Introduction
Type 2 diabetes mellitus (T2DM) is characterized by
beta-cell dysfunction and inadequate insulin release in response to
glucose (1,2). In recent years, due to socioeconomic
developments and the improvement of living standards, the incidence
and prevalence of T2DM is increasing annually. It is becoming a
major social issue, which threatens the lives and health of the
public. Thus, for the effective prevention of T2DM, an in-depth
discussion on the etiology and risk factors associated with T2DM is
considered to be of significance.
Recent studies have elucidated the effects of
glucose or free fatty acids (FFA) on insulin secretion and content
(3,4). Previous studies have reported that
high glucose and FFA may cause impairment of pancreatic beta cells
(5–8). In comparison with glucose or FFA, few
studies have investigated the effect of amino acids on insulin
secretion and content. Leucine, a branched chain amino acid, has
been reported to be closely associated with insulin secretion and
content (9–11). Previous studies have demonstrated
that long-term leucine culture decreases glucose-stimulated insulin
secretion (GSIS) in vitro (11). However, whether this detrimental
effect is reversible, and the underlying mechanism of the effect,
remain unclear.
Pancreatic/duodenal homeobox-1 (PDX-1) is important
in pancreatic beta cell differentiation and insulin secretion
(12–14). It transactivates the expression of
insulin as well as other beta cell-specific genes, including
glucose transporter 2 (GLUT2) (15–18).
Previous studies reported that PDX-1 gene disruption resulted in
aplasia (12,19) and overexpression of PDX-1 restored
beta cell function (20). Numerous
studies reported that prolonged exposure to glucose or FFA induced
a marked decrease in PDX-1 expression, which depressed the activity
of GLUT2 and was associated with a decline in insulin secretion and
content (21–23). However, it remains unclear whether
the effects of leucine on insulin secretion and content are
accompanied by alterations in PDX-1 and GLUT2.
The aim of the present study was to estimate the
effects of sustained leucine exposure on insulin secretion, insulin
content and the protein expression levels of PDX-1, as well as its
downstream target, GLUT2 in INS-1 cells. Furthermore, whether these
effects are reversible or not, following the removal of high
concentrations of leucine, was investigated.
Materials and methods
Cell culture and treatment
INS-1 (rat insulinoma cell line) cells were grown in
a monolayer culture of RPMI-1640 media containing 11.1 mmol/l
glucose supplemented with 10 mmol/l
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10% fetal
bovine serum, 1 mmol/l sodium pyruvate, 2 mmol/l L-glutamine, 50
μmol/l β-mercaptoethanol, 100 IU/ml penicillin and 100 μg/ml
streptomycin at 37°C in a humidified atmosphere with 5%
CO2 (4). The cells
(passages <40) were employed for the experiment at 80–90%
confluence. The INS-1 cells were treated in the presence or absence
of elevated concentrations of leucine (10, 20 or 40 mmol/l) for 24
h.
Insulin secretion and insulin content
assays
INS-1 cells (1.5×105 cells/well) were
firstly incubated overnight in 24-well plates (Corning
Incorporated, Corning, NY, USA) with standard media (no leucine)
and allowed to attach and treated in the presence or absence of 40
mmol/l leucine for 24 h. Secondly, half of the plates that were
cultured in the presence or absence of leucine were used to
determine insulin secretion. The cells in the remaining plates were
incubated further and the cells that received the leucine
pretreatment were divided into two groups: One was maintained in a
media containing leucine and the other was cultured in a
leucine-free media. Following a further 24-h culture, insulin
secretion was determined by an insulin radioimmunoassay (RIA) kit
(Beijing Atom HighTech Co. Ltd., Beijing, China). For insulin
secretion, the cells were gently washed twice with prewarmed
phosphate-buffered saline (PBS) and incubated in prewarmed
Krebs-Ringer bicarbonate buffer (KRB) containing 3 mmol/l glucose
for 20 min at 37°C. The buffer was removed and half of the plates
were cultured in KRB containing 3 mmol/l glucose and the remaining
half were incubated in KRB containing 27.8 mmol/l glucose.
Following an additional 20 min incubation at 37°C, aliquots of the
medium were collected from each well and stored at −20°C for a
subsequent insulin secretion test with the RIA kit. To measure the
protein content, the cells were washed twice with KRB and RIPA
lysis buffer was added (Shenneng Bo Cai Co. Ltd, Shanghai, China).
The intracellular protein concentration was determined using a
bicinchoninic acid (BCA) protein assay kit (Bio-Rad, Hercules, CA,
USA). Insulin secretion was normalized based on the corresponding
protein content.
In order to test the insulin content, 500 μl
acid/ethanol (75% v/v ethanol, 1.5% v/v concentrated HCl) was added
to the plates. The acid ethanol aliquots were used to measure
insulin content with the RIA kit. The total protein content was
determined as described above and the insulin content was
normalized based on the respective cellular protein content.
Protein analysis by western blotting
The cultured INS-1 cells were washed twice with
ice-cold PBS and the cells were lysed using RIPA lysis buffer
supplemented with 1 mmol/l phenylmethylsulfonyl fluoride on ice for
10 min. The lysate solution was centrifuged (Eppendorf, Hamburg,
Germany) at 10,000 × g for 10 min at 4°C. The protein concentration
was determined by a BCA assay. The protein extracts (60 μg total
protein for GLUT2 and 40 μg protein for PDX-1) were separated by
10% and 12% SDS-PAGE for GLUT2 and PDX-1, respectively. The
proteins in the gel were subsequently transferred onto
nitrocellulose membranes (Millipore, Billerica, MA, USA). The
membranes were washed once with 1X TBST (10 mmol/l Tris, 150 mmol/l
NaCl and 0.1% Tween 20) prior to blocking with 5% non-fat milk at
room temperature for 1 h. The membranes were gently agitated
overnight (Biocotek Scientific Instrument Co., Ningbo, China) at
4°C with 1:10,000 PDX-1 antibody (Chemicon, NY, USA) or 1:200 GLUT2
antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The
membranes were washed three times (3×10 min) with 1X TBST and
incubated with the corresponding second antibodies at room
temperature for 1 h. Finally, the proteins were visualized by
enhanced chemiluminescence (Amersham Biosciences UK Limited,
Amersham, England). The membranes were reblocked and incubated with
mouse anti-β-actin monoclonal antibody (Abcam, Cambridge, UK).
Immunofluorescence
The location and expression of PDX-1, insulin and
GLUT2 were examined by immunofluorescence. INS-1 cells were plated
on polyornithine-coated glass coverslips for 24 h. Rabbit
anti-PDX-1 (Santa Cruz Biotechnology, Inc.), mouse anti-insulin
(DakoCytomation, Glostrup, Denmark) and rabbit anti-GLUT2
antibodies (Santa Cruz Biotechnology, Inc.) were used for the
experiment. The resultant immunofluorescence was viewed under a
fluorescent microscope (Leica DMIRE2; Leica Microsystems GmbH,
Wetzlar, Germany).
Statistical analysis
All values are provided as the mean ± standard
deviation. Statistical analysis was calculated using a one-way
analysis of variance and P<0.05 was considered to indicate a
statistically significant difference.
Results
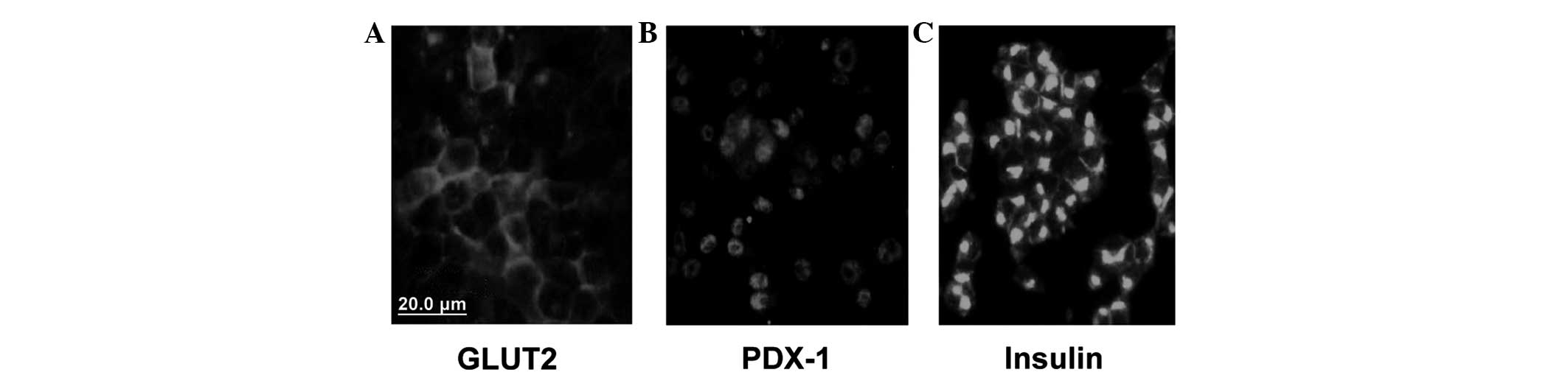
Expression of GLUT2, PDX-1 and insulin in
INS-1 cells
GLUT2, PDX-1 and insulin were all expressed in the
INS-1 cells. GLUT2 was predominantly expressed in the cell membrane
(Fig. 1A), while PDX-1 and insulin
were predominantly expressed in the cytoplasm (Fig. 1B and C).
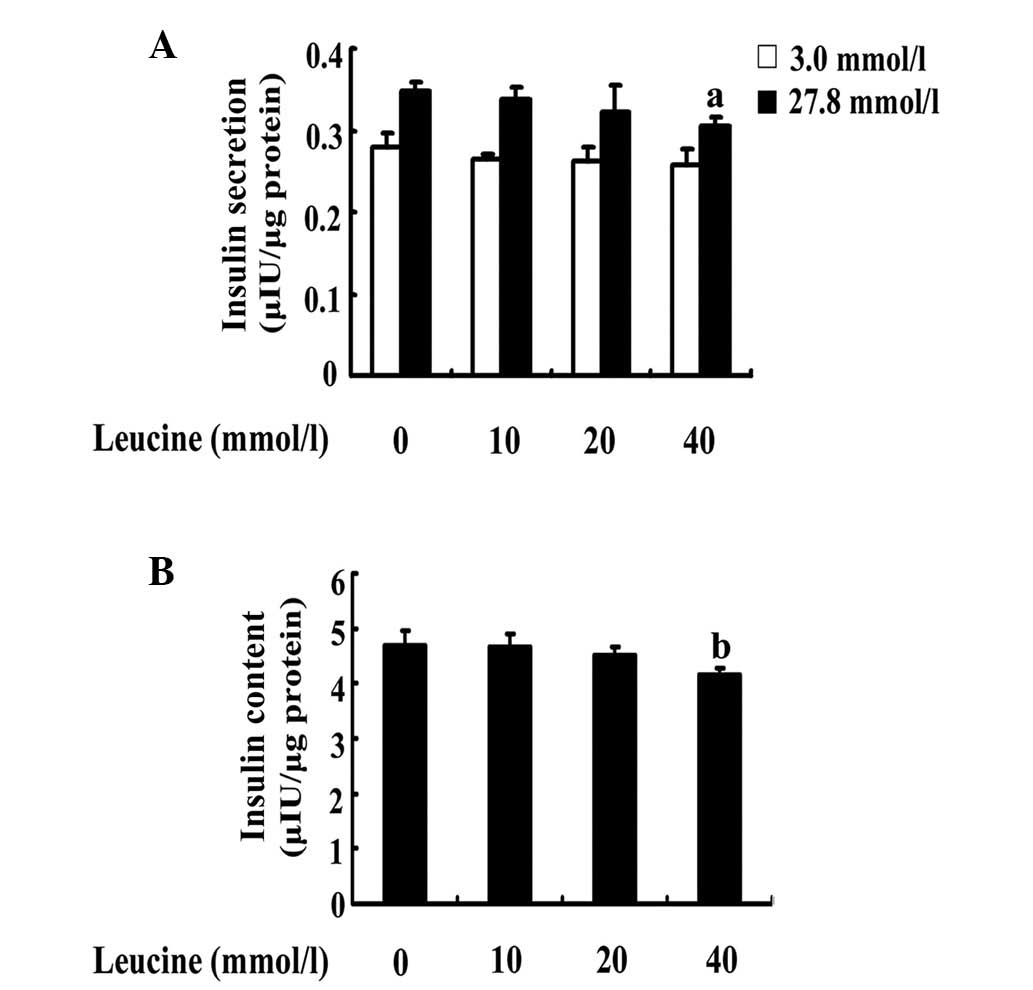
Effects of increasing concentrations of
leucine on insulin secretion and content
In order to investigate the effects of leucine on
insulin secretion and content, INS-1 cells were cultured in a
medium in the presence or absence of increasing concentrations of
leucine for 24 h. The cells were used to determine insulin
secretion and intracellular insulin content at low and high levels
of glucose stimulation. The results demonstrated that 24-h
incubation with increasing concentrations of leucine led to a
decrease of high GSIS and insulin content; the effect was
significant at 40 mmol/l leucine. In contrast to the control, a
40-mmol/l leucine treatment decreased high GSIS by 11% (P=0.026;
Fig. 2A) and insulin content by
14% (P=0.008; Fig. 2B), however,
it did not affect the insulin secretion level at low glucose
stimulation (P=0.01; Fig. 2A). In
contrast to the control, neither 10 nor 20 mmol/l leucine produced
a significant decrease in insulin secretion (P=0.645 and P=0.250;
Fig. 2A) and insulin content
(P=0.870 and P=0.279; Fig.
2B).
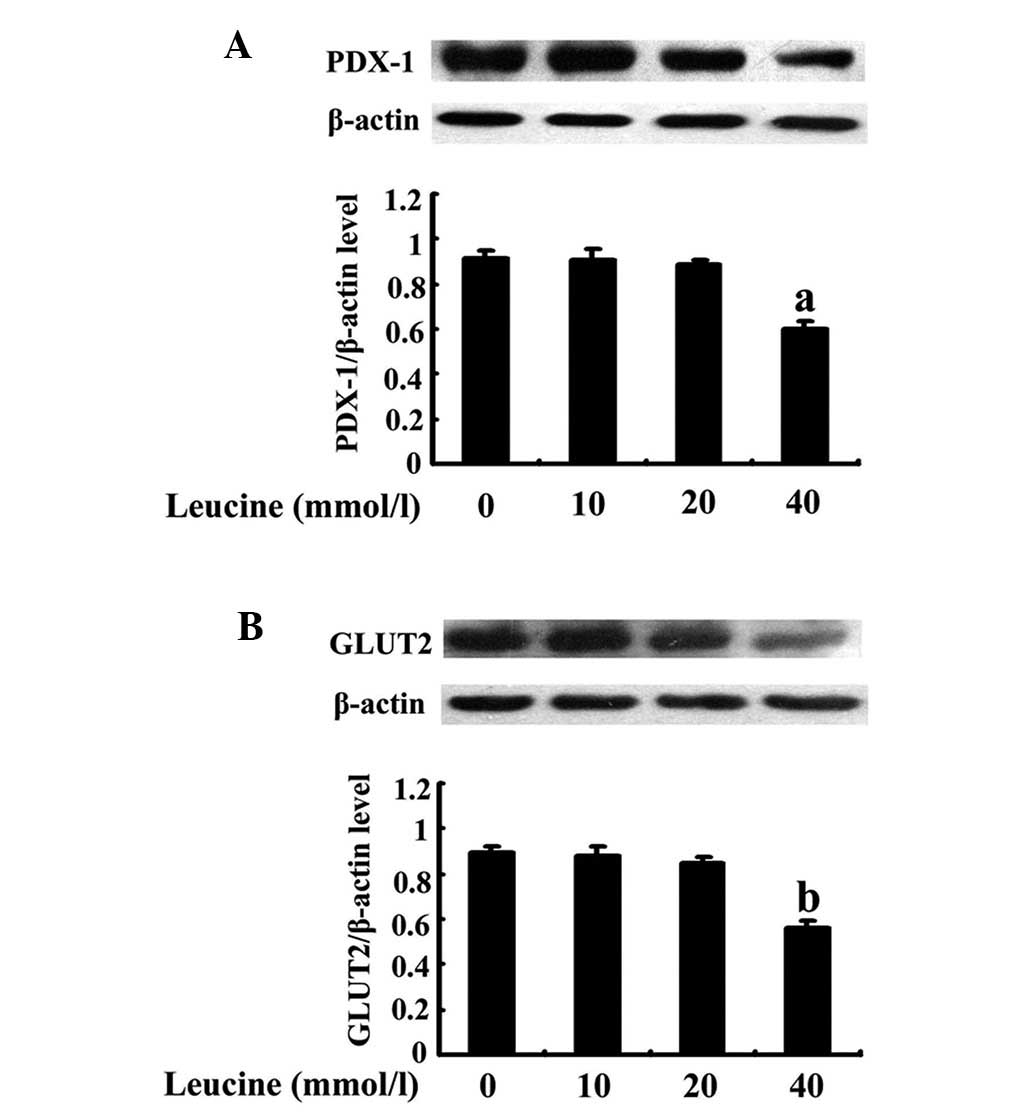
Effects of increasing concentrations of
leucine on PDX-1 and GLUT2 expression
The cells were treated as previously described. The
PDX-1 protein band became weaker with increasing concentration and
the effect was significant with a 40-mmol/l leucine treatment
(P=0.013; Fig. 3A). Similarly,
GLUT2 demonstrated the same trend as PDX-1 and the weakest band was
observed in cells that were treated with 40 mmol/l leucine
(P=0.011; Fig. 3B).
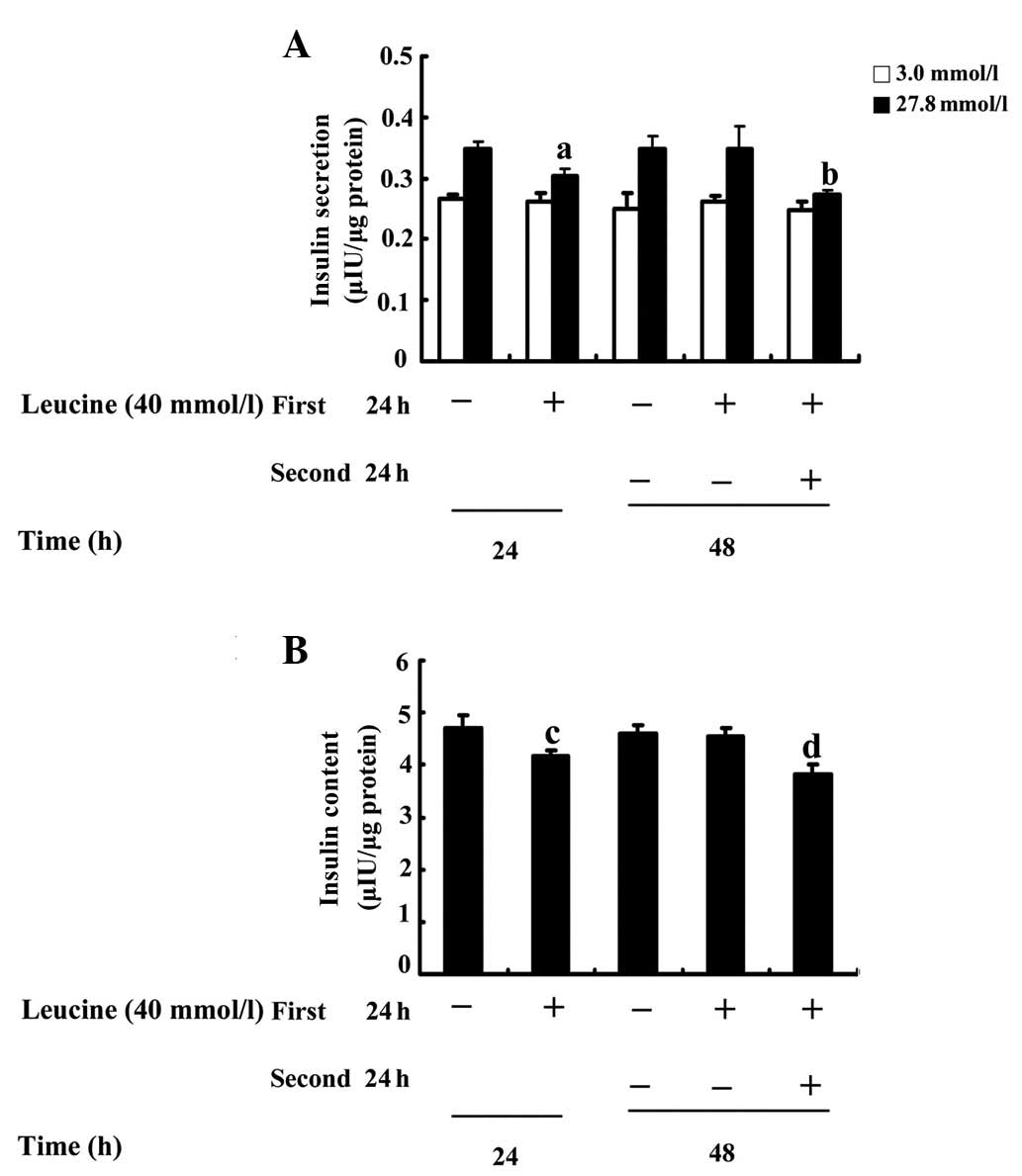
Effects on insulin secretion and content
of a 24-h recovery in a standard medium
To investigate whether GSIS and insulin content is
recoverable following the removal of a high concentration of
leucine, INS-1 cells were firstly treated in the absence or
presence of 40 mmol/l leucine for 24 h and the cells that had
received a leucine pretreatment were divided into two groups: One
was maintained in a medium containing leucine and the other was
cultured in a standard medium for a further 24 h. The results
indicated that, in contrast to the corresponding control, a
40-mmol/l leucine treatment for 24 h decreased GSIS at high glucose
concentrations by 11% (P=0.026; Fig.
4A) and insulin content by 14% (P=0.008; Fig. 4B). In addition, a 40-mmol/l leucine
treatment for 48 h decreased GSIS at high glucose concentrations by
22% (P=0.003; Fig. 4A) and insulin
content by 20% (P=0.002; Fig. 4B).
When leucine was removed from the media and the cells were
incubated for an additional 24 h, the high GSIS was increased by
13% (P=0.032; Fig. 4A) and 27%
(P=0.002; Fig. 4A) and insulin
content was augmented by 10% (P=0.014; Fig. 4B) and 20% (P=0.003; Fig. 4B) compared with those in the cells
that were maintained in the leucine treatment for 24 or 48 h,
respectively.
Effects on PDX-1 and GLUT2 expression of
a 24 h recovery in standard medium
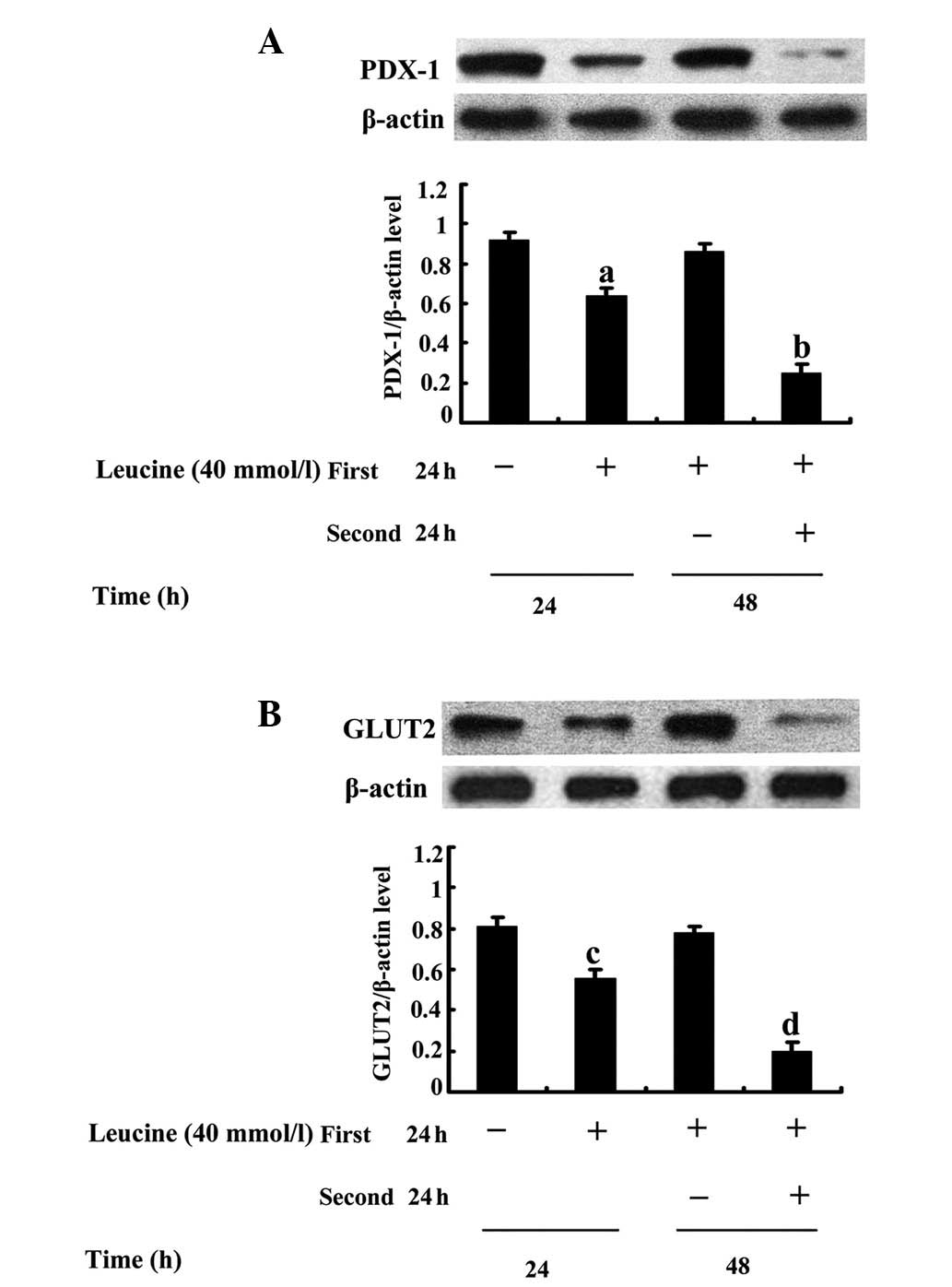
The results demonstrated that in contrast to the
control, a 24-h leucine treatment decreased PDX-1 (Fig. 5A) and GLUT2 (Fig. 5B) protein expression and the
effects were statistically significant in cells that were treated
with leucine for 48 h. The reduced PDX-1 and GLUT2 protein
expression, which was induced by a 24-h leucine treatment almost
recovered to normal in cells that were initially treated with
leucine for 24 h and subsequently allowed a 24-h recovery in
standard medium (P=0.013, Fig. 5A;
P=0.015, Fig. 5B). In comparison
with cells that were maintained in the leucine treatment for 48 h,
the PDX-1 and GLUT2 protein bands in the cells with a 24-h recovery
in standard medium were significantly strengthened (P=0.005,
Fig. 5A; P=0.006, Fig. 5B).
Discussion
In the present study, it was demonstrated that a
24-h incubation of elevated-concentration leucine treatment
resulted in a dose-dependent decrease of GSIS and intracellular
insulin content in INS-1 cells, accompanied by an impaired protein
expression of PDX-1 and its downstream target, GLUT2. It was
identified that in the INS-1 cells, which were able to recover for
a further 24 h in a standard medium following the 24-h high leucine
incubation, the impaired protein expression of PDX-1 and GLUT2 was
completely reversed.
With regard to the effects of leucine on GSIS,
Anello et al (9) reported
that leucine decreased GSIS in a dose-dependent manner and 20
mmol/l leucine significantly reduced GSIS in islets. The present
study demonstrated that a 24-h incubation with increasing
concentrations of leucine decreased high GSIS in a dose-dependent
manner and the effect was significant at 40 mmol/l leucine.
Furthermore, elevated concentrations of leucine decreased the
insulin content in a dose-dependent manner, which further verified
the results. The results were partially in agreement with the study
by Anello et al (9) and the
disparity may be due to different experimental conditions,
including the type of cells. Furthermore, additional studies were
found that used 40 mmol/l leucine (24–26).
In our previous study, it was identified via CCK-8 assay that 40 mM
leucine did not significantly inhibit cell viability, compared with
corresponding controls, in INS-1 cells (27). Although data resulting from high
leucine concentrations have limited physiological significance, the
high concentration may alter pancreatic beta cell function.
Furthermore, the data may provide insight into the signaling
pathway of gene regulation in beta cells, including PDX-1, GCK and
GLUT2, which are important in beta cell function and in the
development of diabetes.
The role of PDX-1 in pancreatic beta-cell insulin
secretion is due to its effect on transactivating the expression of
insulin and other beta cell-specific genes, including GCK and
GLUT2. That is to say, either PDX-1 or GLUT2 is closely correlated
with insulin and may, therefore, be an index of insulin
measurement. Numerous studies in vitro and in vivo
indicated that prolonged exposure to glucose or FFA may induce a
marked decrease in PDX-1 expression (21,22,23).
However, to the best of our knowledge, a study that focuses on the
effect of leucine on PDX-1 has not yet been reported. In the
present study, it was demonstrated that prolonged exposure to
leucine downregulated the protein expression of PDX-1 and its
downstream target, GLUT2, in INS-1 cells indicating a possible role
of leucine in PDX-1 protein expression. These data indicate that
sustained exposure to leucine decreased high GSIS and insulin
content, which was accompanied by the impairment of PDX-1 and GLUT2
protein expression. Furthermore, variations in PDX-1 and GLUT2
protein expression were closely correlated with the leucine
concentration. The concordant changes of PDX-1 and insulin content
observed in the present study were in agreement with the results
from Sun et al (3).
An additional finding of the present study was that
all of the detrimental effects associated with decreased GSIS,
insulin content, PDX-1 and GLUT2 protein expression were completely
recovered to normal once INS-1 cells were allowed a 24-h recovery
in standard medium following the 24-h exposure to leucine. The
detrimental effects that occurred in the cells, which were treated
with leucine continuously for 48 h, were more serious compared with
those observed in cells that had undergone a 24-h leucine
incubation. All results demonstrated that 40 mmol/l leucine
impaired GSIS and insulin content, which accompanied PDX-1 and
GLUT2 protein alterations. It appeared that the impairment of GSIS,
PDX-1 and GLUT2 protein expression that was induced by the
sustained leucine exposure was reversible. At present, the
environment is considered to be an independent factor for the
pathogenesis of diabetes. From the present study, it was
hypothesized that eliminating harmful environmental factors may
enable the recovery of the impaired insulin secretion levels.
However, it remains unclear whether the effect of sustained leucine
exposure on PDX-1 is direct or indirect. Furthermore, whether
leucine requires metabolization for its effect requires further
investigation, which could be conducted via a comparison between
leucine and nonmetabolized leucine analog
2-amino-2-norbornane-carboxylic acid.
In conclusion, the present study demonstrates that
sustained high concentrations of leucine exposure for 24 h induces
the reversible impairment of high GSIS, insulin content, PDX-1 and
GLUT2 expression in INS-1 cells. At present, environmental factors
are deemed independent. The present study demonstrates a novel
approach to the prevention and treatment of diabetes by reducing
high concentrations of amino acid and mediating the PDX-1 and GLUT2
signaling pathway.
Acknowledgements
The authors would like to thank Professor X Han for
providing the INS-1 cells and the teachers at the Science Center of
Shandong Provincial Hospital (Jinan, China) for their technical
assistance. The present study was funded by grants from the
National Natural Science Foundation of China (grant no. 81000325),
the Excellent Young Scientist Award Foundation of Shandong Province
(grant no. BS2010YY050) and the Natural Science Foundation of
Shandong Province (grant no. ZR2009CM101).
References
|
1
|
DeFronzo RA, Bonadonna RC and Ferrannini
E: Pathogenesis of NIDDM. A balanced overview. Diabetes Care.
15:318–368. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Porte D Jr and Kahn SE: beta-cell
dysfunction and failure in type 2 diabetes: potential mechanisms.
Diabetes. 50(Suppl 1): S160–S163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun Y, Zhang L, Gu HF, et al: Peroxisome
proliferator-activated receptor-alpha regulates the expression of
pancreatic/duodenal homeobox-1 in rat insulinoma (INS-1) cells and
ameliorates glucose-induced insulin secretion impaired by
palmitate. Endocrinology. 149:662–671. 2008. View Article : Google Scholar
|
|
4
|
Van de Casteele M, Kefas BA, Cai Y, et al:
Prolonged culture in low glucose induces apoptosis of rat
pancreatic beta-cells through induction of c-myc. Biochem Biophys
Res Commun. 312:937–944. 2003.PubMed/NCBI
|
|
5
|
Unger RH and Zhou YT: Lipotoxicity of
beta-cells in obesity and in other causes of fatty acid spillover.
Diabetes. 50(Suppl 1): S118–S121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ling Z and Pipeleers DG: Prolonged
exposure of human beta cells to elevated glucose levels results in
sustained cellular activation leading to a loss of glucose
regulation. J Clin Invest. 98:2805–2812. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Briaud I, Harmon JS, Kelpe CL, Segu VB and
Poitout V: Lipotoxicity of the pancreatic beta-cell is associated
with glucose-dependent esterification of fatty acids into neutral
lipids. Diabetes. 50:315–321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robertson RP, Harmon J, Tran PO and
Poitout V: Beta-cell glucose toxicity, lipotoxicity, and chronic
oxidative stress in type 2 diabetes. Diabetes. 53(Suppl 1):
S119–S124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anello M, Ucciardello V, Piro S, et al:
Chronic exposure to high leucine impairs glucose-induced insulin
release by lowering the ATP-to-ADP ratio. Am J Physiol Endocrinol
Metab. 281:E1082–E1087. 2001.PubMed/NCBI
|
|
10
|
Yang J, Wong RK, Park M, et al: Leucine
regulation of glucokinase and ATP synthase sensitizes
glucose-induced insulin secretion in pancreatic beta-cells.
Diabetes. 55:193–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang J, Wong RK, Wang X, et al: Leucine
culture reveals that ATP synthase functions as a fuel sensor in
pancreatic beta-cells. J Biol Chem. 279:53915–53923. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaneto H, Miyatsuka T, Fujitani Y, et al:
Role of PDX-1 and MafA as a potential therapeutic target for
diabetes. Diabetes Res Clin Pract. 77(Suppl 1): S127–S137. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernandes A, King LC, Guz Y, Stein R,
Wright CV and Teitelman G: Differentiation of new insulin-producing
cells is induced by injury in adult pancreatic islets.
Endocrinology. 138:1750–1762. 1997.PubMed/NCBI
|
|
14
|
Offield MF, Jetton TL, Labosky PA, et al:
PDX-1 is required for pancreatic outgrowth and differentiation of
the rostral duodenum. Development. 122:983–995. 1996.PubMed/NCBI
|
|
15
|
Waeber G, Thompson N, Nicod P and Bonny C:
Transcriptional activation of the GLUT2 gene by the
IPF-1/STF-1/IDX-1 homeobox factor. Mol Endocrinol. 10:1327–1334.
1996.PubMed/NCBI
|
|
16
|
Marshak S, Totary H, Cerasi E and Melloul
D: Purification of the beta-cell glucose-sensitive factor that
transactivates the insulin gene differentially in normal and
transformed islet cells. Proc Natl Acad Sci USA. 93:15057–15062.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iype T, Francis J, Garmey JC, et al:
Mechanism of insulin gene regulation by the pancreatic
transcription factor Pdx-1: application of pre-mRNA analysis and
chromatin immunoprecipitation to assess formation of functional
transcriptional complexes. J Biol Chem. 280:16798–16807. 2005.
View Article : Google Scholar
|
|
18
|
Melloul D, Marshak S and Cerasi E:
Regulation of insulin gene transcription. Diabetologia. 45:309–326.
2002. View Article : Google Scholar
|
|
19
|
Jonsson J, Carlsson L, Edlund T and Edlund
H: Insulin-promoter-factor 1 is required for pancreas development
in mice. Nature. 371:606–609. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kushner JA, Ye J, Schubert M, et al: Pdx1
restores beta cell function in Irs2 knockout mice. J Clin Invest.
109:1193–1201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gremlich S, Bonny C, Waeber G and Thorens
B: Fatty acids decrease IDX-1 expression in rat pancreatic islets
and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J
Biol Chem. 272:30261–30269. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Leahy JL, Cooper HE, Deal DA and Weir GC:
Chronic hyperglycemia is associated with impaired glucose influence
on insulin secretion. A study in normal rats using chronic in vivo
glucose infusions. J Clin Invest. 77:908–915. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiao CQ, Deng HM and Huang Y: Effects of
supraphysiologic concentration glucose on pancreatic duodenal
homeobox-1 expression and insulin secretion in rats. Chin Med J
(Engl). 120:1020–1023. 2007.PubMed/NCBI
|
|
24
|
Lennernäs H, Nilsson D, Aquilonius SM,
Ahrenstedt O, Knutson L and Paalzow LK: The effect of L-leucine on
the absorption of levodopa, studied by regional jejunal perfusion
in man. Br J Clin Pharmacol. 35:243–250. 1993.PubMed/NCBI
|
|
25
|
Ganapathy V and Radhakrishnan AN:
Interaction of amino acids with glycl-L-leucine hydrolysis and
transport in monkey small intestine. Clin Sci (Lond). 57:521–527.
1979.PubMed/NCBI
|
|
26
|
Rideau N and Simon J: L-leucine or its
keto acid potentiate but do not initiate insulin release in
chicken. Am J Physiol. 257:E15–E19. 1989.PubMed/NCBI
|
|
27
|
Zhang X, Sun N, Wang L, et al:
AMP-activated protein kinase and pancreatic/duodenal homeobox-1
involved in insulin secretion under high leucine exposure in rat
insulinoma beta-cells. J Cell Mol Med. 13:758–770. 2009. View Article : Google Scholar : PubMed/NCBI
|