Introduction
Nitric oxide (NO) is a fundamental
endothelium-derived relaxing factor involved in a variety of
physiological processes within organ systems. Under physiological
conditions, NO has the characteristic of inhibiting leukocyte and
smooth muscle cell hyperplasia, and is also beneficial in
preventing cardiac damage following myocardial ischemia (1). Emerging evidence has indicated that
NO exerts cardioprotection during ischemia-reperfusion events
(2,3). NO is catalyzed from the substrate
L-arginine by nitric oxide synthase (NOS). At least three distinct
subgroups, including neuronal NOS (nNOS or NOS1), inducible NOS
(iNOS or NOS2) and endothelial cell NOS (eNOS or NOS3), are
classified into the NOS family in mammalian cells. Among these
isoforms, eNOS is highly expressed in coronary endothelial cells
and is also rich in the sarcolemma of cardiac myocytes (4). NO released from eNOS in the heart is
considered to have a critical role in coronary vasodilation and
tonic inhibition of mitochondrial O2 consumption. A
study demonstrated that transgenic mice overexpressing eNOS were
protected from myocardial infarction-induced cardiac damage
(5). Conversely, progressive
cardiomyocyte dysfunction was evidently exacerbated in
eNOS−/− mice following myocardial infarction, suggesting
a protective effect of eNOS-derived NO (6,7).
Therefore, inhibition of eNOS may be a useful and promising
approach for attenuating the impairment caused by myocardial
infarction.
Cumulative evidence has also demonstrated that
oxidative stress is implicated in the pathogenesis of myocardial
infarction, and finally contributes to cardiac dysfunction and the
death of cardiomyocytes (8,9). A
study identified that marked generation of oxygen-derived free
radicals following ischemic insults, which destroyed endogenous
antioxidant defenses and resulted in oxidative damage of membrane
lipids, proteins, carbohydrates and DNA, concomitantly leading to
cardiomyocyte necrosis and apoptosis (10). A previous study revealed that
excessive free radicals attack the unsaturated fatty acids within
myocardial membranes and exacerbate the damage following acute
myocardial infarction (11).
Baicalein is a medicinal herb purified from the root
of Scutellaria baicalensis Georgi and has been demonstrated
to possess anti-inflammatory (12)
and anti-oxidative (13)
properties. A previous study revealed that baicalein protected
cardiomyocytes against ischemia-reperfusion injury in a chick
embryonic ventricular myocyte model (14). In addition, Woo et al
(15) also demonstrated that
pretreatment with baicalein evidently diminished intracellular
calcium overload and attenuated the cardiac damage during
myocardial ischemia-reperfusion injury. However, whether baicalein
has is protective against myocardial infarction in rats has not
been clarified. Furthermore, as the eNOS signaling pathway and
oxidative stress are central in the amelioration of ischemic
insults, we hypothesized that they are involved in the
cardioprotective effect of baicalein. The present study was
performed to evaluate the cardiprotective effect of baicalein and
elucidate the roles of the eNOS signaling pathway and oxidative
stress in baicalein-mediated cardioprotection against acute
myocardial infarction in rats.
Materials and methods
Experimental animals
All experiments were conducted with adult male
Wistar rats (weight, 230–260 g), obtained from the Beijing Animal
Center (Beijing, China) and allowed to acclimatize in the animal
cage for four days prior to use, with access to water and food
ad libitum. All experimental protocols were approved by the
Animal Care Committee of the Second Xiangya Hospital of Central
South University, (Changsha, Hunan, China). Efforts were made to
minimize suffering and the number of animals used.
Acute myocardial infarction model and
drug administration
The induction of acute myocardial infarction was
performed as previously described (16). In brief, the male Wistar rats were
anesthetized with pentobarbital sodium (40 mg/kg) intraperitoneally
(i.p.) and then fixed on the operating table for the surgical
procedures. A tracheotomy was performed and an intubation cannula
was connected with a volume-controlled ventilator (Nanjing
Jiancheng Biotechnology Institute, Nanjing, Jiangsu, China). The
normal electrocardiogram (II; Nanjing Jiancheng Biotechnology
Institute) was recorded via a multi-channel recorder (BL-420F;
Chengdu Taimeng, Chengdu, China) after the electrodes were
subcutaneously placed onto the four limbs and connected to an
electrocardiograph. A 5-0 silk suture 1–2 mm (Nanjing Jiancheng
Biotechnology Institute) was employed to encircle the left anterior
descending coronary artery below the left atrial appendage. The
left coronary artery of the rats was ligated. The sham-surgery
animals underwent identical surgical procedures with the exception
of coronary artery occlusion. Baicalein (purity >95%;
Sigma-Aldrich, St. Louis, MO, USA) was dissolved in
dimethylsulfoxide (DMSO; 0.01%; diluted in phosphate-buffered
saline). The rats were randomized into five groups as follows: i)
Sham-surgery group (Sham; n=6), rats underwent identical surgery,
with the exception of coronary artery ligation, and were
administered with 0.01% DMSO (0.1 ml/100 g, i.p.); ii) vehicle
group (Vehicle; n=6), rats underwent occlusion of the left coronary
artery and were administered with 0.01% DMSO (0.1 ml/100 g, i.p.);
iii) baicalein treatment group (BAC30; n=6), rats were subjected to
occlusion of the left coronary artery and treated with baicalein
(30 mg/kg, i.p.) (17); iv) L-NAME
(an inhibitor of NOS) treatment group (L-NAME; n=6), rats were
subjected to occlusion of the left coronary artery and treated with
L-NAME (10 mg/kg, i.p.) (18); v)
baicalein and L-NAME co-administration group (BAC30 + L-NAME; n=6),
rats were subjected to occlusion of the left coronary artery and
treated with baicalein (30 mg/kg, i.p.) and L-NAME (10 mg/kg,
i.p.). Baicalein and DMSO were administered once a day for six
consecutive days and L-NAME was injected once 25 min prior to the
induction of ischemia. After the final administration (30 min), the
rats underwent surgery by occlusion of the left coronary
artery.
Measurement of infarct size
After the occlusion of the coronary artery (6 h),
the animals were anesthetized with pentobarbital sodium and the
hearts were immediately excised, and the left ventricles were then
sectioned into transverse slices (2-mm thick) from the apex to the
atrioventricular groove. The slices were subsequently incubated in
1% triphenyltetrazolium chloride (TTC; Sigma-Aldrich) solution at
37°C for 30 min, photographed with a digital camera (Panasonic
Lumix DMC-TZ40-K; Canon Inc., Tokyo, Japan) and weighed. For each
slice, TTC stained the normal myocardium brick-red while the
infarcted area remained grayish-white. The size of the infarcted
area was calculated by the volume and weight as a percentage of the
left ventricle, according to the method described in a previous
study (16).
Determination of plasma cardiac marker
enzyme levels
The cardiac damage was evaluated by measuring the
levels of myocardial-specific enzymes, including creatine kinase
(CK), MB isoenzyme of creatine kinase (CK-MB), lactate
dehydrogenase (LDH) and cardiac troponin T (cTnT). The blood
samples were acquired 6 h after the occlusion of the coronary
artery. The plasma concentrations of CK, CK-MB and LDH were
measured by the colorimetric method using an automatic biochemical
analyzer (AU-2700; Olympus Corporation, Tokyo, Japan). The plasma
cTnT levels were measured by an immunoassay kit (Elecsys 2010;
Roche Diagnostics, Mannheim, Germany).
Western blotting
The heart samples were collected 6 h after the
occlusion of the coronary artery and homogenized in a standard
lysis buffer. Following centrifugation at 13,200 × g for 20 min at
4°C, the supernatant was collected and the protein concentration
was determined by a bicinchoninic acid assay (Beyotime Institute of
Biotechnology, Shanghai, China). Protein (30 μg) was separated on
8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels
and transferred to nitrocellulose membranes (Nanjing Jiancheng
Biotechnology Institute). The protein was detected using rabbit
anti-eNOS (1:1,000; Sigma-Aldrich) or mouse anti-GAPDH (1:2,000;
KangChen Bio-tech, Shanghai, China) and horseradish
peroxidase-conjugated goat anti-rabbit antibody (1:5,000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or goat anti-mouse
antibody (1:5,000; Santa Cruz Biotechnology, Inc.), respectively.
Detection of the labeled antigens was performed with an enhanced
chemiluminescence kit (Nanjing Jiancheng Biotechnology Institute).
Densitometric analysis was conducted using Quantity One 1-D
analysis software (Bio-Rad, Hercules, CA, USA).
Assay of plasma NO production levels
The blood samples were obtained 6 h after the
occlusion of the coronary artery and centrifuged at 2,000 × g for
20 min at 4°C. The plasma supernatant was collected and the nitrite
concentration was spectrophotometrically determined using Griess
reagent (Nanjing Jiancheng Biotechnology Institute; 1%
sulfanilamide and 0.1% naphthylethylenediamide in 5% phosphoric
acid). The absorbance was measured at 540 nm and the nitrite
concentration was calculated using sodium nitrite as a
standard.
Measurement of malondialdehyde (MDA) and
superoxide dismutase (SOD) levels
The myocardial supernatant was isolated by
centrifugation, at 3,000 × g for 10 min at 4°C, 6 h after the
occlusion of the coronary artery. The MDA levels in the supernatant
were determined by the measurement of thiobarbituric-acid reacting
substances with a commercial kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) according to the manufacturer’s
instructions. The SOD activity levels in the supernatant were
estimated in the heart homogenate by calculating the rate of the
inhibition of nucleotide oxidation using a Superoxide Dismutase
Detection kit (A001–3; Nanjing Jiancheng Bioengineering
Institute).
Detection of plasma 8-isoprostane
levels
The levels of 8-isoprostane in each group were
analyzed using an 8-Isoprostane EIA kit (no. 516351; Cayman
Chemical Company, Ann Arbor, MI, USA) according to the
manufacturer’s instructions. Briefly, the plasma samples were
hydrolyzed by adding 25 μl of 2 M NaOH to each 100 μl plasma sample
and the samples were then incubated at 45°C for 2 h. Following the
addition of 25 μl of 10 M HCl acid, the samples were centrifuged at
12,000 × g for 5 min. The supernatant was collected and the
measurement of 8-isoprostane was performed using the commercial
kit. The results were expressed as pg/ml of plasma.
Statistical analysis
Data were collected and are presented as the mean ±
standard deviation. Comparisons between different groups were
performed using one-way analysis of variance (ANOVA) followed by
Dunnett’s test. All statistical analyses were conducted using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
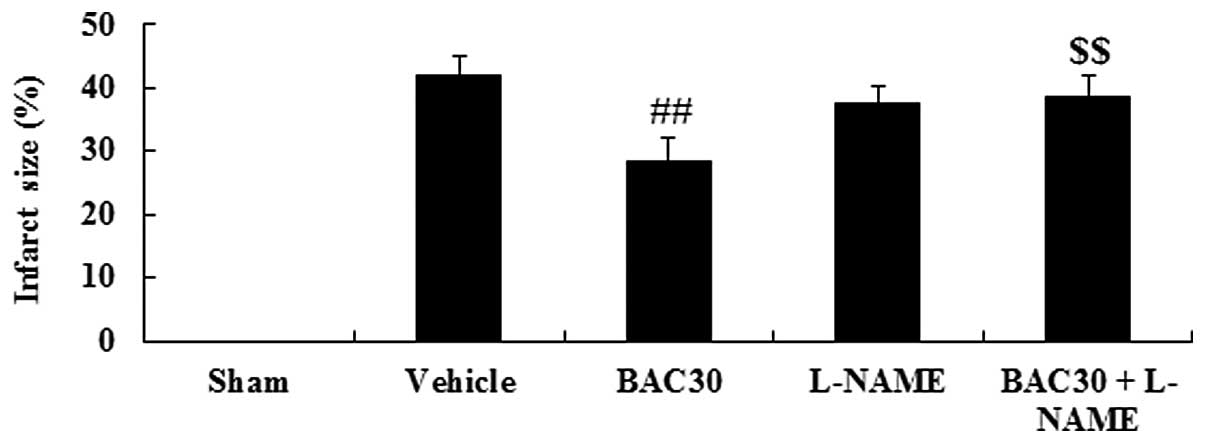
Baicalein reduces myocardial infarct size
in a rat model of acute myocardial infarction
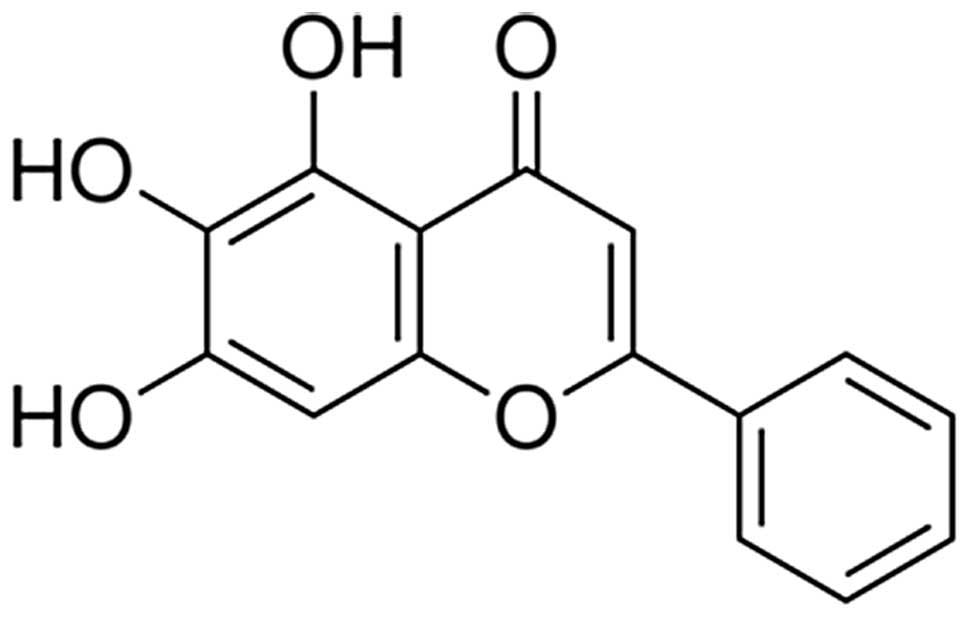
The chemical structure of baicalein is shown in
Fig. 1. As shown in Fig. 2, the infarction size in the
vehicle-treated infarcted group was 41.96±3.20%. However, the
baicalein pretreatment yielded an infarct size of 28.36±3.75%
(P<0.01, versus the vehicle group). Notably, when baicalein and
L-NAME, an inhibitor of NOS, were co-administered, the infarcted
area was significantly increased to 38.59±3.39% (P<0.01),
compared with that of the baicalein-treated group.
Baicalein inhibits plasma CK, CK-MB and
LDH activity and reduces cTnT levels in a rat model of acute
myocardial infarction
To investigate whether baicalein inhibited the
cardiomyocyte necrosis, the activity of plasma CK, CK-MB and LDH,
as well as the cTnT levels were determined at the end of the
surgery. As displayed in Fig. 3,
the levels of CK, CK-MB, LDH and cTnT in the vehicle-treated group
were markedly elevated (P<0.01), in comparison with those in the
sham group. Following preconditioning with baicalein, the specific
cardiac enzymes were all significantly decreased in the plasma
compared with those in the vehicle controls (P<0.01).
Furthermore, pretreatment with L-NAME completely inhibited the
baicalein-induced reduction in the levels of CK, CK-MB, LDH and
cTnT in the plasma of infarcted rats versus those in the baicalein
treatment group (P<0.01).
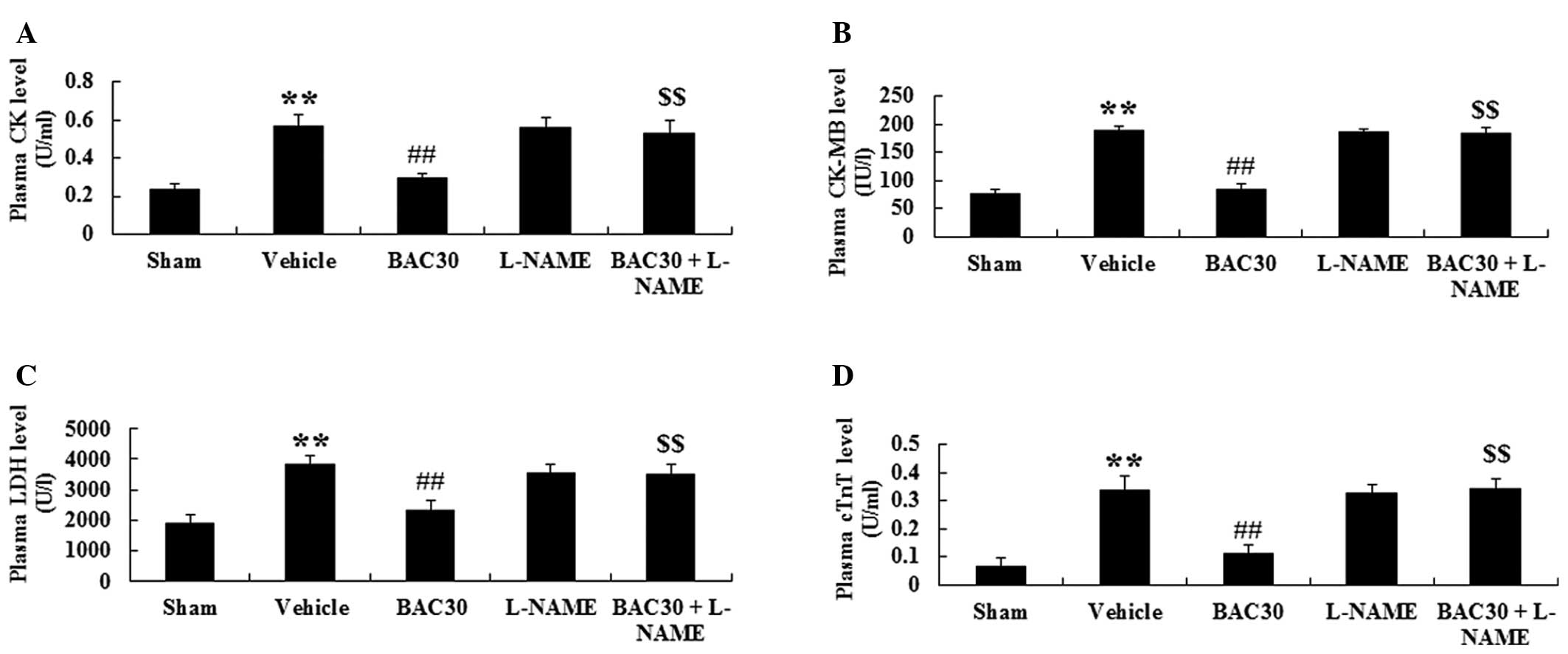 | Figure 3Baicalein inhibits plasma CK, CK-MB
and LDH activity and reduces cTnT levels in a rat model of acute
myocardial infarction (mean ± standard deviation; n=6). The plasma
(A) CK, (B) CK-MB and (C) LDH activity and (D) cTnT levels in the
different groups. **P<0.01 vs. the sham-surgery
group; ##P<0.01 vs. the vehicle-treated group and
$$P<0.01 vs. the baicalein-treated group. Sham,
sham-surgery; vehicle, vehicle-treated; BAC30, baicalein (30
mg/kg)-treated; L-NAME, L-NAME (10 mg/kg)-treated; and BAC30 +
L-NAME, co-administration of baicalein (30 mg/kg) and L-NAME (10
mg/kg)-treated groups. CK, creatine kinase; CK-MB, MB isoenzyme of
creatine kinase; LDH, lactate dehydrogenase; cTnT, cardiac troponin
T. |
Baicalein increases eNOS protein
expression levels and plasma NO concentration in a rat model of
acute myocardial infarction
Whether baicalein exerted protection against acute
myocardial infarction through mediating the eNOS signaling pathway
was further investigated. Fig. 4A
reveals that western blotting with the eNOS antibody produced
specific bands of 133 kDa. Following the one-way ANOVA, an evident
reduction in the levels of eNOS protein was observed in the
vehicle-treated group (P<0.01), versus those of the sham
control, as shown in Fig. 4B.
However, baicalein pretreatment caused a significant elevation in
the eNOS protein levels in the myocardial infarction-induced rats
(P<0.01), compared with those in the vehicle group.
Co-administration of baicalein and L-NAME completely inhibited the
increase in eNOS protein expression levels caused by baicalein
(P<0.01, n=6). In addition, the levels of NO production were
also evaluated. The levels of NO production, which were indicated
as the levels of nitrite formation, were reported to be
significantly reduced following the induction of acute myocardial
infarction (P<0.01). Baicalein pretreatment induced a marked
increase in the nitrite levels (P<0.01) compared with those in
the vehicle group; however, this elevation was suppressed in the
presence of L-NAME (P<0.01; Fig.
4C).
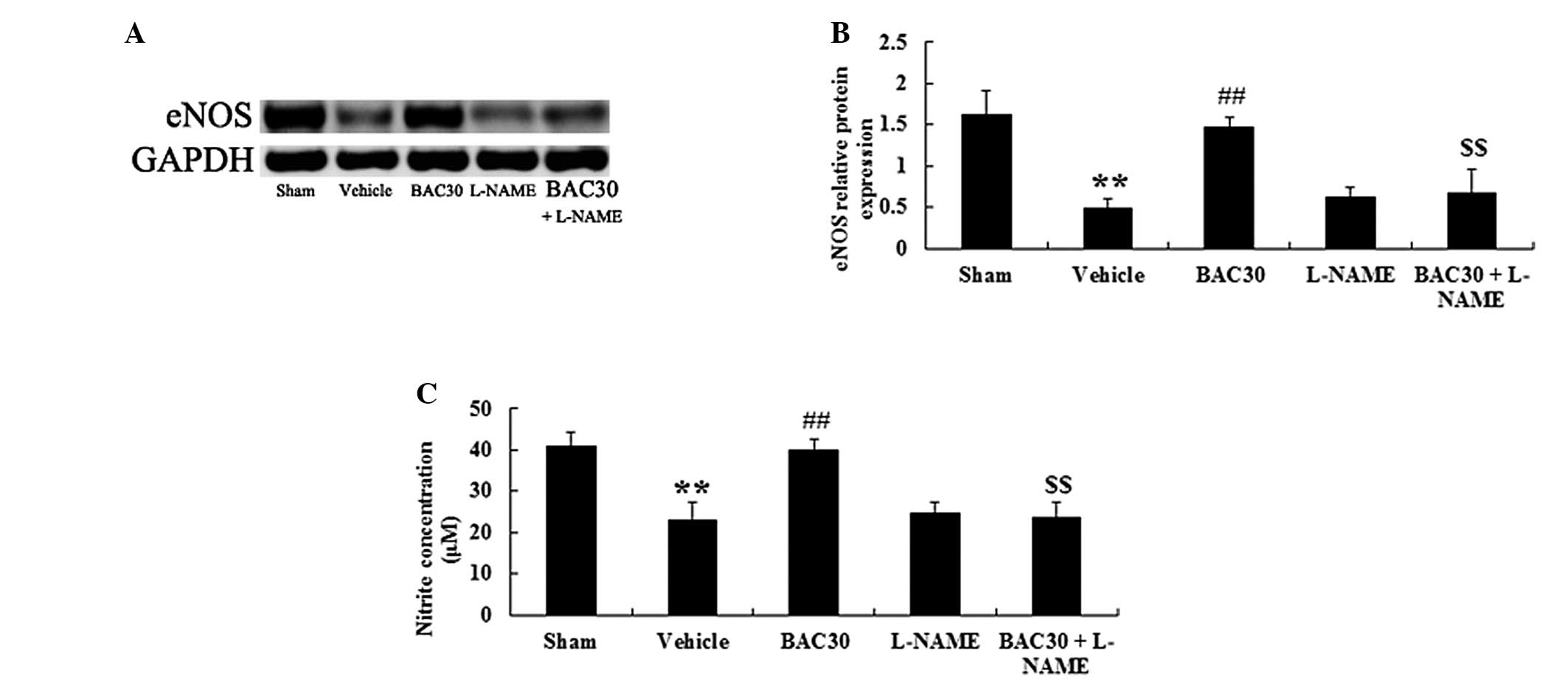 | Figure 4Baicalein increases the eNOS protein
expression levels and plasma NO concentration in a rat model of
acute myocardial infarction (mean ± standard deviation; n=6). (A)
Representative images of the immunoblots with antibodies against
eNOS in hearts of the rats from different groups. eNOS, 133 kDa and
GAPDH, 36 kDa. (B) Quantitative analysis of the protein levels of
eNOS in hearts of the rats from different groups. The data were
normalized to the loading control GAPDH. (C) NO production was
detected spectrophotometrically by measuring the levels of its
metabolite, nitrite. **P<0.01 vs. the sham-surgery
group; ##P<0.01 vs. the vehicle-treated group;
$$P<0.01 vs. the baicalein-treated group. Sham,
sham-surgery; vehicle, vehicle-treated; BAC30, baicalein (30
mg/kg)-treated; L-NAME, L-NAME (10 mg/kg)-treated; and BAC30 +
L-NAME, co-administration of baicalein (30 mg/kg) and L-NAME (10
mg/kg)-treated groups. eNOS, endothelial nitric oxide synthase; NO,
nitric oxide. |
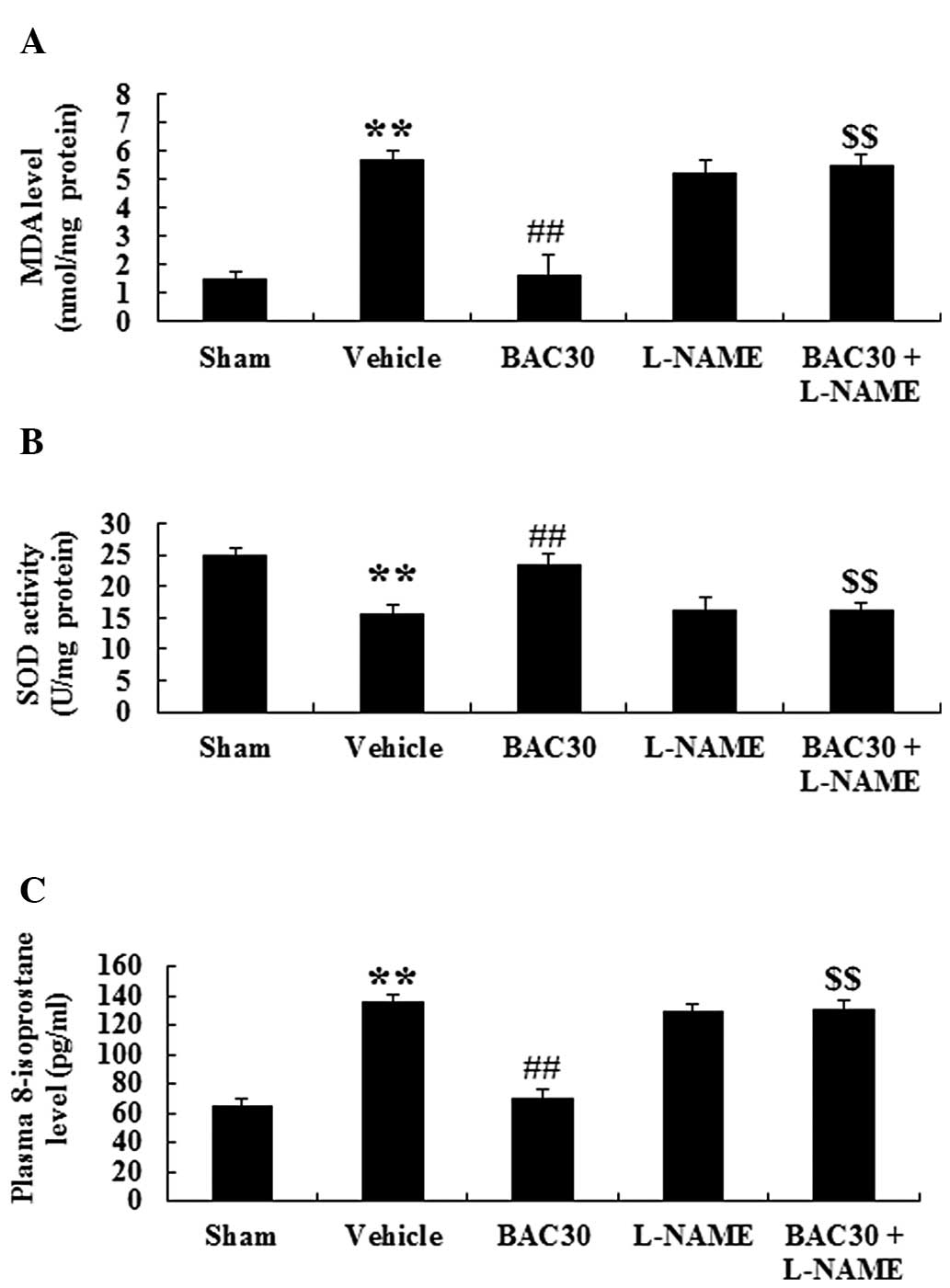
Baicalein inhibits oxidative stress in a
rat model of acute myocardial infarction via eNOS signaling
To examine the effect of baicalein on oxidative
stress during acute myocardial infarction, the MDA and SOD levels
were detected in the myocardium of different groups. Compared with
those of the sham group, the MDA content was markedly increased
while the SOD activity levels were reduced (P<0.01). Following
preconditioning with baicalein, the MDA levels were markedly
reduced (P<0.01) compared with those of the vehicle-treated
group. However, the reduced effect of MDA in the baicalein
treatment group was reversed by the administration of L-NAME
(Fig. 5A). By contrast, baicalein
pretreatment in the infarcted rats induced an evident increase in
the levels of SOD activity compared with those in the vehicle group
and this phenomenon was inhibited by L-NAME (Fig. 5B). The levels of 8-isoprostane, an
important index for evaluating oxidative damage (19), were also determined. As shown in
Fig. 5C, acute myocardial
infarction resulted in a marked augmentation in the levels of
8-isoprostane compared with those in the sham group (P<0.01,
n=6). Baicalein treatment at the dose of 30 mg/kg significantly
reduced the concentration of 8-isoprostane (P<0.01) compared
with that of the vehicle group. However, the suppression of eNOS
signaling by the inhibitor L-NAME completely abolished the
baicalein-induced reduction of 8-isoprostane content (P<0.01).
Collectively, baicalein pretreatment markedly attenuated the
oxidative stress following acute myocardial infarction and this
attenuation may be associated with eNOS signaling.
Discussion
The major findings in the present study demonstrated
that pretreatment with baicalein had the capacity to reduce infarct
size and the activity of specific cardiac enzymes (CK, CK-MB, LDH
and cTnT). The protective effects induced by baicalein
preconditioning may be associated with activation of eNOS and an
increase in NO bioavailability, together with inhibition of
oxidative stress. These protective effects were also abolished by
an eNOS inhibitor (L-NAME), suggesting that an eNOS-dependent
mechanism was involved in the baicalein-mediated
cardioprotection.
In the rat model of acute myocardial infarction in
the present study, notably the baicalein treatment markedly
diminished the infarction size and the levels of cardiac marker
enzymes (CK, CK-MB, LDH and cTnT). During ischemic insults, these
myocardial enzymes and protein are released into the blood and
their concentrations in the plasma directly reflect the extent of
the myocardial injury. Therefore, CK, CK-MB, LDH and cTnT in the
plasma are sensitive biomarkers for assessing cardiac injury
(11). Previous studies have shown
that the infarction size and the levels of myocardial specific
enzymes, including CK, CK-MB and LDH, were all markedly elevated in
rats subjected to acute myocardial infarction (20,21).
cTnT is a contractile protein, which remains at low levels but is
markedly augmented during myocardial necrosis or cell death
(22). In line with the results of
previous studies, the present study demonstrated elevations in
infarct size and the levels of myocardial specific enzymes (CK,
CK-MB, LDH and cTnT) in the infarcted rats compared with those in
the sham group. However, they were all markedly decreased following
baicalein administration, suggesting the cardioprotective effect of
baicalein against acute myocardial infarction. Notably, the
attenuation of cardiac damage was completely inhibited following
co-treatment with baicalein and an eNOS inhibitor (L-NAME). This
implies that baicalein prevents cardiomyocytes from injury during
acute myocardial infarction, at least in part, via mediating eNOS
signaling.
NO is regarded as one of the major regulators during
myocardial damage. A prior study revealed that eNOS-derived NO
exerts protection against myocardium injury caused by myocardial
ischemia-reperfusion (7). In
addition, it has been demonstrated that treatment with statins
significantly attenuates the impairment of ischemia-reperfusion
during myocardial infarction, via eNOS-mediated signaling pathways
with increased NO production (23). Consistent with the results of
previous studies, the present study demonstrated that the levels of
protein expression and NO production were evidently reduced in the
infarcted rats. Administration of baicalein markedly augmented the
protein levels of eNOS and promoted NO generation in the myocardial
infarction model. More importantly, baicalein-induced increases in
the levels of eNOS and NO production in the infarcted rats were
completely inhibited by the presence of an eNOS inhibitor (L-NAME).
These findings suggest that baicalein exerted protective effects
against acute myocardial infarction by activating eNOS and
concomitantly increasing NO bioavailability.
Oxidative stress has also been implicated in the
pathogenesis of cardiac dysfunction and the death of
cardiomyocytes. Excessively produced oxygen free radicals attack
the fatty acids within the myocardial membranes and cause the
release of lipid peroxides (11).
Accumulation of lipid hydroperoxides directly reflects the damage
of the cardiac constituents. MDA is a major lipid peroxidation end
product and an increased MDA concentration has been demonstrated to
facilitate the generation of free radicals and inhibit the activity
of the antioxidant defense system (24). SOD is conceived of as the first
line of cell defense against oxidative stress, which functions by
eliminating reactive oxygen radicals, including superoxide and
hydrogen peroxide, and preventing the generation of more hydroxyl
radicals. In the present study, acute myocardial infarction
resulted in a marked elevation in the MDA content and reduced the
SOD activity levels compared with those in the sham group,
indicating the generation of oxidative stress. The levels of
8-isoprostane, a cardiac marker of oxidative stress, were also
identified as evidently increased in the infarcted rats compared
with those in the sham group and this finding confirmed the
production of oxidative stress during acute myocardial infarction.
Baicalein pretreatment induced significant reductions in the levels
of MDA and 8-isoprostane, while increasing those of SOD activity,
which is attributable to its potent anti-oxidative property. In
agreement with the findings of the present study, a previous study
reported that baicalein preconditioning protected cardiomyocytes
from oxidative damage in a chick embryonic model of
ischemia-reperfusion injury in vitro (25). Furthermore, the present study also
identified that inhibition of eNOS by L-NAME significantly
prevented the baicalein-mediated protection against oxidative
damage, implying the involvement of eNOS signaling in the
attenuation of oxidative stress by baicalein pretreatment following
acute myocardial infarction.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that baicalein, a
natural occurring flavonoid, has potent cardioprotective effects in
rats with acute myocardial infarction, and that the
cardioprotective effects may be associated with activation of eNOS
signaling and inhibition of oxidative stress. Furthermore, the
inhibition of oxidative stress by baicalein treatment is
eNOS-dependent. These findings support the use of baicalein as a
promising cardioprotective agent for the prevention or treatment of
myocardial injury induced by acute myocardial infarction. This
study also suggests that eNOS signaling may serve as a potential
therapeutic target in ischemic heart disease in the future.
References
|
1
|
Bulhak AA, Sjöquist PO, Xu CB, Edvinsson L
and Pernow J: Protection against myocardial ischaemia/reperfusion
injury by PPAR-alpha activation is related to production of nitric
oxide and endothelin-1. Basic Res Cardiol. 101:244–252. 2006.
View Article : Google Scholar
|
|
2
|
Gourine AV, Bulhak AA, Gonon AT, Pernow J
and Sjöquist PO: Cardioprotective effect induced by brief exposure
to nitric oxide before myocardial ischemia-reperfusion in vivo.
Nitric Oxide. 7:210–216. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Andelová E, Barteková M, Pancza D, Styk J
and Ravingerová T: The role of NO in ischemia/reperfusion injury in
isolated rat heart. Gen Physiol Biophys. 24:411–426.
2005.PubMed/NCBI
|
|
4
|
Balligand JL and Cannon PJ: Nitric oxide
synthases and cardiac muscle. Autocrine and paracrine influences.
Arterioscler Thromb Vasc Biol. 17:1846–1858. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones SP, Greer JJ, van Haperen R, Duncker
DJ, de Crom R and Lefer DJ: Endothelial nitric oxide synthase
overexpression attenuates congestive heart failure in mice. Proc
Natl Acad Sci USA. 100:4891–4896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sumeray MS, Rees DD and Yellon DM: Infarct
size and nitric oxide synthase in murine myocardium. J Mol Cell
Cardiol. 32:35–42. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones SP, Girod WG, Palazzo AJ, et al:
Myocardial ischemia-reperfusion injury is exacerbated in absence of
endothelial cell nitric oxide synthase. Am J Physiol.
276:H1567–H1573. 1999.PubMed/NCBI
|
|
8
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collard CD and Gelman S: Pathophysiology,
clinical manifestations, and prevention of ischemia-reperfusion
injury. Anesthesiology. 94:1133–1138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Panda VS and Naik SR: Cardioprotective
activity of Ginkgo biloba Phytosomes in
isoproterenol-induced myocardial necrosis in rats: a biochemical
and histoarchitectural evaluation. Exp Toxicol Pathol. 60:397–404.
2008.
|
|
11
|
Priscilla DH and Prince PS:
Cardioprotective effect of gallic acid on cardiac troponin-T,
cardiac marker enzymes, lipid peroxidation products and
antioxidants in experimentally induced myocardial infarction in
Wistar rats. Chem Biol Interact. 179:118–124. 2009. View Article : Google Scholar
|
|
12
|
Lin CC and Shieh DE: The anti-inflammatory
activity of Scutellaria rivularis extracts and its active
components, baicalin, baicalein and wogonin. Am J Chin Med.
24:31–36. 1996.
|
|
13
|
Gabrielska J, Oszmiański J, Zyłka R and
Komorowska M: Antioxidant activity of flavones from Scutellaria
baicalensis in lecithin liposomes. Z Naturforsch C. 52:817–823.
1997.
|
|
14
|
Shao ZH, Vanden Hoek TL, Qin Y, et al:
Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol
Heart Circ Physiol. 282:H999–H1006. 2002.PubMed/NCBI
|
|
15
|
Woo AY, Cheng CH and Waye MM: Baicalein
protects rat cardiomyocytes from hypoxia/reoxygenation damage via a
prooxidant mechanism. Cardiovasc Res. 65:244–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu W, Liu Q and Zhu S: Carvacrol protects
against acute myocardial infarction of rats via anti-oxidative and
anti-apoptotic pathways. Biol Pharm Bull. 36:579–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo CP, Wen LL, Chen CM, et al:
Attenuation of neurological injury with early baicalein treatment
following subarachnoid hemorrhage in rats. J Neurosurg.
199:1028–1037. 2013.PubMed/NCBI
|
|
18
|
Maslov LN, Lishmanov YB, Oeltgen PR, et
al: Activation of peripheral delta2 opioid receptors increases
cardiac tolerance to ischemia/reperfusion injury: Involvement of
protein kinase C, NO-synthase, KATP channels and the autonomic
nervous system. Life Sci. 84:657–663. 2009. View Article : Google Scholar
|
|
19
|
Papazzo A, Conlan X, Lexis L and
Lewandowski P: The effect of short-term canola oil ingestion on
oxidative stress in the vasculature of stroke-prone spontaneously
hypertensive rats. Lipids Health Dis. 10:1802011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo J, Li HZ, Wang LC, et al: Increased
expression of calcium-sensing receptors in atherosclerosis confers
hypersensitivity to acute myocardial infarction in rats. Mol Cell
Biochem. 366:345–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ming X, Tongshen W, Delin W and Ronghua Z:
Cardioprotective effect of the compound yangshen granule in rat
models with acute myocardial infarction. Evid Based Complement
Alternat Med. 2012:7171232012.PubMed/NCBI
|
|
22
|
Katus HA, Remppis A, Scheffold T,
Diederich KW and Kuebler W: Intracellular compartmentation of
cardiac troponin T and its release kinetics in patients with
reperfused and nonreperfused myocardial infarction. Am J Cardiol.
67:1360–1367. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wolfrum S, Dendorfer A, Schutt M, et al:
Simvastatin acutely reduces myocardial reperfusion injury in vivo
by activating the phosphatidylinositide 3-kinase/Akt pathway. J
Cardiovasc Pharmacol. 44:348–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pipaliya H and Vaghasiya J: Cardio
protective effect of vitamin A against isoproterenol-induced
myocardial infarction. J Nutr Sci Vitaminol (Tokyo). 58:402–407.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang WT, Li J, Vanden Hoek MS, et al:
Baicalein preconditioning protects cardiomyocytes from
ischemia-reperfusion injury via mitochondrial oxidant signaling. Am
J Chin Med. 41:315–331. 2013. View Article : Google Scholar : PubMed/NCBI
|