Introduction
Cardiovascular diseases and stroke are among the
most important leading causes of morbidity and mortality worldwide.
The vascular lumen, either from the peripheral organs or the
central nervous system, is covered with endothelial cells.
Endothelial cells not only respond to but also produce and release
substances that relax or constrict the blood vessels, which may
contribute to the development of vascular failure (1). Endothelial cells are also of great
importance in disease recovery. The formation of new blood vessels,
or angiogenesis, is necessary to fully supply tissues with their
metabolic and functional requirements in the long-term. The
proliferation and migration of endothelial cells is essential for
angiogenesis (2). Systemic
administration of human cord blood-derived CD34+ cells
to immunocompromised mice subjected to stroke 48 h earlier induces
neovascularization in the ischemic zone and provides a favorable
environment for neuronal regeneration (3). Although CD34+ circulating
endothelial progenitor cells have the capacity to participate in
neovascularization in ischemic tissues (4–5), the
outgrowth of pre-existing vasculature is assumed to be
indispensable in the postnatal development of neovessels. Thus, the
capacity for proliferation and migration in endothelial cells is
crucial for the recovery of vascular diseases, including
cardiovascular diseases and stroke.
Tetramethylpyrazine (TMP), a biologically active
alkaloid isolated from the rhizome of the traditional herbal
medicine Ligusticum walliichi (Chuanxiong), has been used
routinely in China for the treatment of stroke and other vascular
diseases. TMP, or its derivatives, was reported to scavenge free
radicals, inhibit Ca2+ influx (6), increase the transcription of
thioredoxin (7) and suppress the
inflammatory response (8), thus
protecting the neurons in rat ischemic stroke models. The effects
of TMP on the proliferation and migration of endothelial cells,
however, have not been well explored. Therefore, the present study
focused on the proliferation and migration of endothelial cells
induced by TMP and its mechanisms with the aim of identifying
possible novel targets for the treatment of cardiovascular diseases
and stroke.
Materials and methods
Endothelial cell proliferation assay
The immortalized mouse brain microvascular
endothelial cell line bEnd.3, purchased from the American Type
Culture Collection (Manassas, VA, USA), were grown in complete
medium consisting of DMEM GlutaMAX, supplemented with 1%
penicillin/streptomycin and 10% FCS (Gibco-BRL, Melbourne,
Australia).
For the proliferation assay, 1×104 cells
in 100 μl complete medium were seeded into each well of a 96-well
plate. Following 24 h, different concentrations of TMP (Chinese
National Institute for the Control of Pharmaceutical and Biological
Products, Beijing, China) or combined with soluble FasL (Sigma, St.
Louis, MO, USA) were used to stimulate endothelial cells for 48 h.
At the end of the cell culture, 20 μl of CCK-8 solution (5 mg/ml;
Dojindo, Kumamoto, Japan) was added into each well and cells were
incubated for an additional 4 h. Cell proliferation was measured
with a microplate reader at 450 nm and the proliferation index was
calculated as follows: (OD450 in the presence of TMP - OD450 in the
blank control)/OD450 in the blank control × 100%.
Endothelial cell migration assay
The migration of endothelial cells were assayed in
an in vitro scraping experiment as described elsewhere.
Briefly, cells were grown in a 6-well plate. When the cell
confluence reached 60%, cells were injured by a deliberate scratch
with a 1,000 μl pipette tip and stimulated with TMP alone (0.25
ng/ml) or combined with sFasL (0.16 ng/ml). Cells were washed with
PBS softly and images were captured, 24 h after injury. Distance of
the scratch was calibrated in triplicate in a blinded manner.
ELISA for VEGF
Cells grown in the 6-well plate were treated with
TMP alone or combined with soluble FasL for 72 h. The supernatant
was harvested and stored in −80°C until further analysis. ELISA for
the VEGF was performed according to the manufacturer’s instructions
(R&D Systems, Minneapolis, MN, USA). Briefly, 50 μl assay
diluents were added to each well, followed by the addition of 50 μl
standard, control or cell culture supernatant and the plate was
incubated at room temperature for 2 h. Following incubation, each
well was aspirated and washed five times and 100 μl conjugated
secondary antibody was added into each well for 2 h at room
temperature. Following extensive washing, 100 μl of substrate
solution was added to each well for 30 min at room temperature.
Finally, 100 μl of stop solution was added to each well and the
absorbance was read at 450 nm on an ELISA reader (Bio-Rad,
Hercules, CA, USA) within 30 min. All readings were repeated at
least three times.
Western blotting for the Fas protein
Cells grown in the 6-well plate were treated with
TMP alone or combined with soluble FasL for 72 h. Following being
washed twice with ice-cold PBS, an ice-cold cell lysis buffer was
added to the cells and the solution was passed through a pipette
several times. The homogenates were centrifuged at 4°C and 18,188 ×
g for 30 min. Proteins were measured using the BCA assay. Equal
amounts of protein samples were separated using electrophoresis on
10% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and
transferred onto polyvinylidene difluoride (PVDF) membranes. The
membranes were inhibited with Tris-buffered saline Tween-20 (TBST)
containing 5% (w/v) skimmed milk at room temperature for 2 h. The
membranes were incubated with anti-Fas (dilution 1:2,000; KeyGen
Biotech, Nanjing, Jiangsu, China) overnight at 4°C. Following
washing, the membranes were incubated with HRP-conjugated secondary
antibodies for 1 h at room temperature. Antigen was detected using
enhanced chemiluminescence (Pierce Biotechnology, Inc., Rockford,
IL, USA). All the samples were normalized to β-actin.
Imaging and statistical analysis
Blots were scanned and quantified using Image J
software. Blots were quantified as follows: the value attained for
each sample was divided by the value of the corresponding β-actin
and then expressed as a normalized ratio.
Data are expressed as the mean ± SEM. Intergroup
comparisons were performed using one-way analysis of variance
(ANOVA) for multiple comparisons followed by Fisher’s protected
least significant difference (PLSD). P<0.05 was considered to
indicate a statistically significant difference.
Results
Proliferation of endothelial cells
stimulated by low-dosage TMP
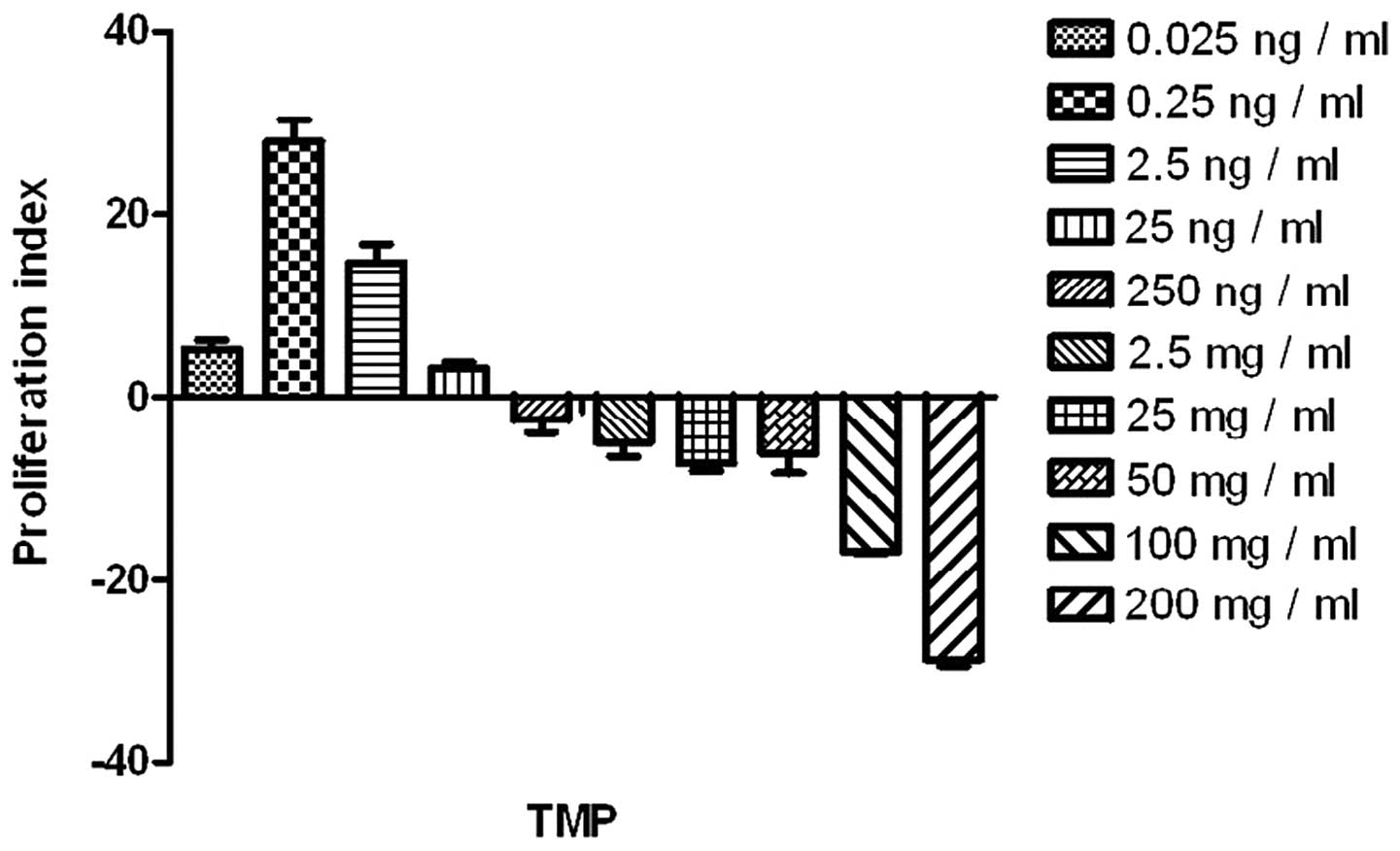
TMP at a low-dosage, ranging from 0.025 ng/ml to
0.25 ng/ml, promoted the proliferation of the endothelial cells
significantly (compared with the blank control; P<0.05). TMP at
a higher dosage, particularly >100 ng/ml, impaired the
endothelial cells (Fig. 1). Thus,
TMP at 0.25 ng/ml was selected for the analysis of the combined
effects with sFasL.
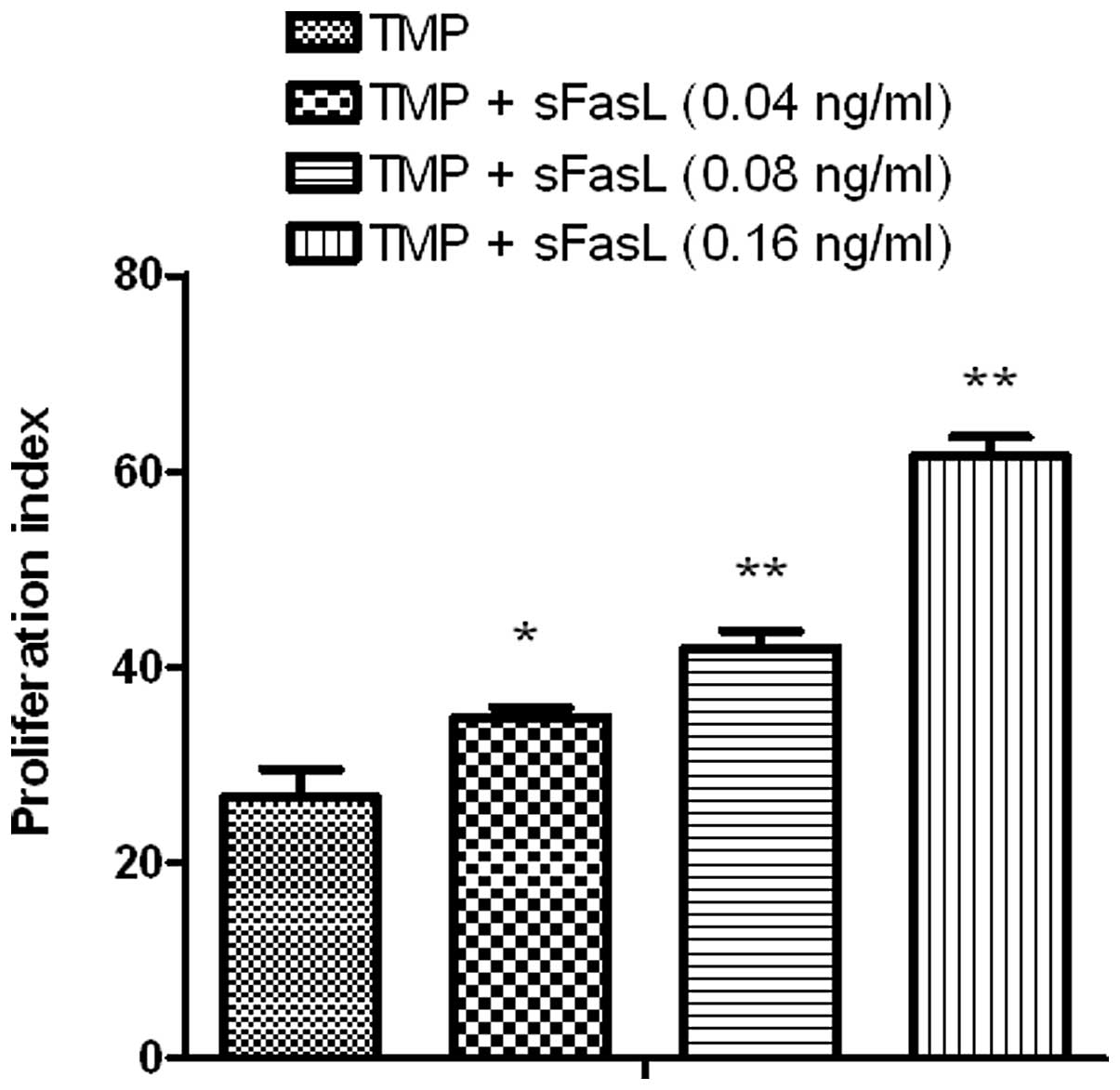
Effect of sFasL on the TMP-stimulated
proliferation of endothelial cells
As depicted in Fig.
1, TMP at 0.25 ng/ml could stimulate the division of
endothelial cells and sFasL (0.16 ng/ml) could also stimulate the
proliferation of endothelial cells via the Fas-FasL pathway.
Notably, compared with TMP alone, TMP combined with sFasL was a
more powerful stimulant for the proliferation of endothelial cells
(P<0.05; Fig. 2).
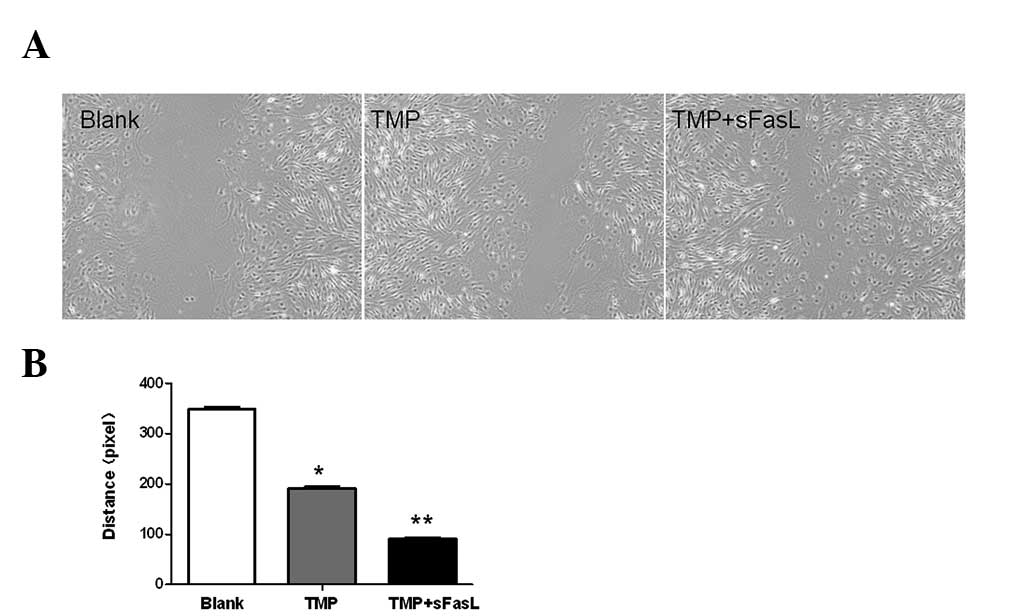
TMP combined with sFasL enhances the
migration of endothelial cells
TMP promoted the proliferation of endothelial cells.
Endothelial cell migration is also essential for angiogenesis. In
the present study, TMP could also enhance the migration of
endothelial cells, which is more evident if combined with sFasL
(P<0.05; Fig. 3).
Fas expression increases upon stimulation
of TMP
TMP and sFasL could promote the proliferation and
migration of endothelial cells. In addition, TMP also increased the
expression of Fas on endothelial cells (P<0.05; Fig. 4).
TMP promotes the autocrine signaling of
VEGF by endothelial cells
The proliferation and migration of endothelial cells
were mediated by VEGF. Accordingly, the endothelial cells secreted
significantly more VEGF upon the stimulation of TMP. sFasL
combination further increased the autocrine signaling of VEGF
(Fig. 5).
Discussion
Previously, it was verified that TMP suppressed the
production of nitric oxide in human umbilical vein endothelial
cells (HUVECs) stimulated with TNF-α (9). TMP also decreased the expression of
intracellular adhesion molecule-1 and heat shock protein 60,
suggesting its anti-inflammatory role in endothelial cells
(9). As a reactive oxygen species
antagonist, TMP protected the rat pulmonary microvascular
endothelial cells from hypoxia and TMP-treated animals demonstrated
less pulmonary vascular leakage compared with those exposed to
hypoxia alone (10). It was also
reported that TMP, or its derivatives, suppressed the apoptosis of
HUVECs induced by hydrogen peroxide (11). Whether TMP could modulate the
proliferation and migration of endothelial cells, however, is
largely unknown.
The dosage effects of TMP on endothelial cells were
first investigated. TMP at a rather lower dosage (0.025 ng/ml or
0.25 ng/ml) significantly promoted the proliferation of the
endothelial cells, while TMP at a concentration >100 ng/ml
appeared negative for the proliferation of endothelial cells in the
physiological condition. Plasma concentration may affect the
efficiency of TMP in the treatment of stroke (12). The results presented in the present
study suggest that TMP overdose may also be harmful, which is
occasionally reported in clinical practice.
TMP promoted not only the proliferation but also the
migration of the endothelial cells, which was further improved by
sFasL. Considering the Fas-FasL pathway in angiogenesis, it may be
valuable to detect whether TMP could regulate the Fas-FasL pathway
in endothelial cells. Fas-FasL ligation induced proliferation by
recruiting the Fas-associated death domain protein and the
Flice-like inhibitory protein (FLIP). FLIP further recruited and
activated the downstream molecules TNF-receptor-associated factor
and nuclear factor κB (NF-κB), which eventually promoted the
proliferation of the cells. In the photoreceptor cells damaged by
N-methyl-N-nitrosourea, TMP increased the expression of NF-κB and
suppressed the apoptosis of cells (13). NF-κB is one of the downstream
molecules of the Fas-FasL pathway. As verified in the present
study, TMP also directly upregulated the expression of Fas, which
may expand our understanding of the underlying mechanisms of TMP on
endothelial cells.
In summary, TMP promoted the proliferation and
migration of endothelial cells, which was further improved by
sFasL. The positive roles of TMP on the endothelial cells was
partially dependent on the increased expression of Fas and enhanced
secretion of VEGF. Further study may elucidate whether Fas could be
a novel target in the treatment of vascular diseases and
stroke.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81171659) and the Natural
Science Foundation of Jiangsu Province (no. BE2010768) to C.B.
Zhang.
References
|
1
|
Hirase T and Node K: Endothelial
dysfunction as a cellular mechanism for vascular failure. Am J
Physiol Heart Circ Physiol. 302:H499–H505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fahmy RG, Dass CR, Sun LQ, et al:
Transcription factor Egr-1 supports FGF-dependent angiogenesis
during neovascularization and tumor growth. Nat Med. 9:1026–1032.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taguchi A, Soma T, Tanaka H, et al:
Administration of CD34+cells after stroke enhances
neurogenesis via angiogenesis in a mouse model. J Clin Invest.
114:330–338. 2004.
|
|
4
|
Asahara T, Masuda H, Takahashi T, et al:
Bone marrow origin of endothelial progenitor cells responsible for
postnatal vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar
|
|
5
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun Y, Yu P, Zhang G, et al: Therapeutic
effects of tetramethylpyrazine nitrone in rat ischemic stroke
models. J Neurosci Res. 90:1662–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu XL, Xiong LZ, Wang Q, et al:
Therapeutic time window and mechanism of tetramethylpyrazine on
transient focal cerebral ischemia/reperfusion injury in rats.
Neurosci Lett. 449:24–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao SL, Kao TK, Chen WY, et al:
Tetramethylpyrazine reduces ischemic brain injury in rats. Neurosci
Lett. 372:40–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu HJ, Hao J, Wang SQ, et al: Protective
effects of ligustrazine on TNF-alpha-induced endothelial
dysfunction. Eur J Pharmacol. 674:365–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Deng M and Zhou S:
Tetramethylpyrazine inhibits hypoxia-induced pulmonary vascular
leakage in rats via the ROS-HIF-VEGF pathway. Pharmacology.
87:265–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhai L, Zhang P, Sun RY, et al:
Cytoprotective effects of CSTMP, a novel stilbene derivative,
against H2O2-induced oxidative stress in human endothelial cells.
Pharmacol Rep. 63:1469–1480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ho WK, Wen HL and Lee CM:
Tetramethylpyrazine for treatment of experimentally induced stroke
in Mongolian gerbils. Stroke. 20:96–99. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang JN, Chen JM, Luo L, et al:
Tetramethylpyrazine protected photoreceptor cells of rats by
modulating nuclear translocation of NF-kappaB. Acta Pharmacol Sin.
26:887–892. 2005. View Article : Google Scholar : PubMed/NCBI
|