Introduction
In July 2009, the World Health Organization (WHO)
declared a pandemic due to a novel influenza virus, haemagglutinin
type 1 and neuraminidase type 1 (H1N1) (1), which raised serious public concern
(2). At present, vaccines and
anti-influenza drugs are used for the treatment and prevention of
influenza (3,4). However, the number of vaccines
currently available is insufficient. Failure to anticipate the
circulating strains also reduces the efficacy of the vaccines
(5,6). Two classes of approved antiviral
drugs are available at present, neuraminidase inhibitors and M2
channel inhibitors (7). However,
the use of these drugs is limited due to the emergence of
drug-resistant viruses (8,9); therefore, the development of novel,
effective antiviral drugs is critical in order to control and treat
influenza infections.
Statins are 3-hydroxy-3-methylglutaryl coenzyme A
(HMG-CoA) reductase inhibitors, and are widely used in
lipid-lowering therapy. Studies have shown that statins have
pleiotropic effects (10–12), including anti-inflammatory and
immunomodulatory effects, which may provide a potential therapeutic
treatment against influenza (13).
Influenza infection is characterized by increased plasma
concentrations of proinflammatory cytokines, known as a ‘cytokine
storm’ (14). Anti-inflammatory
and immunomodulatory agents are potential antiviral drugs. Statins
have been shown to exert antimicrobial effects against certain
organisms, and they have also been reported to be active against a
broad spectrum of viruses (15,16).
In addition, observational studies have suggested that statins may
reduce mortality in patients with influenza (17,18).
We previously reported that a statin/caffeine combination protects
BALB/c mice against H5N1, H3N2 and H1N1 infection. The effect of
statin alone on influenza virus infection; however, is not known.
Fluvastatin is a member of the statin family. In this study, the
inhibition effect of fluvastatin on influenza A virus infection was
investigated, as well as the mechanism of action in cultured
cells.
Materials and methods
Cells, virus strains and reagents
Madin-Darby canine kidney (MDCK) cells and a human
epithelial lung cell line (A549) obtained from the Experimental
Animal Center of Sun Yat-sen University (Guangzhou, China) were
grown in minimum essential medium (MEM; Invitrogen Life
Technologies, Carlsbad, CA, USA) and Dulbecco’s Modified Eagle
medium/Nutrient Mixture F12 (DMEM/F12; Hyclone, Thermo Fisher
Scientific, Waltham, MA, USA), respectively, with 10%
heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml),
and streptomycin (100 μg/ml). Pandemic 2009 strain
A/Guangdong/03/2009 (H1N1) was provided by Guangzhou Center for
Disease Control and Prevention (Guangzhou, China) and was
propagated in MDCK cells. The fluvastatin and Oseltamivir was
supplied by LGC (Teddington, UK). All virus work was performed
under BL2+ safety conditions.
Viral titer assay
Monolayer MDCK cells (6×105 cells/well)
in six-well plates were infected with a serially diluted virus.
Following adsorption for 2 h at 37°C, cells were washed twice and
supplemented with MEM containing 0.9% agarose (Oxoid Ltd.,
Basingstoke, UK), 0.4% bovine serum albumin (Gibco®-BRL,
Carlsbad, CA, USA) and 4 μg/ml trypsin (Gibco®-BRL).
Plaques were visualized 2–3 days later, then fixed with 10%
formaldehyde and stained with 1% crystal violet.
Cytotoxicity test and IC50
determination
MDCK or A549 cells were seeded onto 96-well plates
(5×105 cells/well) and incubated for 24 h. Following
treatment with MEM containing two-fold serially diluted fluvastatin
for 48 h, the medium was removed and 50 μl MTT (Sigma, St. Louis,
MO, USA) was added into each well and incubated for 3 h at 37°C, 5%
CO2. Dimethyl sulphoxide (DMSO; 150 μl/well; Sigma) was
then added, and the absorbance of the cells was determined at 490
nm. The 50% cytotoxic concentration (CC50) was
calculated. Anti-influenza virus activity of fluvastatin was
analysed and the 50% inhibition concentration (IC50) was
determined. MDCK cells were infected with H1N1 and a multiplicity
of infection (MOI) of 0.1 for 1 h. Maintenance medium containing
serial two-fold dilutions of fluvastatin (0–20 μg/ml) was added and
the cells were then incubated for 48 h at 37°C, 5% CO2.
The MTT assay was performed as described above. Oseltamivir (0–4
μg/ml; ) was used as the positive control.
Quantitative polymerase chain reaction
(qPCR)
MDCK cells were infected with H1N1 (MOI of 0.1), and
were treated with or without fluvastatin (0–20 μg/ml). Total RNA
was extracted 12 and 24 h following infection using the
QIAamp® MinElute Virus Spin kit (Qiagen, Venlo, The
Netherlands). The primer sequences used for the qPCR of viral RNA
were as follows: Forward, 5′-GAGGATGGTGCTTTCTGCTTTT-3′ and reverse,
5′-AGTTCTCTCATCCACTTTCCGTCT-3′. β-actin was used as an internal
control of cellular RNA, and the primer sequences used were as
follows: Forward, 5′-CGTGCGTGACATCAAGGAAGAAG-3′ and reverse,
5′-GGAACCGCTCGTTGCCAATG-3′.
Total RNA was reverse transcribed into cDNA using
PrimeScript® RT Master Mix (Perfect Real Time) (Takara
Bio Inc., Shiga, Japan). qPCR was conducted using 2 μl cDNA and
SYBR® Premix Ex Taq II (Takara Bio Inc.). Cycling
conditions for qPCR were as follows: 95°C for 30 sec, followed by
40 cycles of 95°C for 5 sec and 60°C for 34 sec. β-actin mRNA was
used as a loading control. qPCR was conducted using the ABI
Prism® 7500 sequence detection system (Applied
Biosystems, Foster City, CA, USA). Data are expressed as the
relative differences between control and treated cells following
normalization to β-actin expression.
Immunofluorescence assay
MDCK cells were plated on glass coverslips and were
exposed to the H1N1 virus at an MOI of 0.1 for 1 h, then
maintenance medium in the presence or absence of 20 μg/ml
fluvastatin were added. Twelve hours following infection, the cells
were fixed with 4% paraformaldehyde for 10 min and permeabilised
with 1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) in
phosphate-buffered saline (PBS) for 10 min. Cells were then
incubated with rat anti-nucleoprotein (anti-NP) monoclonal
antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at
37°C for 1 h, then washed with 0.5% NP-40 in PBS and incubated with
fluorescein isothiocyanate-conjugated goat anti-rabbit monoclonal
antibody (Santa Cruz Biotechnology, Inc.) for 1 h. Subsequently,
cells were stained with 4′,6′-diamidino-2-phenylindole
dihydrochloride (Roche, Mannheim, Germany). The cells were mounted
and observed using a fluorescence microscope (DMI3000B; Leica
Microsystems, Wetzlar, Germany).
Time-of-addition assay
MDCK cells (5×105 cells/well) were seeded
in six-well plates and incubated for 24 h, and the virus (MOI of 1)
was added to cells for 1 h at 37°C. Fluvastatin was added from -9
to -1 h (prior to adsorption), -1 to 0 h (during adsorption) and 0
to 8 h (post-adsorption). Following administration of fluvastatin,
the cells were washed with PBS and incubated with fresh medium
until 8 h post-infection. The supernatant was collected and frozen
at −80°C prior to determination of the viral yield using the plaque
assay.
Analysis of cytokine production
A549 cells in a six-well plate were infected with
H1N1 with an MOI of 1 for 1 h, then maintenance medium in the
presence or absence of 20 μg/ml fluvastatin was added. Culture
supernatants were collected and the concentration of cytokines was
analysed 24, 36 and 48 h following infection. Cytokine assays were
performed using Quantibody Human Inflammation Array (product
number, QAH-INF-1; RayBiotech Inc., Norcross, GA, USA).
Neuraminidase inhibition assay
Equal volumes of virus and fluvastatin that were
two-fold serially diluted with PBS were mixed and incubated for 30
min at room temperature, and the reaction was initiated by addition
of 2′-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (Sigma). A
total of 500 mM carbonate buffer (pH 10.7) was added to terminate
the reaction. The fluorescence was measured by a Promega GloMax 96
Microplate Luminescence detector (Promega Corporation, Madison, WI,
USA) at 365 nm (excitation) and 450 nm (emission).
Statistical analysis
A one-way analysis of variance followed by
Bonferroni’s method was used to compare the differences between the
groups. P<0.05 was considered to indicate a statistically
significant difference. The IC50 and CC50
values were calculated using GraphPad Prism 5 (GraphPad Software,
Inc., La Jolla, CA, USA).
Results
Cytotoxicity of fluvastatin and
IC50 determination
MDCK cells were incubated in the presence of
two-fold fluvastatin serial dilutions for 48 h. The viability of
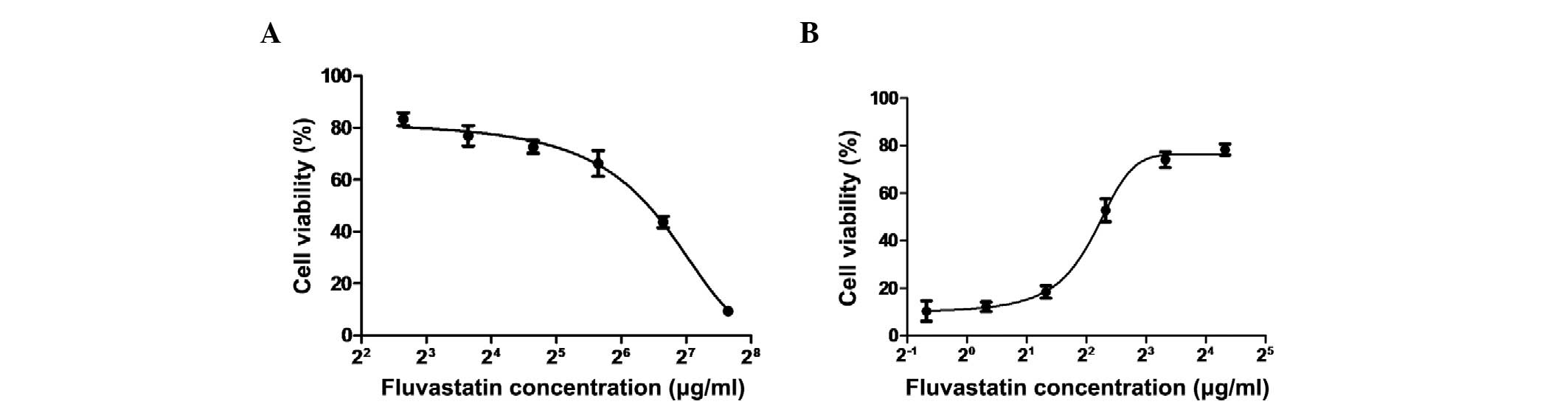
cells was then estimated using the MTT assay. As shown in Fig. 1A, the CC50 value was
90.3 μg/ml. When MDCK cells were incubated with fluvastatin
following virus adsorption, the cytopathic effect formation was
decreased in MDCK cells that were infected with pandemic 2009
strain A/Guangdong/03/2009 (H1N1) at an MOI of 0.1 (data not
shown). To investigate the effect of fluvastatin on influenza, MDCK
cells were incubated with different concentrations of fluvastatin
following virus adsorption, and the viability of the cells was
determined. It was found that at the concentration range of <20
μg/ml, fluvastatin exerted an inhibitory effect on influenza virus
infection, and the IC50 value of fluvastatin was ~4.3
μg/ml (Fig. 1B). The
IC50 value of the positive control (oseltamivir) was 0.8
μg/ml. The selectivity index (CC50/IC50) for
fluvastatin was 21.
The cytotoxicity of fluvastatin on A549 cells was
also investigated. At a concentration of 300 μg/ml, fluvastatin did
not exhibit any detrimental effects on cell viability (data not
shown).
Fluvastatin inhibits viral RNA and
protein synthesis
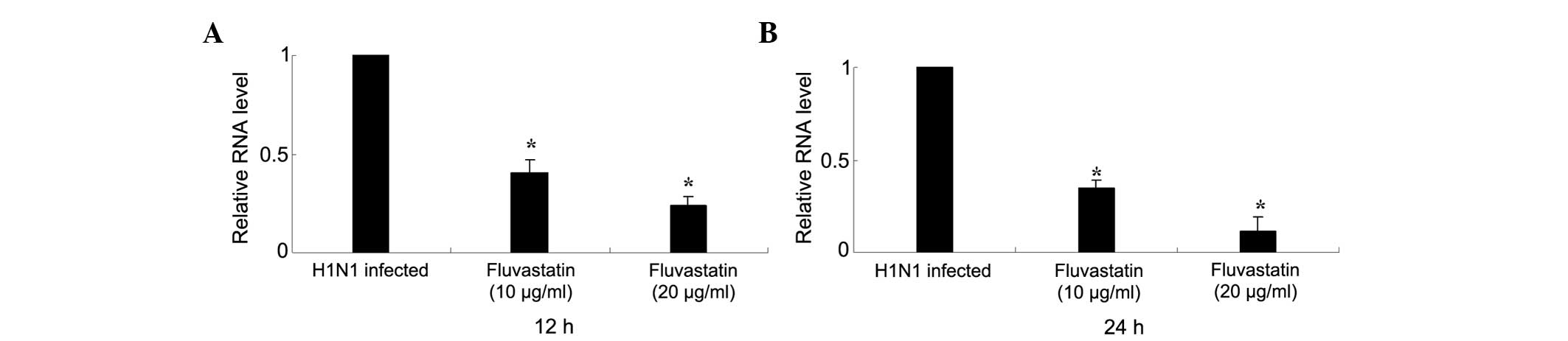
The inhibitory effect of fluvastatin on influenza
virus replication was determined by qPCR. Fluvastatin (<20
μg/ml) significantly reduced intracellular RNA expression of
influenza virus NP in H1N1-infected MDCK cells 12 h post-infection.
Expression of NP was further reduced 24 h post-infection (Fig. 2).
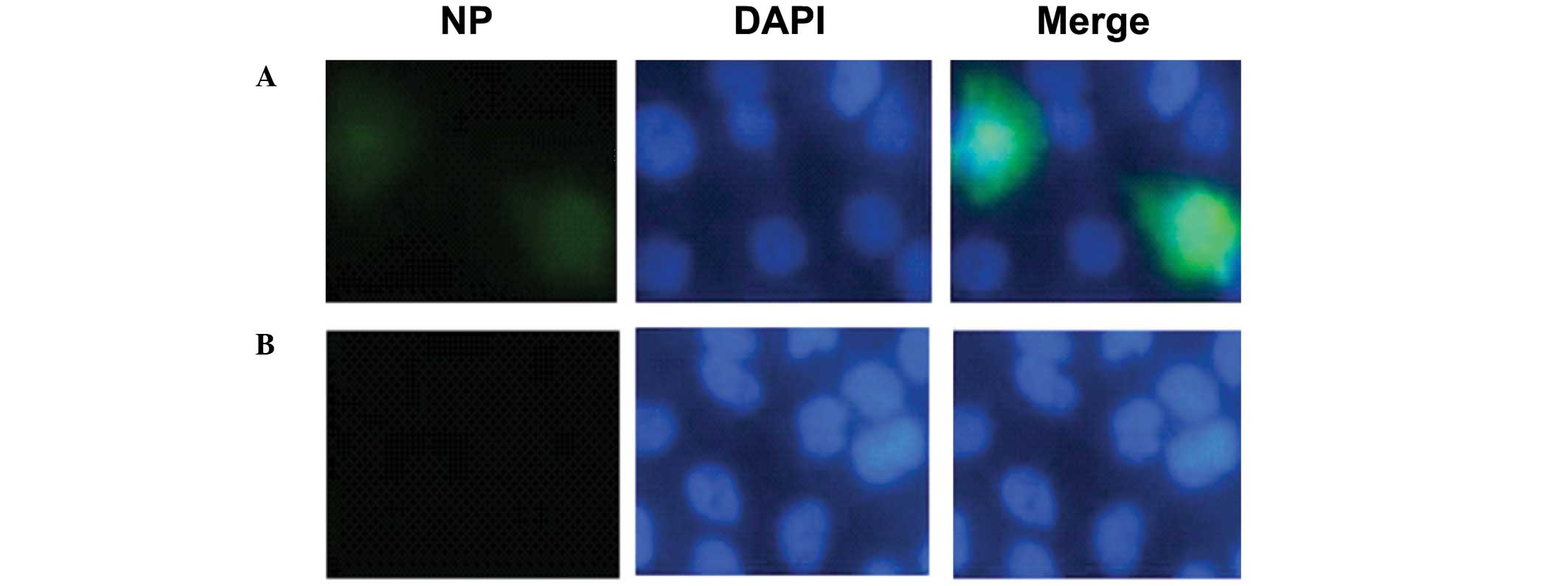
The inhibition of fluvastatin on viral NP expression
was further investigated using immunofluorescence microscopy. MDCK
cells were exposed to H1N1 virus (MOI of 0.1) for 1 h and then
incubated with fluvastatin (20 μg/ml). Cells were stained for NP 12
h post-infection. The results showed that NP expression was
decreased in the presence of fluvastatin (Fig. 3).
Inhibitory effects of fluvastatin on
different stages of viral replication
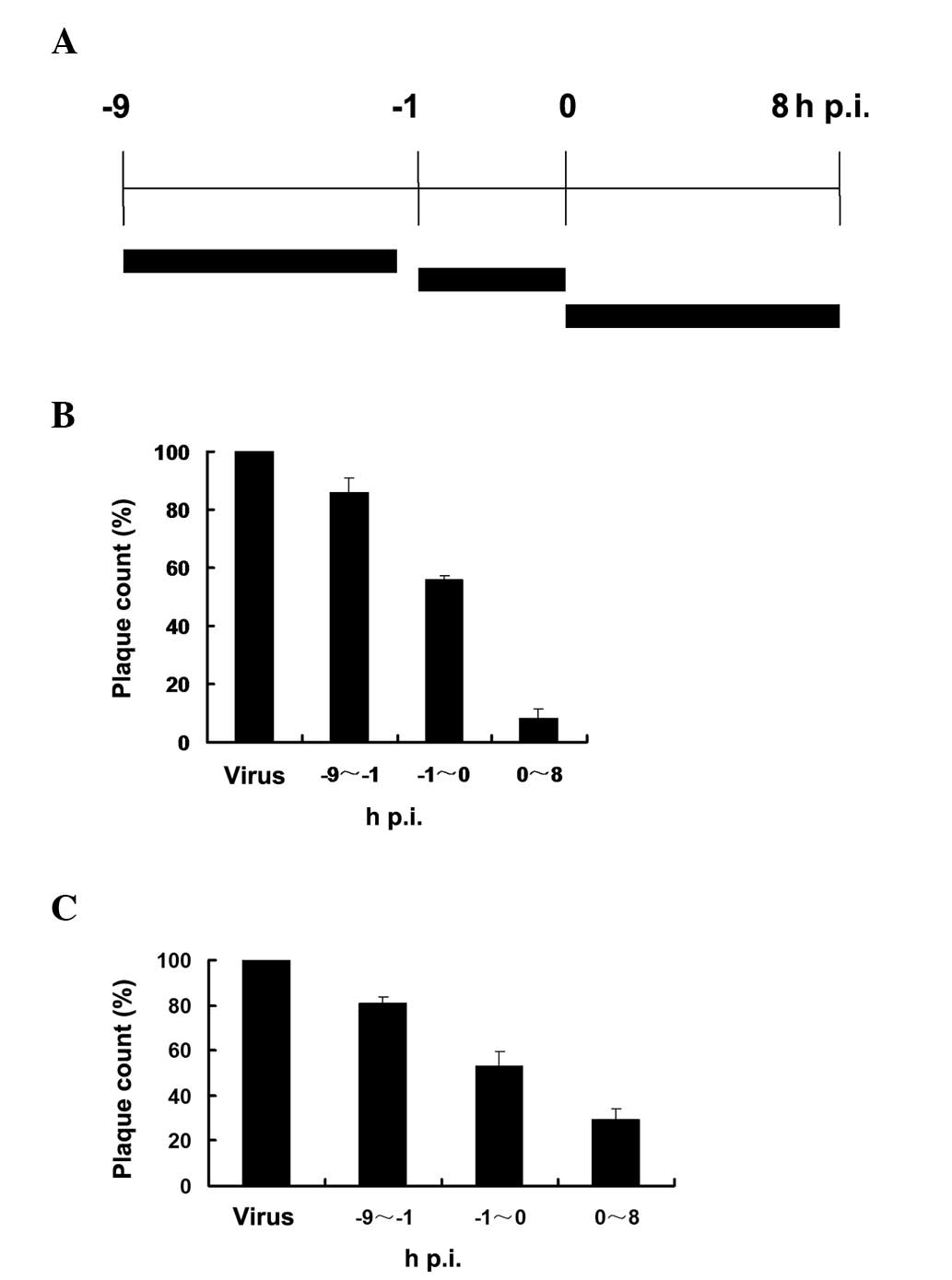
The time-of-addition assay was used to analyse the
effect of fluvastatin on virus yield in MDCK cells when fluvastatin
was administered at different times relative to virus infection.
The anti-viral effect of fluvastatin was determined following
administration of fluvastin at three time-points, respectively
(Fig. 4A). The viral plaque
formation was observed and the plaques were counted by visual
examination.
When fluvastatin (20 μg/ml) was added prior to virus
adsorption (Fig. 4B), a partial
reduction in progeny virus production was detected compared with
the untreated virus-infected cells. Fluvastatin exhibited an
inhibitory effect when added during the adsorption period, and the
addition of fluvastatin following viral adsorption caused a further
decrease in progeny virus production. The inhibitory effects were
also detected at the final concentration of 10 μg/ml fluvastatin
(Fig. 4C).
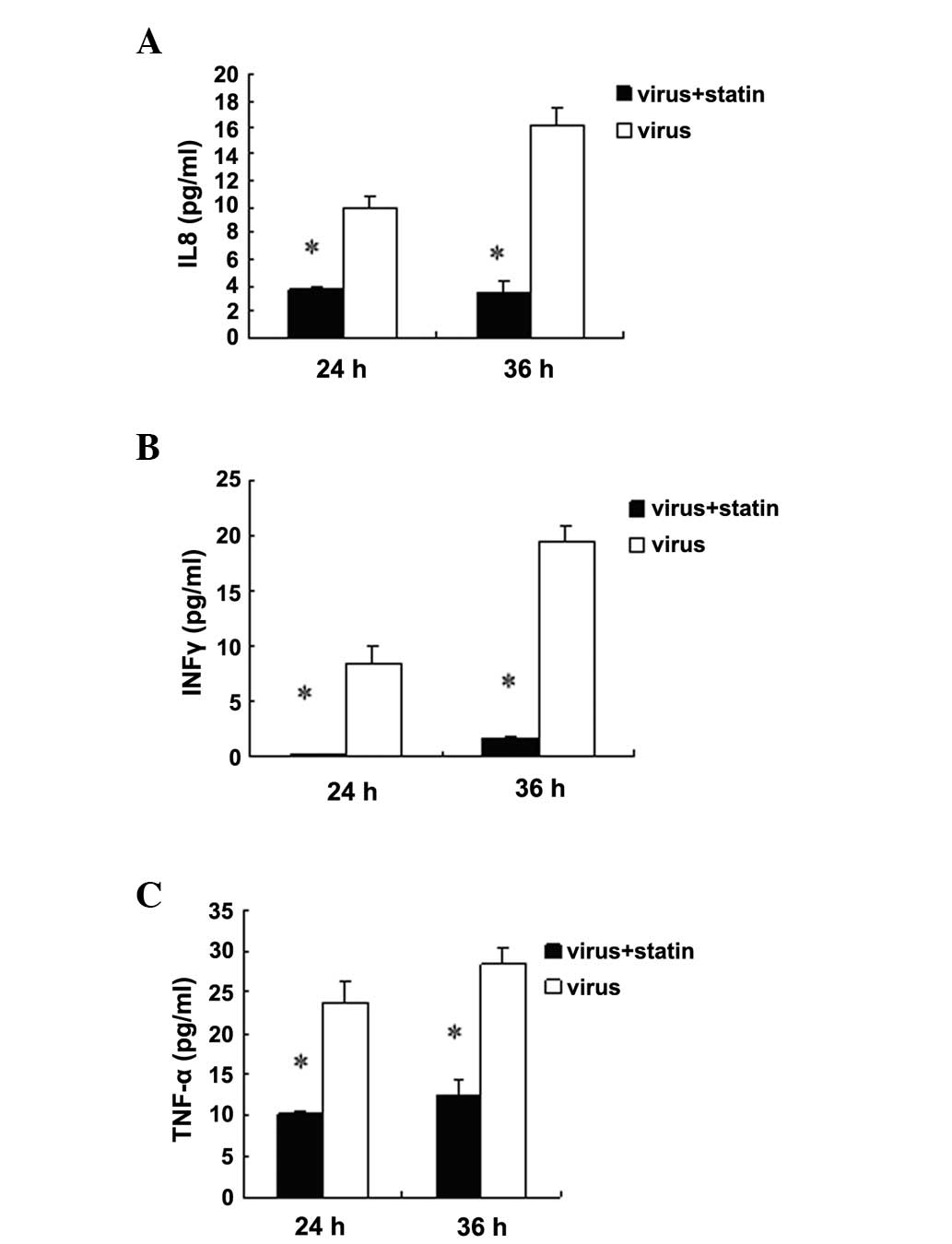
Fluvastatin decreases the release of
cytokines in H1N1-infected A549 cells
Influenza infection results in the increased
production of cytokines; therefore, it was investigated whether
this response was altered by fluvastatin treatment. The results
showed that fluvastatin did not suppress cytokine expression in
non-infected cells (data not shown). Baselines of cytokine were
very low in non-infected cells. It was found that fluvastatin (20
μg/ml) reduced the release of tumour necrosis factor-α (TNF-α),
interleukin 8 (IL8) and interferon γ (IFNγ) in the supernatants of
H1N1-infected A549 cells 24 and 36 h post-infection (Fig. 5). By contrast, fluvastatin did not
suppress secretion of seven other cytokines (data not shown). In
addition, since the enhancement of the neuraminidase activity in
host cells may reflect the virus infection, the inhibitory effect
of fluvastatin on neuraminidase was evaluated in this study.
However, no significant effect was observed (Fig. 6).
Discussion
At present, influenza treatment options are
expensive and result in side effects; therefore, novel, effective
and inexpensive treatments against influenza are required (19). Statins have been of recent interest
due to their pleiotropic effects (20). An in vitro study reported
that statins disrupt the replication of hepatitis C virus by
inhibition of prenylation (21).
In other studies, it was shown in vivo that statins exhibit
direct anti-human immunodeficiency virus 1 (HIV-1) effects by
targeting Rho (22,23). In addition, another study
demonstrated in vivo and in vitro that lovastatin may
inhibit respiratory syncytial virus by reducing cell-to-cell fusion
(16). Epidemiological evidence
has also shown that statins improve poor clinical outcomes in
observational studies of patients with sepsis, community-acquired
pneumonia and influenza-related pneumonia (24–28).
We previously found that a statin/caffeine combination protects
BALB/c mice against H5N1, H3N2 and H1N1 infection (29); however, it was not elucidated
whether statins alone had an effect on viral infection. Therefore,
in the current study, it was investigated whether statin is capable
of inhibiting influenza virus replication.
The results of this study demonstrated that
fluvastatin inhibited H1N1 influenza virus replication in
H1N1-infected MDCK and A549 cells; however, the cytotoxicity of
fluvastatin was higher in MDCK cells compared with that in A549
cells. This may be due to the different origins of the MDCK and
A549 cells. Fluvastatin was shown to have an inhibitory effect
post-infection, possibly by inhibiting the expression of cytokines.
In particular, fluvastatin diminished the expression of TNF-α, IL8
and IFNγ. Several anti-inflammatory and immunomodulatory drugs,
including celecoxib and mesalazine, have previously been reported
to influence influenza virus replication (30,31).
In this study it was shown that fluvastatin also inhibits viral RNA
replication. However, the value of selectivity index was only 21;
therefore, the inhibitory effect was limited.
Node et al (32) demonstrated that the administration
of statins was associated with a reduction in the plasma
concentrations of TNF-α and IL6 (32). Radigan et al (33) observed that rosuvastatin did not
attenuate the severity of influenza A-induced lung injury in
influenza A-infected C57Bl/6 mice, which is inconsistent with our
results. However, a longer duration of treatment may have had an
effect (33). Belser et al
(34) reported that simvastatin
showed relatively little efficacy in treating the influenza virus
infection in BALB/c mice. Additionally, simvastatin treatment
appeared to increase the time to death, but these findings were not
statistically significant (34).
Different virus types and statins were used in these studies, which
may explain the conflicting results observed.
Cytokines, including TNF-α and IL6, have been
implicated in weight loss during influenza infection (35). Further in vivo investigation
is required to enhance the understanding of antiviral activity of
fluvastatin. It is not yet known whether fluvastatin also
attenuates the severity of influenza A infection by reducing the
levels of these cytokines in vivo.
In conclusion, the results of the present study
suggest that fluvastatin exerts a minor inhibitory effect on
influenza virus infection in vitro. However, these results
require further investigation in vivo.
Acknowledgements
The study was supported by the National Natural
Science Foundation of China (nos. 81172735 and 81201283) and
Guangdong Provincial Scientific and Technological Planning Project
(no. 2010B031600076).
References
|
1
|
Kok J and Dwyer DE: How common was 2009
pandemic influenza A H1N1? Lancet Infect Dis. 11:423–424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization. Pandemic (H1N1)
- update 112. http://www.who.int/csr/don/2010_08_06/en/indexhtml.
Accessed November 10, 2011
|
|
3
|
Memoli MJ, Morens DM and Taubenberger JK:
Pandemic and seasonal influenza: therapeutic challenges. Drug
Discov Today. 13:590–595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cox MM: Pandemic influenza: overview of
vaccines and antiviral drugs. Yale J Biol Med. 78:321–328.
2005.PubMed/NCBI
|
|
5
|
Podolec P and Kopeć G: Influenza vaccines
for prevention of cardiovascular diseases. Kardiol Pol. 65:612–615.
2007.(In Polish).
|
|
6
|
Lambert LC and Fauci AS: Influenza
vaccines for the future. N Engl J Med. 363:2036–2044. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sugrue RJ, Tan BH, Yeo DS and Sutejo R:
Antiviral drugs for the control of pandemic influenza virus. Ann
Acad Med Singapore. 37:518–524. 2008.PubMed/NCBI
|
|
8
|
Moss RB, Davey RT, Steigbigel RT and Fang
F: Targeting pandemic influenza: a primer on influenza antivirals
and drug resistance. J Antimicrob Chemother. 65:1086–1093. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fedson DS: Confronting an influenza
pandemic with inexpensive generic agents: can it be done? Lancet
Infect Dis. 8:571–576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tleyjeh IM, Kashour T, Hakim FA, et al:
Statins for the prevention and treatment of infections: a
systematic review and meta-analysis. Arch Intern Med.
169:1658–1667. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Q and Liao JK: Pleiotropic effects of
statins: basic research and clinical perspectives. Circ J.
74:818–826. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Corsini A, Ferri N and Cortellaro M: Are
pleiotropic effects of statins real? Vasc Health Risk Manag.
3:611–613. 2007.PubMed/NCBI
|
|
13
|
Terblanche M, Smith TS and Adhikari NK:
Statins, bugs and prophylaxis: intriguing possibilities. Crit Care.
10:1682006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tisoncik JR, Korth MJ, Simmons CP, Farrar
J, Martin TR and Katze MG: Into the eye of the cytokine storm.
Microbiol Mol Biol Rev. 76:16–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montoya CJ, Jaimes F, Higuita EA, et al:
Antiretroviral effect of lovastatin on HIV-1-infected individuals
without highly active antiretroviral therapy (The LIVE study): a
phase-II randomized clinical trial. Trials. 10:412009. View Article : Google Scholar
|
|
16
|
Gower TL and Graham BS: Antiviral activity
of lovastatin against respiratory syncytial virus in vivo and in
vitro. Antimicrob Agents Chemother. 45:1231–1237. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frost FJ, Petersen H, Tollestrup K and
Skipper B: Influenza and COPD mortality protection as pleiotropic,
dose-dependent effects of statins. Chest. 131:1006–1012. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kwong JC, Li P and Redelmeier DA:
Influenza morbidity and mortality in elderly patients receiving
statins: a cohort study. PLoS One. 4:e80872009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hayden F: Developing new antiviral agents
for influenza treatment: what does the future hold? Clin Infect
Dis. 48(Suppl 1): S3–S13. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nair P and Roguin A: Statins and the acute
coronary syndrome: ‘the early bird catches the worm’. Int J Clin
Pract. 60:716–727. 2006.
|
|
21
|
Ye J, Wang C, Sumpter R Jr, Brown MS,
Goldstein JL and Gale M Jr: Disruption of hepatitis C virus RNA
replication through inhibition of host protein geranylgeranylation.
Proc Nat Acad Sci USA. 100:15865–15870. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
del Real G, Jiménez-Baranda S, Mira E, et
al: Statins inhibit HIV-1 infection by down-regulating Rho
activity. J Exp Med. 200:541–547. 2004.PubMed/NCBI
|
|
23
|
Moncunill G, Negredo E, Bosch L, et al:
Evaluation of the anti-HIV activity of statins. AIDS. 19:1697–1700.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Janssen NE, van Lelyveld SF, Hoepelman AI,
Gras L, Groenwold RH and Oosterheert JJ: The effect of statin
therapy on pneumonia in an HIV-infected population in the
Netherlands. J Infect. 67:238–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grau AC and George SM: Improved outcomes
in community-acquired pneumonia with prior statin use. Am J Med.
122:e15author reply e17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chalmers JD, Singanayagam A, Murray MP and
Hill AT: Prior statin use is associated with improved outcomes in
community-acquired pneumonia. Am J Med. 121:1002–1007. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo YF and Hu DY: Statin use and reduced
prevalence of sepsis. Zhonghua Nei Ke Za Zhi. 46:798–800. 2007.(In
Chinese).
|
|
28
|
Almog Y, Shefer A, Novack V, et al: Prior
statin therapy is associated with a decreased rate of severe
sepsis. Circulation. 110:880–885. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Z, Guo Z, Wang G, et al: Evaluation of
the efficacy and safety of a statin/caffeine combination against
H5N1, H3N2 and H1N1 virus infection in BALB/c mice. Eur J Pharm
Sci. 38:215–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin Y, Zhang G, Hu Y, et al: Inhibition of
highly pathogenic avian H5N1 influenza virus propagation by RNA
oligonucleotides targeting the PB2 gene in combination with
celecoxib. J Gene Med. 13:243–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hui DS, Lee N and Chan PK: Adjunctive
therapies and immunomodulatory agents in the management of severe
influenza. Antiviral Res. 98:410–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Node K, Fujita M, Kitakaze M, Hori M and
Liao JK: Short-term statin therapy improves cardiac function and
symptoms in patients with idiopathic dilated cardiomyopathy.
Circulation. 108:839–843. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Radigan KA, Urich D, Misharin AV, et al:
The effect of rosuvastatin in a murine model of influenza A
infection. PLoS One. 7:e357882012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Belser JA, Szretter KJ, Katz JM and Tumpey
TM: Simvastatin and oseltamivir combination therapy does not
improve the effectiveness of oseltamivir alone following highly
pathogenic avian H5N1 influenza virus infection in mice. Virology.
439:42–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zarogiannis SG, Noah JW, Jurkuvenaite A,
Steele C, Matalon S and Noah DL: Comparison of ribavirin and
oseltamivir in reducing mortality and lung injury in mice infected
with mouse adapted A/California/04/2009 (H1N1). Life Sci.
90:440–445. 2012. View Article : Google Scholar : PubMed/NCBI
|