as an endogenous modulator in mitochondria and mitochondria
dysfunction. Oxid Med Cell Longev. 2012:8780522012.PubMed/NCBI
|
|
5
|
Ha T, Liu L, Kelley J, Kao R, Williams D
and Li C: Toll-like receptors: new players in myocardial
ischemia/reperfusion injury. Antioxid Redox Signal. 15:1875–1893.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Young RW: Hyperoxia: a review of the risks
and benefits in adult cardiac surgery. J Extra Corpor Technol.
44:241–249. 2012.PubMed/NCBI
|
|
7
|
Nagai T, Anzai T, Kaneko H, et al: Impact
of systemic acidosis on the development of malignant ventricular
arrhythmias after reperfusion therapy for ST-elevation myocardial
infarction. Circ J. 74:1808–1814. 2010. View Article : Google Scholar
|
|
8
|
Porrello ER: microRNAs in cardiac
development and regeneration. Clin Sci (Lond). 125:151–166. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen LJ, Lim SH, Yeh YT, Lien SC and Chiu
JJ: Roles of microRNAs in atherosclerosis and restenosis. J Biomed
Sci. 19:792012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu H and Fan GC: Role of microRNAs in the
reperfused myocardium towards post-infarct remodelling. Cardiovasc
Res. 94:284–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang KC, Ku YC, Lovett M and Nerbonne JM:
Combined deep microRNA and mRNA sequencing identifies protective
transcriptomal signature of enhanced PI3Kalpha signaling in cardiac
hypertrophy. J Mol Cell Cardiol. 53:101–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng Y and Zhang C: MicroRNA-21 in
cardiovascular disease. J Cardiovasc Transl Res. 3:251–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hers I, Vincent EE and Tavare JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomek M, Akiyama T and Dass CR: Role of
Bcl-2 in tumour cell survival and implications for pharmacotherapy.
J Pharm Pharmacol. 64:1695–1702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zakeri Z and Lockshin RA: Cell death:
history and future. Adv Exp Med Biol. 615:1–11. 2008. View Article : Google Scholar
|
|
16
|
Cheng Y, Ji R, Yue J, et al: MicroRNAs are
aberrantly expressed in hypertrophic heart: do they play a role in
cardiac hypertrophy? Am J Pathol. 170:1831–1840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin Y, Yu Y, Dong H, Bian X, Guo X and
Dong S: MicroRNA 21 inhibits left ventricular remodeling in the
early phase of rat model with ischemia-reperfusion injury by
suppressing cell apoptosis. Int J Med Sci. 9:413–423. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sayed D, He M, Hong C, et al: MicroRNA-21
is a downstream effector of AKT that mediates its antiapoptotic
effects via suppression of Fas ligand. J Biol Chem.
285:20281–20290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng Y, Liu X, Zhang S, Lin Y, Yang J and
Zhang C: MicroRNA-21 protects against the H(2)O(2)-induced injury
on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol.
47:5–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu CZ, Liu W, Zheng Y, et al: PTEN and
PDCD4 are bona fide targets of microRNA-21 in human
cholangiocarcinoma. Chin Med Sci J. 27:65–72. 2012.PubMed/NCBI
|
|
21
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McCubrey JA, Steelman LS, Chappell WH, et
al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how
mutations can result in therapy resistance and how to overcome
resistance. Oncotarget. 3:1068–1111. 2012.PubMed/NCBI
|
|
23
|
Tang Y and Wang MH: MicroRNA-21 protects
cardiomyocytes from tumor necrosis factor-alpha induced apoptosis
in vitro via modulating PTEN/AKT/FOXO3a pathway. Chinese Journal of
Cardiovascular Diseases. 41:135–142. 2013.(In Chinese).
|
|
24
|
Ma YX, Guo Z and Sun T: CGRP inhibits
norepinephrine induced apoptosis with restoration of Bcl-2/Bax in
cultured cardiomyocytes of rat. Neurosci Lett. 549:130–134. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu XP, Zhai D, Kim E, et al:
Three-dimensional structure of Bax-mediated pores in membrane
bilayers. Cell Death Dis. 4:e6832013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walensky LD and Gavathiotis E: BAX
unleashed: the biochemical transformation of an inactive cytosolic
monomer into a toxic mitochondrial pore. Trends Biochem Sci.
36:642–652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Renault TT and Manon S: Bax: Addressed to
kill. Biochimie. 93:1379–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fiandalo MV and Kyprianou N: Caspase
control: protagonists of cancer cell apoptosis. Exp Oncol.
34:165–175. 2012.PubMed/NCBI
|
|
29
|
Snigdha S, Smith ED, Prieto GA and Cotman
CW: Caspase-3 activation as a bifurcation point between plasticity
and cell death. Neurosci Bull. 28:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Poreba M, Strózyk A, Salvesen GS and Drag
M: Caspase substrates and inhibitors. Cold Spring Harb Perspect
Biol. 5:a0086802013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kale J, Liu Q, Leber B and Andrews DW:
Shedding light on apoptosis at subcellular membranes. Cell.
151:1179–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|