Introduction
Autophagy is an evolutionarily conserved biological
process in which under certain types of cellular stress, damaged
organelles and long-lived proteins are encapsidated and directed to
the lysosome for degradation (1,2). The
cytoplasmic content is sequestered in autophagosomes, which are
double-membraned vesicles that fuse with lysosomes to form
autophagolysosomes, in which the cellular material is degraded by
acidic lysosomal hydrolases. In addition to its physiological role
in the elimination of aged or damaged cell components, autophagy
acts as an important mechanism for cellular homeostasis and a
survival mechanism for cells undergoing nutrient deprivation or
other stresses (3). Furthermore,
an increasing number of studies have shown that autophagy is
involved in programmed cell death and may lead to cell death or
have a cytoprotective function (4,5). As
an important mediator of pathological responses, autophagy has
attracted increasing attention, particularly in cancer research
(6,7).
Curcumin is a yellow, dietary polyphenol derived
from the rhizomes of Curcuma longa. Numerous studies have
demonstrated that curcumin has anti-inflammatory, -oxidative and
-carcinogenic effects in various types of tumor cells (8–11).
However, based on its instability under certain physiological
conditions, poor bioavailability and rapid metabolism, the
application of curcumin in anticancer therapy has been limited
(12). The generation of synthetic
curcumin analogs may overcome the limitations associated with
curcumin. Several studies have generated novel synthetic analogs or
derivatives of curcumin in order to enhance the anti-proliferative
activity of curcumin (13–15); however, such analogs have generally
led to cancer cell death through apoptosis.
In the present study, a novel derivative of curcumin
was chemically synthesized, termed 2E,6E-2-(1H-indol-3-yl)
methylene)-6-(4-hydroxy-3-methoxy benzylidene)-cyclohexanone (IHCH)
(Fig. 1). The present study aimed
to investigate the cell death pathway induced by IHCH in A549
cells. IHCH was found to have an anti-proliferative effect in A549
cells by inducing autophagy. Acridine orange staining and
monodansylcadaverine (MDC) fluorescence analysis were used to
monitor autophagolysosomes and autophagic vacuoles, respectively.
Immunocytochemistry of light chain (LC) 3 localization detected
recruitment of LC3 to autophagic vesicles. Furthermore, western
blot analysis was used to assess the conversion of LC3-I to LC3-II
in the IHCH treated A549 cells. The present study identified that
autophagy is an important process involved in IHCH-induced cell
death.
Materials and methods
Chemicals and cell culture
IHCH was obtained from the College of Chemistry and
Chemical Engineering, Henan University of Technology (Zhengzhou,
China) and was dissolved in dimethylsulfoxide (DMSO; stock
solution, 0.1 M). Wortmannin was purchased from the Beyotime
Institute of Biotechnology (Shanghai, China). MDC and acridine
orange were purchased from Sigma-Aldrich (St. Louis, MO, USA) and
fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit
antibodies were purchased from Jackson ImmunoResearch Inc. (West
Grove, PA, USA). A549 cells were maintained at Henan University of
Technology (Zhengzhou, China) in RPMI-1640 medium (Gibco-BRL, Grand
Island, NY, USA) containing 1% penicillin and 10% (v/v) fetal
bovine serum (Hyclone, Rockford, IL, USA) in a humidified
atmosphere containing 5% CO2 at 37°C.
Cell proliferation assays
A549 cells were plated in 24-well culture plates
(Corning Inc., Corning, NY, USA) and treated with IHCH at different
concentrations (1, 5, 10, 20 and 40 mM) for different time periods
(1, 3, 6, 12, 24 and 36 h). Cell morphology was analyzed using an
inverted microscope (Nikon Eclipse TS100; Nikon Corporation, Tokyo,
Japan). All experiments were repeated at least three times.
2E,6E-2-(1H-indol-3-yl)
methylene)-6-(4-hydroxy-3-methoxy benzylidene)-cyclohexanone (MTT)
analysis
Cells were seeded on 96-well plates and treated with
IHCH at 1, 5, 10, 20 or 40 mM for 6, 12, 24 or 48 h. MTT (5 mg/ml)
was filter sterilized and added to each well (20 μl). Plates were
kept in the dark for 4 h at 37°C until a purple precipitate was
visible. DMSO (100 μl/well) was then added and the absorbance was
read using an ELISA reader (BioTek, Winooski, VT, USA) at 490 nm.
The percentage of cell viability was assessed using the following
formula: Cell viability (%) = (100−Am/An) ×
100. Am and An represent the absorbance of
the test substances and solvent control, respectively.
Acridine orange staining
Acridine orange staining was used to analyze
autophagic vacuoles (16). A549
cells were treated with IHCH at 1, 5, 10, 20 or 40 μM for 3, 6, 12
or 24 h. Cells were then washed twice in phosphate-buffered saline
(PBS) and stained with 5 μg/ml acridine orange for 40 sec at room
temperature. Cell micrographs were analyzed using a fluorescence
microscope (Nikon Eclipse TE2000-U; Nikon Corporation).
MDC labeling assay
Cells were seeded on 24-well flat bottomed plates
over night followed by treatment with different concentrations of
IHCH for 3 h. Subsequent to treatment with hydrazinobenzoylcurcumin
(IHCH) at identical concentrations and time points, cells were
incubated for 10 min with 50 μM MDC at 37°C and observed using
fluorescence microscopy with a 380 nm excitation filter.
Immunocytochemistry analysis
Cells were seeded on coverslips in six-well plates
and treated with IHCH for 3 h. Subsequent to washing with PBS and
fixing in 4% paraformaldehyde for 15 min at room temperature, A549
cells were blocked using 5 mg/ml bovine serum albumin for 30 min.
Cells were then incubated with anti-LC3 antibodies (Beyotime
Institute of Biotechnology) diluted 1:500 in PBS, followed by
FITC-conjugated goat anti-rabbit antibodies diluted 1:100 in PBS
for 2 h. Slides were then analyzed using a fluorescence microscope
(Nikon TE2000-U; Nikon Corporation).
Western blot analysis
A549 cells were cultured in 60 mm round dishes in
the presence of 1, 10, 20 or 40 μM IHCH for 3 h, and harvested and
lysed in cold lysis buffer (150 mM NaCl, 1% NP-40, 20 mM Tris-HCl,
20 mg/ml aprotinin, 20 mg/ml leupeptin, 1 mM orthovanadate and 100
mM phenylmethanesulfonyl fluoride, pH 7.4). The cell lysates were
electrophoresed using 15% SDS-PAGE and transferred to
nitrocellulose membranes (Immobilon™; Millipore, Billerica, MA).
Membranes were blocked with 8% non-fat dry milk in Tris-buffered
saline containing Tween-20 at room temperature for 1 h, then
incubated with rabbit anti-LC3B antibodies diluted 1:1,000 in PBS,
followed by alkaline phosphatase-conjugated goat anti-rabbit
immunoglobulin G (Vector Laboratories Inc., Burlingame, CA, USA)
diluted 1:1,000 in PBS at 4°C for 18 h. Tubulin was used as an
internal control. Membranes were washed and subsequently incubated
with substrate solution containing nitroblue tetrazolium and
bromo-4-chloro-3-indoxyl-phosphate (Boster Biological Technology,
Ltd., Wuhan, China). Image J (National Institutes of Health,
Bethesda, MA, USA) was used to quantify the intensity of each
protein band. Band intensity values were presented as the fold
increase or decrease with respect to the control bands.
Statistical analysis
Experiments were performed three times and data are
presented as the mean ± standard deviation. Differences between
mean values were analyzed using Student’s t-test. P<0.05 or
<0.01 were considered to indicate statistically significant
differences.
Results
IHCH inhibits A549 cell
proliferation
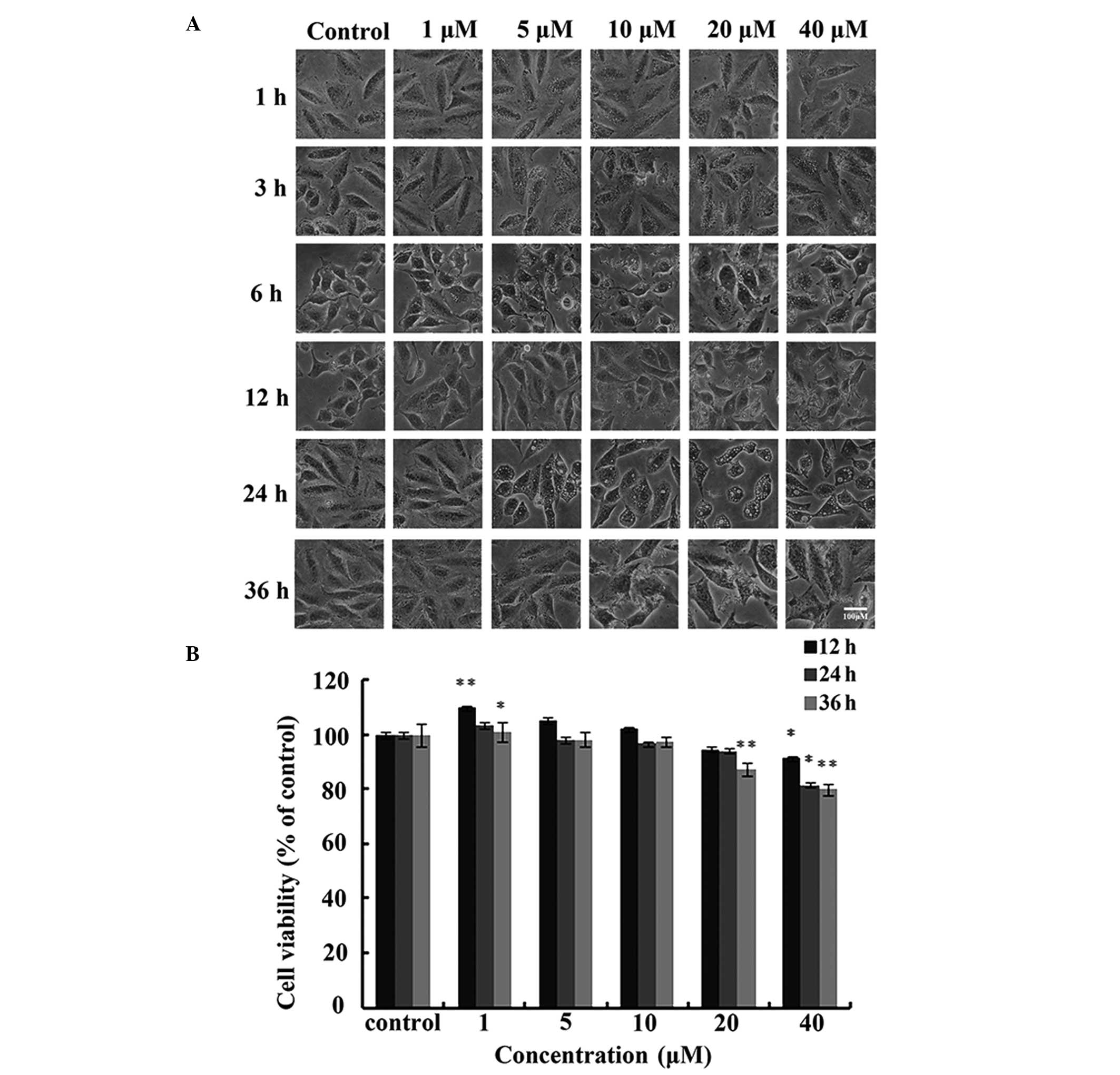
A549 cells were treated with different
concentrations of IHCH (1–40 μM) for 36 h. DMSO was used as a
negative control. After 3 h, the IHCH-treated cells exhibited
vacuole-like structures. Furthermore, significant changes were
observed in cell morphology after 6 h of IHCH treatment (Fig. 2A). MTT was used to assess the
effect of IHCH on A549 cell viability and showed that cell
viability was reduced to 77.34% after 36 h of treatment with 40 μM
IHCH (Fig. 2B).
IHCH induces the formation of acidic
vesicular organelles (AVOs) in A549 cells
Autophagolysosomes generate an acidic compartment,
which may be fluorescently stained red or green using acridine
orange (17). To determine whether
IHCH induced autophagy in A549 cells, acridine orange staining was
performed in IHCH-treated A549 cells. Autophagy is associated with
an increase in acridine orange positive AVOs. Red AVOs were
observed following 3 h of IHCH treatment (1–40 μM) in the A549
cells (Fig. 3A), suggesting AVO
formation. Furthermore, the percentage of cells exhibiting AVOs was
analyzed among 100 IHCH-treated cells. After 3 h of IHCH treatment,
the percentage of cells with AVOs was found to increase in a
concentration-dependent manner (1–20 μM) (Fig. 3B).
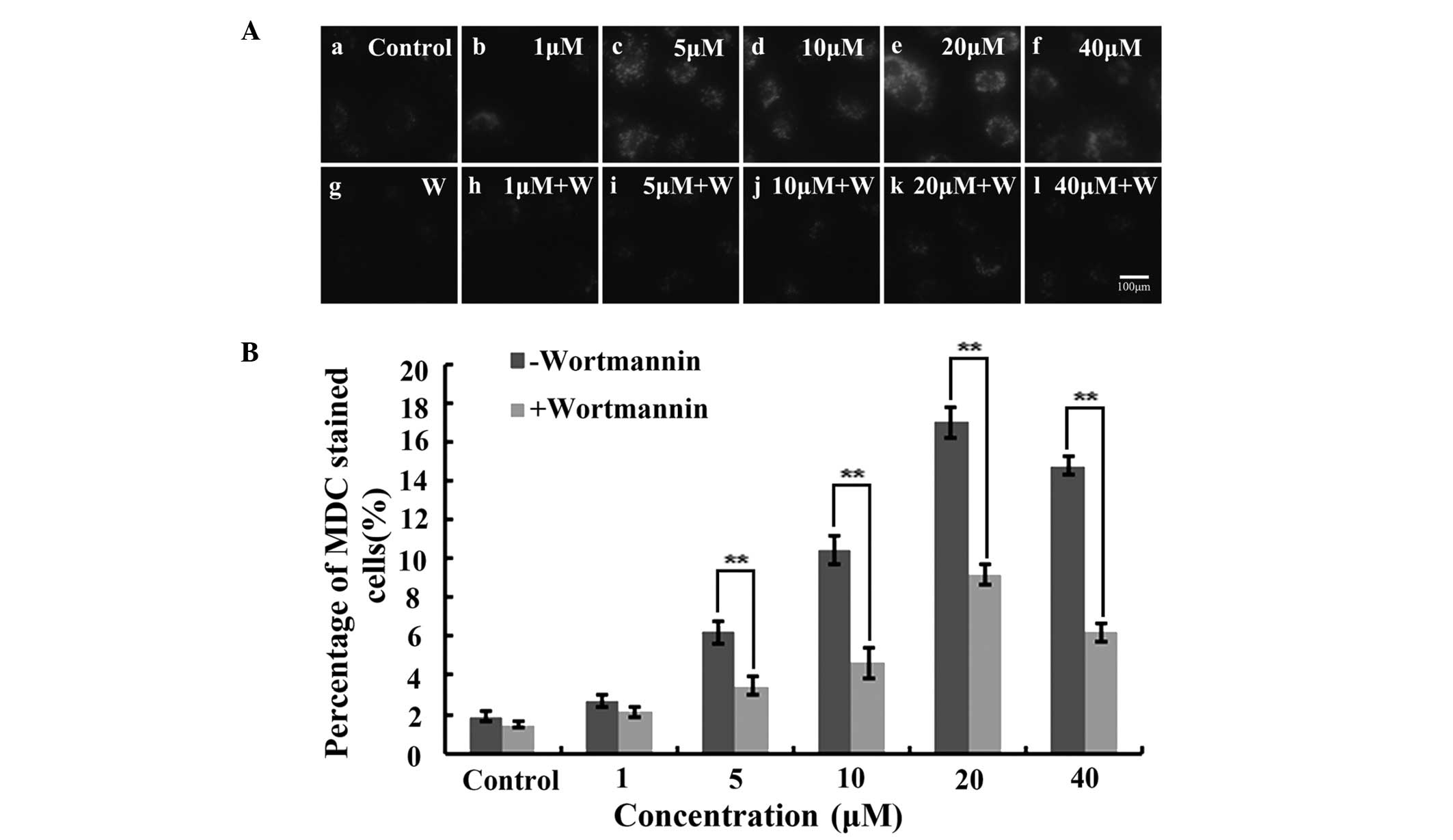
Wortmannin inhibits IHCH-induced A549
cell autophagy
As a specific dye for autophagosomes, MDC
accumulates in mature autophagic vacuoles (AVs) and
autophagolysosomes, but not in the early endosome compartment. In
the present study, MDC-stained AVs appeared as distinct dot-like
structures distributed within the cytoplasm or localized in the
perinuclear region. Furthermore, the highest number of dot-like
structures were observed after 3 h of treatment with 20 μM IHCH
(Fig. 4A). Wortmannin was also
used to treat the IHCH-incubated A549 cells. As a highly specific
inhibitor of phosphatidylinositol-4,5-bisphosphate 3-kinase,
wortmannin is capable of inhibiting the Akt signaling pathway and
cell autophagy (18). In the
present study, wortmannin was found to decrease the intensity of
the MDC fluorescence at the different concentrations of IHCH
(Fig. 4A). Furthermore, the rate
of cell autophagy following wortmannin treatment was observed to be
lower than that in the untreated cells (Fig. 4B).
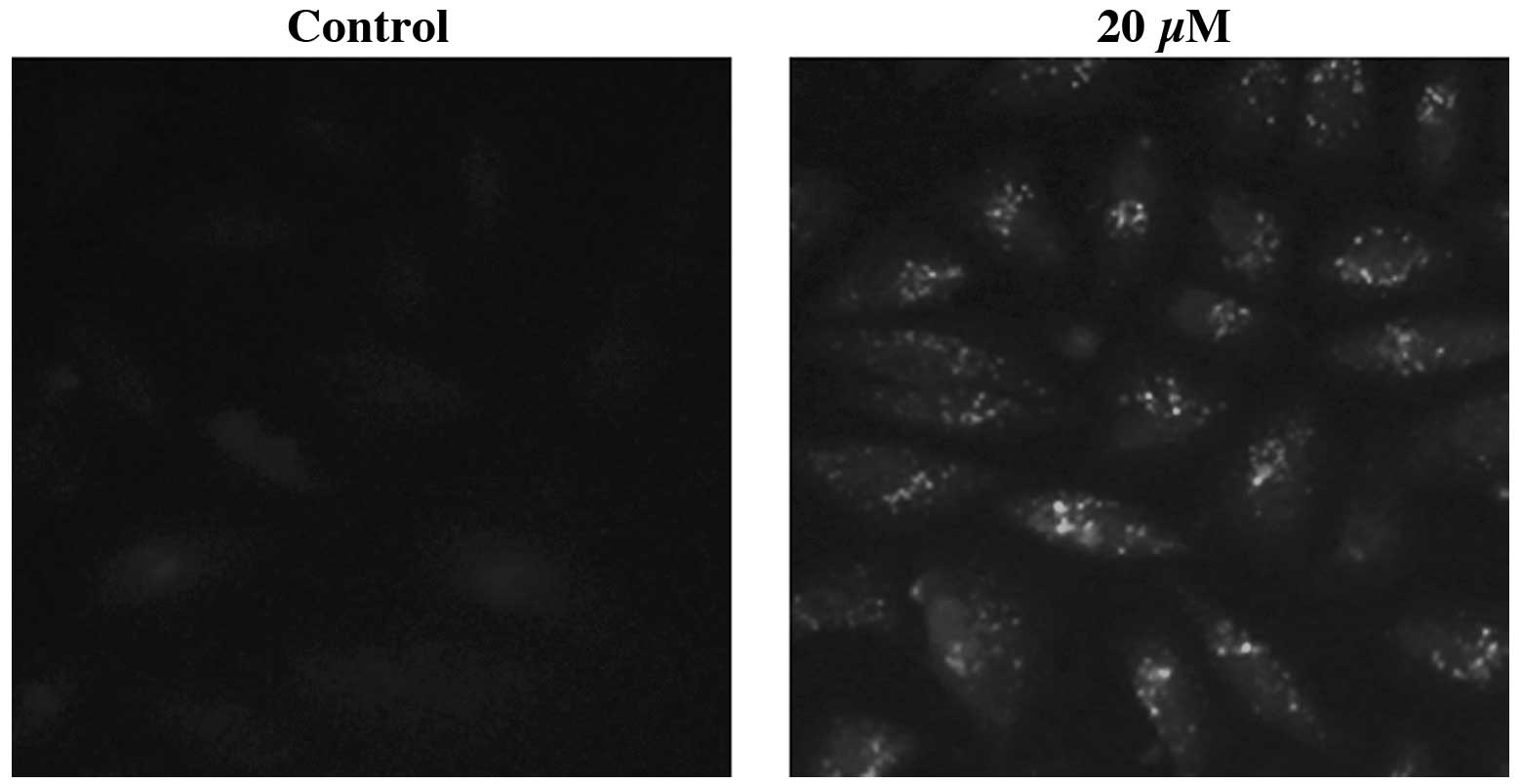
IHCH induces LC3 protein
accumulation
The intracellular localization of the LC3 protein
was analyzed using immunocytochemistry. IHCH-treated (20 μM; 3 h)
A549 cells were immunostained using anti-LC3 primary antibodies.
Fluorescence microscopy revealed that the specific fluorescent
signals were punctate, which is typical of the distribution of
LC3-II within autophagosomes (Fig.
5). The DMSO-treated control cells showed no LC3
immunofluorescence.
IHCH induces the conversion of LC3-I to
LC3-II
Western blot analysis was used to detect LC3-II and
-I, with increases in the LC3-II/LC3-I ratio indicative of
autophagy (19). Lysates of A549
cells treated with DMSO (40 μM) or IHCH (1–40 μM) for 3 h were
subjected to western blot analysis. Fig. 6A shows the conversion of LC3-I to
LC3-II. The level of autophagy was represented as the ratio of
LC3-II expression to tubulin expression (Fig. 6B). LC3-II protein expression was
observed to significantly increase in the IHCH-treated cells in a
dose-dependent manner. This finding suggests IHCH induced autophagy
in the A549 cells.
Discussion
A number of bioactive compounds are phytochemicals,
which have been found to demonstrate growth suppressive activity as
well as chemopreventive properties against various types of cancer
(20). One of the most widely
characterized phytochemicals is curcumin, whose inhibitory effects
on tumorigenesis and tumor growth have been confirmed in
vitro and in vivo (21). Due to the low bioavailability of
curcumin, curcumin alternatives have been investigated. In our
previous study, an analog of curcumin, HBC, was chemically
synthesized and its inhibitory effect on A549 lung cancer cells was
identified (22).
In the present study, an MTT assay revealed that 40
μM IHCH inhibited cell proliferation after 36 h. In order to
investigate the cell death mechanism associated with this
IHCH-induced decrease in cell proliferation, IHCH-treated A549
cells were subjected to acridine orange and MDC staining, as well
as immunofluorescent and western blot analyses. Acridine orange
staining revealed a concentration-dependent increase in red
fluorescent structures after 3 h of IHCH treatment. However, the
red fluorescence in the A549 cells was found to decrease with
increasing IHCH treatment duration. Thus, all subsequent
experiments were performed using A549 cells treated with IHCH for 3
h. MDC staining revealed a concentration-dependent increase in
fluorescent structures in the IHCH-treated A549 cells, which was
inhibited upon pretreatment with wortmannin (100 nM). Wortmannin is
a typical inhibitor of autophagy (18). These findings suggest that IHCH may
lead to A549 cell death through inducing autophagy.
As a key marker of cell autophagy, LC3 is associated
with autophagosome expression. In order to investigate whether IHCH
induces A549 cell autophagy, immunofluoresence and western blot
analyses of LC3 protein expression were performed.
Immunofluorescence revealed punctate accumulation of LC3 in the
cytoplasm of A549 cells in response to IHCH. Furthermore, western
blot analysis showed a dose-dependent increase of LC3-II expression
in IHCH-treated A549 cells. These findings show that IHCH induces
A549 cell death through autophagy; however, the specific autophagy
pathway involved requires further investigation. The present study
has shown that IHCH may have potential as a therapeutic,
anti-proliferative agent in cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 31101931 and
81172240), the High-Level Talents Fund from Henan University of
Technology (grant no. 2010BS016) and the Plan for Scientific
Innovation Talent of Henan University of Technology (grant no.
11CXRC13).
References
|
1
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiøtz BL, Roos N, Rishovd AL and Gjøen
T: Formation of autophagosomes and redistribution of LC3 upon in
vitro infection with infectious salmon anemia virus. Virus Res.
151:104–107. 2010.PubMed/NCBI
|
|
4
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gurusamy N and Das DK: Autophagy, redox
signaling, and ventricular remodeling. Antioxid Redox Signal.
11:1975–1988. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Liang B, Shirwany NA and Zou MH:
2-Deoxy-D-glucose treatment of endothelial cell induces autophagy
by reactive oxygen species-mediated activation of the AMP-activated
protein kinase. PLoS One. 6:e172342011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang PY, Peng SF, Lee CY, et al:
Curcumin-loaded nanoparticles induce apoptotic cell death through
regulation of the function of MDR1 and reactive oxygen species in
cisplatin-resistant CAR human oral cancer cells. Int J Oncol.
43:1141–1150. 2013.
|
|
9
|
Norris L, Karmokar A, Howells L, Steward
WP, Gescher A and Brown K: The role of cancer stem cells in the
anti-carcinogenicity of curcumin. Mol Nutr Food Res. 57:1630–1637.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ranjan AP, Mukerjee A, Helson L, Gupta R
and Vishwanatha JK: Efficacy of liposomal curcumin in a human
pancreatic tumor xenograft model: inhibition of tumor growth and
angiogenesis. Anticancer Res. 33:3603–3609. 2013.PubMed/NCBI
|
|
11
|
Doggui S, Belkacemi A, Paka GD, Perrotte
M, Pi R and Ramassamy C: Curcumin protects neuronal-like cells
against acrolein by restoring Akt and redox signaling pathways. Mol
Nutr Food Res. 57:1660–1670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumaravel M, Sankar P and Rukkumani R:
Antiproliferative effect of an analog of curcumin
bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione in human breast
cancer cells. Eur Rev Med Pharmacol Sci. 16:1900–1907.
2012.PubMed/NCBI
|
|
14
|
Xiao J, Wang Y, Peng J, et al: A synthetic
compound, 1,5-bis(2-methoxyphenyl)penta-1,4-dien-3-one (B63),
induces apoptosis and activates endoplasmic reticulum stress in
non-small cell lung cancer cells. Int J Cancer. 131:1455–1465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faião-Flores F, Suarez JA, Maria-Engler
SS, Soto-Cerrato V, Pérez-Tomás R and Maria DA: The curcumin analog
DM-1 induces apoptotic cell death in melanoma. Tumor Biol.
34:1119–1129. 2013.PubMed/NCBI
|
|
16
|
Arsikin K, Kravic-Stevovic T, Jovanovic M,
et al: Autophagy-dependent and -independent involvement of
AMP-activated protein kinase in 6-hydroxydopamine toxicity to
SH-SY5Y neuroblastoma cells. Biochim Biophys Acta. 1822:1826–1836.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paglin S, Hollister T, Delohery T, et al:
A novel response of cancer cells to radiation involves autophagy
and formation of acidic vesicles. Cancer Res. 61:439–444.
2001.PubMed/NCBI
|
|
18
|
Buchanan CM, Dickson JM, Lee WJ, Guthridge
MA, Kendall JD and Shepherd PR: Oncogenic mutations of p110a
isoform of PI 3-kinase upregulate its protein kinase activity. PLoS
One. 8:e713372013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kabeya Y, Mizushima N, Yamamoto A,
Oshitani-Okamoto S, Ohsumi Y and Yoshimori T: LC3, GABARAP and
GATE16 localize to autophagosomal membrane depending on form-II
formation. J Cell Sci. 117:2805–2812. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar
|
|
22
|
Zhou GZ, Zhang SN, Zhang L, Sun GC and
Chen XB: A synthetic curcumin derivative hydrazinobenzoylcurcumin
induces autophagy in A549 lung cancer cells. Pharm Biol.
52:111–116. 2014. View Article : Google Scholar : PubMed/NCBI
|